Abstract
The polyphagous shot hole borer beetle (PSHB, Euwallacea fornicatus) is a pest of global significance. PSHB is an ambrosia beetle which, together with its mutualistic fungi (including Fusarium euwallaceae), can cause the death of more than 100 tree species in invaded ranges. Management of PSHB mostly relies on the removal of infested plant material. Chemical control options have been investigated only in the USA and Israel and only on a few tree species. This study evaluated four chemical treatments for the therapeutic control of PSHB on American sweetgum (Liquidambar styraciflua) in South Africa (1) bifenthrin + surfactant (alcohol ethoxylate), (2) cypermethrin + surfactant (vitamin E) + salicylic acid, (3) emamectin benzoate, and (4) propiconazole. Trees were inoculated with F. euwallaceae and mature PSHB females using a novel technique to document fungal lesion development and PSHB colony establishment success. The bifenthrin and cypermethrin treatments reduced additional PSHB colonisation attempts on treated trees by ca. 40%, while the other treatments had no effect. Colony establishment success was reduced in all treatments by between 20 and 40%. Fungal growth was inhibited only after the application of propiconazole by ca. 36%. Gallery length and the number of PSHB individuals in successful colonies were unaffected by any of the chemical treatments. These results indicate that chemical control of PSHB is only partially effective. Successful PSHB management will likely depend on a combination of chemical control options and other control strategies in an integrated pest management program.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The polyphagous shot hole borer (PSHB, Euwallacea fornicatus, Coleoptera: Curculionidae: Scolytinae) is an ambrosia beetle that has become a global pest within the past few decades. Originating from Southeast Asia, it has now been found in Israel (Mendel et al. 2012), the United States (Eskalen et al. 2012), South Africa (Paap et al. 2018), Australia (Government of Western Australia 2021), Poland, Italy, Germany and the Netherlands (Schuler et al. 2023). The PSHB vectors multiple species of fungi, which grow within galleries that the beetle constructs in the xylem tissues of woody hosts (Freeman et al. 2019). The beetle carries these fungi in specialised organs called mycangia (Hulcr and Dunn 2011; Six 2012). Mutualistic fungal species vectored, such as Fusarium euwallaceae, grow into the xylem tissues and along gallery walls, providing nutrition for developing larvae and adults (Freeman et al. 2016). The tunnelling action of the beetle combined with xylem tissues becoming blocked by F. euwallaceae causes Fusarium dieback, a disease that can lead to the death of highly susceptible host trees (Umeda et al. 2016).
This pest-disease complex can affect a wide range of tree species in invaded regions (Gomez et al. 2019). Since its first report in South Africa in 2017 (Paap et al. 2018), 161 species have been found to host the beetle and/or F. euwallaceae (FABI 2023). However, not all attempts on trees result in successful colony formation. For many tree species, the beetle attempts colonisation and successfully establishes F. euwallaceae in the xylem, but thereafter it fails to establish a viable colony (Eskalen et al. 2013; Lynch et al. 2021). The fungus may persist and cause disease symptoms, but fungal establishment alone is usually not fatal. In other hosts, the beetle readily establishes breeding colonies. These hosts are more likely to develop symptoms of Fusarium dieback, and many can die from the disease (Eskalen et al. 2013; Lynch et al. 2021). Currently, there are 83 of these PSHB reproductive host species (41 indigenous and 42 alien) reported in South Africa (FABI 2023), though more will likely be added to the list as the invasion extends (van Rooyen et al. 2021). Without implementing effective management strategies, the ecological and economic implications of the PSHB for South Africa will be severe (de Wit et al. 2022; Paap et al. 2020; van Rooyen et al. 2021). Current mitigation methods are centered on the removal of affected material, either by pruning of infested branches (Lynch et al. 2018; Mendel et al. 2017) or felling of highly infested trees, followed by chipping and solarisation (Jones and Paine 2015). While these techniques are important for mitigating the beetle’s spread and impact, they are expensive and impractical at large scale (Byrne et al. 2020; Mayorquin et al. 2018).
Chemical management of PSHB and its close relatives is challenging, though it has been attempted in a few trials as preventative, prophylactic, or therapeutic control on various tree species (Byrne et al. 2020; Grosman et al. 2019; Jones et al. 2017; Jones and Paine 2018; Mayorquin et al. 2018). Alone, traditional contact pesticides may be largely ineffectual, especially as therapeutic treatments, because the PSHB and its fungal symbiont spend most of their life cycle inside the host tree (Umeda et al. 2016). However, combining contact insecticides with a surfactant has been proposed to increase the translocation of the active ingredient through the bark when applied as a trunk spray (Jones et al. 2017; Mayorquin et al. 2018). Systemic insecticides and fungicides, applied through soil drench, soil injection or trunk injection, have also been evaluated in a few laboratory (Freeman et al. 2012; Mayorquin et al. 2018) and field settings (Byrne et al. 2020; Grosman et al. 2019; Jones et al. 2017; Mayorquin et al. 2018). Systemic treatment methods are seen as potentially more effective at reaching their target (Cooperband et al. 2016). However, since they are xylem or phloem mobile, systemic chemicals may be less effective in heavily infested trees with vasculature obstructed by the boring activity of the beetle and growth of its fungal symbiont (Byrne et al. 2020; Grosman et al. 2019; Mayorquin et al. 2018).
Results of studies on contact and systemic pesticides have not yet presented a definitive option for chemical control, especially as long-term and/or therapeutic treatments (Umeda et al. 2016; Grosman et al. 2019). Contact applications of bifenthrin and a surfactant as well as systemic applications of emamectin benzoate and propiconazole have shown promise as suitable control agents (Byrne et al. 2020; Grosman et al. 2019; Jones et al. 2017; Mayorquin et al. 2018), though they must still be tested under a variety of field settings and across a broader host range. In South Africa, the contact insecticide cypermethrin has been used in conjunction with a surfactant (vitamin E) and salicylic acid (fungicide) for PSHB management, however, it has not been scientifically evaluated for efficacy against the species (PanAfrican Farms [PanAf], Parys, South Africa, https://www.panafricanfarms.co.za).
The aims of this study were to test the efficacy of (1) bifenthrin + surfactant (alcohol ethoxylate), (2) cypermethrin + surfactant (vitamin E) + salicylic acid, (3) emamectin benzoate, and (4) propiconazole as therapeutic treatments for PSHB/F. euwallaceae infested American sweetgum (Liquidambar styraciflua) trees in the Western Cape province of South Africa. This tree species is a reproductive host of PSHB, which can suffer Fusarium dieback disease symptoms and even death (FABI 2023; Gomez et al. 2019; van Rooyen et al. 2021). While studies abroad have trialed and/or recommended the use of some of these chemicals in combination, here they were evaluated individually, in the event that one or more fails to become registered for PSHB control in South Africa. It is hypothesised that bifenthrin + surfactant, cypermethrin + surfactant + salicylic acid, and emamectin benzoate will be moderately effective as temporary therapeutic treatments against PSHB and that propiconazole will reduce F. euwallaceae growth rate.
Methods and materials
Study site and host species
Experimental trees were situated at Lourensford Estate in Somerset West (S34°04’47.0” E18°53’12.3”) in the Western Cape province of South Africa. Lourensford Estate’s land use ranges from commercial agriculture (pome fruit, stone fruit, wine grapes and avocados) to residential and recreational use. The first observation of PSHB on the estate occurred in the residential and recreational zones, in January 2021. Since then, it has spread rapidly and killed several trees on the estate (notably Boxelder [Acer negundo] and English oak [Quercus robur]). This study used American sweetgum, a prominent tree species planted on the estate. It is a deciduous species native to the USA, but it is extensively planted as an ornamental tree in gardens, parks and agricultural areas throughout South Africa. Although often infested with PSHB, this tree species has not yet been used in any evaluations of chemical treatments against the beetle/Fusarium complex.
The selected experimental trees (n = 40) were all mildly infested (< 40 PSHB holes up to a 3 m trunk height) and were planted at the same time (same age) along a grassy field in a recreational zone on the estate. All trees were in good health and showed no signs of canopy loss or dieback, as would be expected in reaction to severe vascular blockage (Mayorquin et al. 2018). Eight trees were selected for each of the four treatments and the control. Each tree was numbered and then randomly assigned to one of the four therapeutic treatments, or the control group, using a random number generator (Research Randomizer). Before applying treatments, the diameter at breast height (DBH) and the number of PSHB holes (up to a 3 m trunk height) were recorded for each tree on November 4, 2022.
Fungal growth and inoculation
Three isolates of F. euwallaceae (CMW52826, CMW53018 and CMW-1A3) were used for inoculation using the toothpick method (Scandiani et al. 2011; Twiddy et al. 2021). These isolates were obtained from PSHB-infested pear, apple and plum trees in South Africa (de Jager and Roets 2022; Jager and Roets 2023). Inoculum was prepared by placing autoclaved bamboo toothpicks onto potato dextrose agar (PDA; Bioloab, Midrand, South Africa) plates, inoculating these with the fungal isolates and allowing the fungus to overgrow for ca. 2 weeks at 25 °C in the dark (Scandiani et al. 2011). Toothpicks for controls were treated similarly but left uncolonised by fungi.
The lowest branch (ca. 1.5 m height and 10 cm diameter) on each selected tree was inoculated with the three F. euwallaceae isolates, and a control (n = 4 inoculations per branch per tree; Twiddy et al. 2021). All inoculations were conducted on November 5th, 2022 (late Spring for this location). Inoculation points were made by drilling 2 cm deep into the branch using a sterile 2 mm diameter drill bit. Inoculation points were at least 5 cm apart and were rotated around the branch to prevent overlap of expanding fungal lesions (Twiddy et al. 2021; Fig. 1A). Toothpicks were placed into the holes, cut off flush with the branch, and sealed with parafilm to reduce desiccation and contamination (Fig. 1B).
(A) Branch of Liquidambar styraciflua inoculated with three Fusarium euwallaceae isolates and a control (back of branch) using fungus-overgrown toothpicks. (B) Branch inoculated with three Fusarium euwallaceae isolates and control where toothpicks were cut flush to the surface of the bark and thereafter sealed with parafilm to prevent contamination and desiccation. (C) 3-D printed beetle entry device, with collection tube attached. One end of the trap was sealed with spongy double-sided tape to prevent beetles from escaping. (D) Beetle entry devices secured to the selected branch using horticultural tubing and ready to receive living Euwallacea fornicatus beetles. (E) Spring-loaded injectors filled with treatments and placed into predrilled holes around the tree trunk, 15 cm up from the base and 10 cm apart. (F) Fungal lesion (brown staining) caused by Fusarium euwallaceae three months after inoculation into Liquidambar styraciflua. (G) Brown fungal staining on a 1 cm disk cut around the introduction hole of PSHB in the xylem of Liquidambar styraciflua following gallery formation of the beetle three months after introduction. (H) Gallery contents three months after Euwallacea fornicatus introduction into Liquidambar styraciflua. A pupa (p), larva (l) and an egg (e) can be seen
Beetle collection and introduction
PSHB beetles were introduced onto the same branch as fungal inoculation on each (experimental and control) tree. Beetle introductions were conducted on November 6th and 7th, 2022. Beetles were introduced using 3-D printed entry devices (Berry et al. 2016; Fig. 1C) secured to the branch using horticultural tubing. Beetles were placed in the vile of each device and allowed to enter the branch through a small, predrilled hole (2 mm diameter and ca. 5 mm deep) located beneath the device (Berry et al. 2016). Three devices were used per branch, placed in between fungal inoculation points (n = 3 introduction points per branch per tree; Fig. 1D). Individual beetles used for introduction onto selected branches were collected using funnel traps equipped with a Quercivorol ((1 S,4R)-p-menth-2-en-1-ol) (Synergy Semiochemicals Corp.) lure and containing a moist paper towel (Berry et al. 2016). Traps were erected near the experimental trees one day before beetle introductions to maximise the use of healthy PSHB individuals in experiments.
Chemical treatment
One week after fungal inoculations and beetle introductions (November 14th, 2022), the parafilm and beetle introduction traps were removed and experimental trees were treated with one of four treatments (Table 1). For the two systemic treatments (emamectin benzoate and propiconazole), the trunk injection method was applied (n = 8 trees per treatment). Spring-loaded injectors (Chemjet tree injectors, Queensland Plastics) were filled with the treatment and placed into holes drilled 5 cm deep (using a 4 mm thick drill bit) and spaced 10 cm apart around the diameter of each selected tree, 15 cm up from the soil surface (Byrne et al. 2020; Fig. 1E). After being placed into the holes, injectors were released, and the tree was allowed to absorb the chemical. Injectors remained inside the tree until the entirety of the solution was taken up.
For the two contact insecticide treatments (bifenthrin + surfactant and cypermethrin + surfactant), the trunk spray method was used. For both, hand sprayers were rotated around the trunk of the tree, up to a 3 m trunk height (including the branches into which the fungus and beetles were introduced), and sprayed until runoff was seen (Jones et al. 2017; Mayorquin et al. 2018). The treatments were allowed to dry for 24 h. The fungicide component of the cypermethrin treatment, salicylic acid, was applied in the same fashion, one week after the cypermethrin + surfactant, to avoid possible chemical interactions that may cause the breakdown of active ingredients. The entire cypermethrin treatment was repeated twice (n = 3 total applications; cypermethrin + surfactant at weeks 1, 3 and 5; salicylic acid at weeks 2, 4 and 6) following application instructions (PanAf n.d.).
Branch removal and assessment
Three months after trial initiation, the number of PSHB holes was re-recorded for every tree (February 10th, 2023), and the inoculated branch of each tree was removed. For each F. euwallaceae inoculation point, the bark layer was removed, and the fungal lesion length was measured (Fig. 1F). Samples of discoloured woody tissue were then recovered from the lesions. They were surface sterilised, grown on PDA plates for 2 weeks at 25 °C, and confirmed as F. euwallaceae through morphological assessment (De Jager and Roets 2022). Branches were cut into ca. 1 cm thick disks around each beetle introduction point, which enabled visualisation of fungal colonisation by following the gallery system (Fig. 1G). Total gallery length was measured for each established colony. The galleries were then carefully opened and excavated using a utility knife to record the numbers of dead adult PSHB beetles, living adult PSHB beetles, pupae, larvae and eggs (Fig. 1H).
Statistical analyses
The data were analysed using R programming software (version 3.6.3). To exclude confounding effects on beetle colonisation and fungal growth among the trees, tree size data (DBH) was compared between the treatments. This data was tested for normality using a Shapiro-Wilks (1965) test (W = 0.9232; P = 0.009), which was followed by a Kruskal-Wallis (1952) test for the non-parametric data. To investigate whether aggregation behaviour by PSHB affected the number of additional colonisation attempts of PSHB on individual trees, a Pearson’s correlation (Cohen et al. 2009) was used to determine if the initial number of PSHB holes on the trees were significantly correlated with the additional holes gained over the experimental period.
Thereafter the data was tested for treatment effects on the various factors of beetle and fungal success. Treatment effects on PSHB colony establishment success were evaluated using chi2 tests for each treatment versus control, and p values were adjusted for multiple testing using the Holm-Bonferroni method for multiple testing (Holm 1979). A colony was considered successful when it contained at least one living PSHB individual after the trial period, regardless of life stage. Colony successes and failures were summed across all trees in each treatment (n = 3 chances per tree; 8 trees per treatment = 24 total chances per treatment). The number of successes and failures for control trees were used as expected values and the number of successes and failures for treatments were used as observed values.
Additional PSHB attempts and average gallery length of successfully established colonies per tree were compared across the treatments using one-way Analysis of Variance (ANOVA; Girden 1992) for the parametric data (Shapiro-Wilks, W = 0.959 and P = 0.157; W = 0.941 and P = 0.086, respectively). The average number of living individuals in successful colonies per tree (regardless of life stage) was compared between treatments using a Kruskal-Wallis test (Shapiro-Wilks W = 0.928 and P = 0.013).
Fungal lesion length data were analysed using linear regression mixed model analyses within the “lme4” package. The data were log-transformed to enforce normality (Shapiro-Wilks W = 0.986; P = 0.259). The full model contained isolate and treatment as fixed effects and individual trees as random effect. After model selection, the best-fit model (based on the lowest REML value) included the fixed effect of treatment and the random effect of individual tree (REML = -0.6; AIC = -3.88; BIC = 15.63). Post hoc tests were used for pairwise comparisons between treatments for factors that proved significant after main tests (Tukey [1949] HSD tests for parametric data and Dunn [1964] tests for nonparametric data). For all analyses, a confidence level of 5% (P ≤ 0.05) was used to determine statistical significance.
Results
Tree size did not differ between the various treatments and controls (Chi2 = 6.491; Df = 4; P = 0.165). Also, the initial number of PSHB holes in the trees was not significantly correlated with the additional holes gained over the experimental period (Pearson’s r = 0.193; t = 1.212; Df = 38; P = 0.233). Tree size and possible aggregation behaviour by PSHB could therefore be excluded as explanatory variables for PSHB colonisation success and differences in fungal growth between treatments and controls.
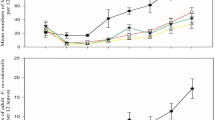
Colony establishment success on control trees was relatively high (ca. 71%; Fig. 2). Colonies that failed to establish often contained the dead remains of the founding female beetle (4 colonies in the control and emamectin benzoate treatment trees, 3 each for the cypermethrin and propiconazole treatment trees, and 6 for the bifenthrin treatment trees). Five of the colonies that were successfully established on control trees also contained a dead female beetle, presumably the foundress, while only a single established colony in each of the chemical treatments also contained a dead foundress. The numbers of failed colonies that contained no trace of any PSHB individuals were 2, 8, 12, 13 and 8 for the control, bifenthrin, cypermethrin, emamectin benzoate and propiconazole treatments, respectively. The total number of successful colonies established for all treatments was significantly lower than for the controls (Table 2; Fig. 2). Percentage colony success after chemical treatment was reduced to between ca. 46% and 33% depending on the treatment (Fig. 2).
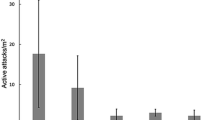
Control trees had a mean number of 8.12 (+/- 1.96 SD) additional colonisation attempts after the experimental period. Treatment had a significant effect on the number of additional attempts recorded on trees after the trial period (F = 5.69; Df = 4; P = 0.001). Treatments with contact insecticides, bifenthrin + surfactant and cypermethrin + surfactant + salicylic acid, had significantly fewer additional PSHB colonisation attempts than the control trees but did not differ from each other (Fig. 3). The mean number of additional attempts was reduced to 5 (+/- 2.56 SD) and 4.88 (+/- 1.73 SD) for these two treatments respectively. Other treatments had no significant effect on the additional number of PSHB attempts after treatment (Fig. 3).
The mean gallery length for established colonies on control trees was 27.79 mm (+/- 7.41 SD). There were no significant differences in gallery length for established colonies between treatment and control trees (F = 0.437; Df = 4; P = 0.781). The median number of individuals per successful colony per tree in controls (irrespective of life stage) was low at 1.83 (± 0.667 IQR). Treatment had no significant effect on the median number of individuals in successful colonies per tree (Chi2 = 6.95, Df = 4, P = 0.138).
The mean fungal lesion length was 32.71 mm (+/- 7.5 SD) in control trees. Treatment had a significant impact on lesion length (Chi2 = 18.5; Df = 4; P = < 0.001), but this was only evident for the systemic fungicide (propiconazole) treatment (Z = -4.292, P = < 0.001). The average fungal lesion length for this treatment was reduced to 20.83 mm (+/- 3.94 SD; Fig. 4).
Discussion
PSHB and its primary fungal symbiont, F. euwallaceae, form part of a pest-disease complex that poses a serious threat to a wide range of tree species in invaded regions. In South Africa, current mitigation methods are still largely centred on infested branch and tree removal. This study evaluated four chemicals or combinations as therapeutic treatments against the proliferation of PSHB/F. euwallaceae (1) bifenthrin + surfactant (alcohol ethoxylate), (2) emamectin benzoate, (3) propiconazole, and (4) cypermethrin + surfactant (vitamin E) + salicylic acid. The first three treatments have shown success in other trials for PSHB or its close relatives in previous experiments, but this is the first time these were evaluated in South Africa. This is also the first time evaluating these treatments for the management of PSHB on American sweetgum trees, which are commonly planted in South Africa. The cypermethrin treatment is often used for PSHB management in South Africa, but to the best of our knowledge, this is the first time evaluating it against other treatments. Overall, the results show that all four treatments are moderately effective as therapeutic measures on American sweetgum. Propiconazole is also moderately effective at reducing the growth of F. euwallaceae on American sweetgum. However, none of the treatments alone demonstrated the ability for complete control of PSHB on this host. Rather, all treatments merely reduced infestation rates over the experimental period. These treatments should therefore be considered viable options as part of a management program against PSHB on American sweetgum.
The two contact treatments, bifenthrin and cypermethrin, significantly reduced new PSHB colonisation attempts. These are both pyrethroids and though they may have different modes of action, they are both synthetic compounds that impact the nervous system of insects (Gammon et al. 2019). Insects may be repelled or even killed when exposed to high enough concentrations of these compounds. In previous studies, contact pyrethroids have had inconsistent results for reducing PSHB colonisation attempts. Jones and Paine (2018) found bifenthrin to be the most effective in reducing PSHB attempts in cut Caster Bean (Ricinus communis) logs, but Jones et al. (2017) showed significant reduction using bifenthrin on California sycamore (Platanus racemosa) only when it was used in combination with other pesticides. Mayorquin et al. (2018) found that bifenthrin significantly reduced beetle colonisation attempts in moderate, but not heavily infested California sycamore trees. The variable success of contact insecticides is likely due to the limited exposure of ambrosia beetles to the surface of the host tree (and therefore these chemicals) and because, unlike bark beetles, ambrosia beetles only ingest small amounts of tree tissues (Beaver 1989; Mayorquin et al. 2018). The results of this study, therefore, echo previous studies in that infestation attempts can be reduced, but not halted altogether, even after multiple applications of these contact insecticides (as was observed for the cypermethrin treatment in the current study).
The systemic treatments, emamectin benzoate and propiconazole, did not reduce colonisation attempts. This contrasts with other studies surrounding the control of Euwallacea species, which have trialed emamectin benzoate alone and in combination with other pesticides (including propiconazole), with positive results. Jones et al. (2017) found emamectin benzoate to be effective for their entire trial period when combined with two other chemicals (bifenthrin and metconazole). Mayorquin et al. (2018) found that emamectin benzoate (alone and in combination with propiconazole) significantly reduced beetle colonisation attempts, and Grosman et al. (2019) had longer-term success using emamectin benzoate and propiconazole as a control measure. One reason for the difference seen here could be a difference in host species’ vasculature or resistance to uptake of these chemicals, as the above studies were conducted on California sycamore trees, and this study was done on American sweetgum trees. It has also been cautioned that systemic treatments could take a long time to distribute throughout the tree (Fettig et al. 2013), and thus the effects of emamectin benzoate and propiconazole may improve over time (Grosman et al. 2019). The chemical formulations of these systemic chemicals may have also had an impact, as emulsifiable concentrates of pesticides have demonstrated phytotoxic effects in other studies using trunk injection (Archer et al. 2022; Kiss et al. 2023), which could have inhibited uptake if tree transport tissues were affected. In this study, observation 12 months after injection showed that 3 (of 16 total) trees had bark discoloration up to ca. 10 cm surrounding injection sites, but no visible impacts on overall tree health were observed. Nonetheless, it is recommended that chemical formulations created for trunk injection be used in future research iterations, especially in the case of repeat applications.
While emamectin benzoate and propiconazole were evaluated individually here, efficacy may be increased when these are used in combination (Mayorquin et al. 2018; Grosman et al. 2019). It is therefore recommended that these systemic treatments should be evaluated in combination, and in longer-term control studies on American sweetgum and other PSHB hosts in South Africa.
All treatments significantly lowered the colonisation success for introduced PSHB beetles. All of these can therefore be considered therapeutic treatments in the management of PSHB, at least early on in the colonisation process. The success of bifenthrin and cypermethrin in reducing colonisation success may suggest that they have some absorbance within the tree, aided by the addition of surfactants (Mayorquin et al. 2018; Schnabel et al. 2012). However, it is likely that these contact insecticides killed or repelled some foundress females before they were able to start a colony. This is because no dead larvae or teneral adult beetles were observed in this study despite often observing dead or missing foundress females. The posterior end of foundress females is often found blocking the entrance of holes at the bark surface, a posture that helps them to protect their gallery and offspring from external threats (Dodge 2019; Parthiban 1992). In this position, pesticides applied as sprays can more easily encounter foundress females. The success of the two contact insecticides here indicates that they may be useful candidates for PSHB management and could be included in further therapeutic assessments on American sweetgum and other hosts in South Africa.
Like the contact insecticides, the systemic insecticide, emamectin benzoate, and the systemic fungicide, propiconazole, were able to reduce (but not halt) PSHB colony establishment success, indicating that they are suitable, but not entirely efficacious candidates for therapeutic treatment. This echoes the results of other laboratory and field trials (Byrne et al. 2020; Grosman et al. 2019; Jones et al. 2017; Mayorquin et al. 2018). Therefore, it is possible that a combination of a contact treatment, like bifenthrin or cypermethrin, along with a systemic treatment like emamectin benzoate or propiconazole, might offer greater protection against both new colonisation attempts and colonisation success stemming from those attempts. Because emamectin benzoate and propiconazole reduced PSHB colonisation success, but not new attempts, visible signs of PSHB holes on the outside of trees may not be an accurate indication of successful colony establishment. The novel methodology used in the present study to characterise PSHB colonisation success may therefore be more relevant than visual inspections in future evaluations of chemical trials against this pest.
Treatment had no impact on the gallery length or the abundance of individuals within successful colonies. Therefore, chemical treatments did not deter established beetle colonies from expanding in size or in population numbers. However, the median abundance of individuals within successful PSHB colonies was only 1.87. Cooperband et al. (2016) found that, when reared on host tree sawdust, 32 adult female offspring could be produced by one PSHB foundress in 22 days at 24 °C, and this number almost doubled in 7 weeks due to generational overlap. In comparison, given the 3.5-month trial period in the present study and that ambient temperatures were within PSHB’s optimal range, the abundance of PSHB individuals was lower than expected in all colonies. This may mean that American sweetgum, while being a reproductive host for PSHB, is not a preferred host species. It is therefore recommended that these experiments should be expanded to a variety of different host tree species, including preferred hosts such as Boxelder and English oak.
There was no evidence of fungicidal activity of salicylic acid towards F. euwallaceae within American sweetgum hosts. Propiconazole was the only treatment that reduced the fungal growth rate within this host, and its success aligns with previous in vitro trials using triazole fungicides against Fusarium sp. (Freeman et al. 2012; Mayorquin et al. 2018). The propiconazole treatment also reduced PSHB colonisation success, and may therefore be a strong candidate to use in conjunction with a systemic insecticide such as emamectin benzoate to manage PSHB infestations (Mayorquin et al. 2018; Jones et al. 2017; Grosman et al. 2019). However, it is unclear whether propiconazole reduced colonisation success due to reduced fungal growth (the food source of the beetle), or because propiconazole may have insecticidal properties (Drummond 2022; Haizhen et al. 2006). It seems plausible that during the current study, propiconazole concentration in sapwood tissues were sufficient to reduce F. euwallaceae growth, but insufficient to entirely halt growth (Peyton et al. 2015). Therefore, the fungus was likely still available as a food source to the beetles in the galleries. Beetle population numbers in successfully established colonies on trees treated with propiconazole were also the same as those in all other treatments, indicating that suitable resources were still available for beetle development. For these reasons, and because of the relatively high numbers of dead beetles observed within PSHB galleries in propiconazole-treated trees, we suspect that propiconazole may have insecticidal properties which aided the reduction in PSHB colony success. This may be the reason why control of PSHB can be enhanced when combining propiconazole with an insecticide, as the effective concentration of insecticide in the treatment would ultimately be increased.
Conclusion
All the treatments evaluated here may be considered in a program for the management of PSHB. Bifenthrin and cypermethrin reduced new PSHB colonisation attempts, all treatments reduced colonisation success, and propiconazole reduced F. euwallaceae growth rate. More research will have to continue in South Africa to determine if these pesticides are useful in the longer term against PSHB on American sweetgum and other important host species. Because not one of the evaluated treatments offered complete control, even when applied regularly (e.g. the cypermethrin treatment), different combinations of these treatments or combinations of these with untested control measures should be undertaken to determine whether greater efficacy could be attained. Importantly, the environmental impact of these treatments, especially the contact chemicals, as well as the possible phytotoxic effects, especially for the systemic chemicals, should be evaluated. These chemical treatments should also be evaluated as part of an integrated pest management program that includes other forms of management, such as monitoring, biological control (Guevara-Avendaño et al. 2018; Nel et al. 2023), and attractants and repellents (Byers et al. 2020, 2021, 2022).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on request.
References
Archer L, Crane JH, Albrecht U (2022) Trunk injection as a tool to deliver plant protection materials—An overview of basic principles and practical considerations. Horticulturae 8:552
Beaver RA, Wilding N, Collins N, Hammond P, Webber J (1989) Insect-fungus relationships in the bark and ambrosia beetles. In: Wilding N, Collins NM, Hammond PM, Webber JF (eds) Insect-fungus interactions, vol 6. Academic Press, Cambridge, Massachusetts, pp 121–143. https://doi.org/10.1016/B978-0-12-751800-8.50011-2
Berry D, Selby RD, Horvath JC, Cameron RH, Porqueras D, Stouthamer R (2016) A modular system of 3D printed emergence traps for studying the biology of shot hole borers and other Scolytinae. J Econ Entomol 109:969–972. https://doi.org/10.1093/jee/tov407
Byers JA, Levi-Zada A (2022) Modelling push‐pull management of pest insects using repellents and attractive traps in fruit tree orchards. Pest Manag Sci 78:3630–3637. https://doi.org/10.1002/ps.7005
Byers JA, Maoz Y, Fefer D, Levi-Zada A (2020) Semiochemicals affecting attraction of ambrosia beetle Euwallacea fornicatus (Coleoptera: Curculionidae: Scolytinae) to quercivorol: developing push-pull control. J Econ Entomol 113:2120–2127. https://doi.org/10.1093/jee/toaa127
Byers JA, Maoz Y, Cohen B, Golani M, Fefer D, Levi-Zada A (2021) Protecting avocado trees from ambrosia beetles by repellents and mass trapping (push–pull): experiments and simulations. J Pest Sci 94:991–1002. https://doi.org/10.1007/s10340-020-01310-x
Byrne FJ, Almanzor J, Tellez I, Eskalen A, Grosman DM, Morse JG (2020) Evaluation of trunk-injected emamectin benzoate as a potential management strategy for Kuroshio shot hole borer in avocado trees. Crop Prot 132:105–136. https://doi.org/10.1016/j.cropro.2020.105136
Cohen I, Huang Y, Chen J, Benesty J (2009) Pearson correlation coefficient. In: Benesty J (ed) Noise reduction in speech processing. Springer, Berlin, pp 1–4. https://doi.org/10.1109/5.771073
Cooperband MF, Stouthamer R, Carrillo D, Eskalen A, Thibault T, Cossé AA, Castrillo LA, Vandenberg JD, Rugman-Jones PF (2016) Biology of two members of the Euwallacea fornicatus species complex (Coleoptera: Curculionidae: Scolytinae), recently invasive in the USA, reared on an ambrosia beetle artificial diet. Agric for Entomol 18:223–237. https://doi.org/10.1111/afe.12155
de Jager MM, Roets F (2022) Pathogenicity of Fusarium euwallaceae towards apple (Malus domestica) and grapevine (Vitis vinifera). Australas Plant Dis Notes 17:8. https://doi.org/10.1007/s13314-022-00456-0
de Jager MM, Roets F (2023) Pathogenicity of Fusarium euwallaceae, symbiont of the polyphagous shot hole borer beetle, to selected stone fruit trees in South Africa. J Plant Pathol 105:5–13. https://doi.org/10.1007/s42161-022-01258-0
de Wit MP, Crookes DJ, Blignaut JN, de Beer ZW, Paap T, Roets F, van der Merwe C, van Wilgen BW, Richardson DM (2022) An assessment of the potential economic impacts of the invasive Polyphagous Shot Hole Borer (Coleoptera: Curculionidae) in South Africa. J Econ Entomol 1–11. https://doi.org/10.1093/jee/toac061
Dodge CE (2019) Biology of Two Invasive Ambrosia Beetles, the Polyphagous and Kuroshio Shot Hole Borers (Euwallacea spp.), in California. Dissertation, University of California, Riverside
Drummond FA (2022) Honey bee exposure to the fungicide propiconazole in lowbush blueberry fields. Agron 12:3081. https://doi.org/10.3390/agronomy12123081
Dunn OJ (1964) Multiple comparisons using rank sums. Technometrics 6:241–252
Eskalen A, Gonzalez A, Wang DH, Twizeyimana M, Mayorquin SJ, Lynch SC (2012) First report of a Fusarium sp. and its vector tea shot hole borer (Euwallacea fornicatus) causing fusarium dieback on avocado in California. Plant Dis 96:1070. https://doi.org/10.1094/PDIS-03-12-0276-PDN
Eskalen A, Stouthamer R, Lynch SC, Rugman-Jones PF, Twizeyimana M, Gonzalez A, Thibault T (2013) Host range of fusarium dieback and its ambrosia beetle (Coleoptera: Scolytinae) vector in southern California. Plant Dis 97:938–951. https://doi.org/10.1094/PDIS-11-12-1026-RE
FABI (2023) Polyphagous Shot Hole Borer South African host list. https://www.fabinet.up.ac.za/images/PSHB/PSHB_host_list_v6_20230417.pdf. Accessed 6 June 2023
Fettig CJ, Grosman DM, Munson AS (2013) Advances in insecticide tools and tactics for protecting conifers from bark beetle Attack in the western United States. In: Trdan S (ed) Insecticides—development of safer and more effective technologies. IntechOpen, London, pp 472–492. https://doi.org/10.5772/54178
Freeman S, Sharon M, Okon-Levy N, Protasov A, Eliyahu M, Mendel Z (2012) Fungicide screening for inhibition of the fungal symbiont Fusarium sp.nov. in Israel. http://www.avocadosource.com/Journals/IABC_2012/S5_04_Freeman.pdf. Accessed 6 June 2023
Freeman S, Sharon M, Dori-Bachash M, Maymon M, Belausov E, Maoz Y, Margalit O, Protasov A, Mendel Z (2016) Symbiotic association of three fungal species throughout the life cycle of the ambrosia beetle Euwallacea Nr. Fornicatus. Symbiosis 68:115–128. https://doi.org/10.1007/s13199-015-0356-9
Freeman S, Miller G, Protasov A, Maymon M, Elazar M, David-Schwartz R, Zhou J, Mendel Z (2019) Aposymbiotic interactions of three ambrosia beetle fungi with avocado trees. Fungal Ecol 39:117–130. https://doi.org/10.1016/j.funeco.2018.11.007
Gammon DW, Liu Z, Chandrasekaran A, El-Naggar SF, Kuryshev YA, Jackson S (2019) Pyrethroid neurotoxicity studies with bifenthrin indicate a mixed type I/II mode of action. Pest Manag Sci 75:1190–1197. https://doi.org/10.1002/ps.5300
Girden ER (1992) ANOVA: repeated measures. Sage, Newburry Park, California. https://doi.org/10.4135/9781412983419
Gomez DF, Lin W, Gao L, Li Y (2019) New host plant records for the Euwallacea fornicatus (Eichhoff) species complex (Coleoptera: Curculionidae: Scolytinae) across its natural and introduced distribution. J Asia Pac Entomol 22:338–340. https://doi.org/10.1016/j.aspen.2019.01.013
Government of Western Australia (2021) Fremantle residents asked to look for exotic insect borer. https://www.wa.gov.au/government/announcements/fremantle-residents-asked-look-exotic-insect-borer. Accessed 6 June 2023
Grosman DM, Eskalen A, Brownie C (2019) Evaluation of emamectin benzoate and propiconazole for management of a new invasive shot hole borer (Euwallacea Nr. Fornicatus, Coleoptera: Curculionidae) and symbiotic fungi in California sycamores. J Econ Entomol 112:1267–1273. https://doi.org/10.1093/jee/toy423
Guevara-Avendaño E, Carrillo JD, Ndinga-Muniania C, Moreno K, Méndez-Bravo A, Guerrero-Analco JA, Eskalen A, Reverchon F (2018) Antifungal activity of avocado rhizobacteria against Fusarium euwallaceae and Graphium spp., associated with Euwallacea spp. Nr. Fornicatus, and Phytophthora Cinnamomi. Antonie Van Leeuwenhoek 111:563–572. https://doi.org/10.1007/s10482-017-0977-5
Haizhen Z, Zhixiang Z, Meide L, Jingli Z, Hanhong X (2006) Insecticidal activities of propiconazole against {\sl Spodoptera litura}(Lepidoptera: Noctuidae). Acta Entomologica Sinica 49:265–270
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70. https://doi.org/10.2307/4615733
Hulcr J, Dunn RR (2011) The sudden emergence of pathogenicity in insect–fungus symbioses threatens naive forest ecosystems. Proc Royal Soc B-Biol Sci 278:2866–2873. https://doi.org/10.1098/rspb.2011.1130
Jansson RK, Brown R, Cartwright B, Cox D, Dunbar DM, Dybas RA, Eckel C, Lasota JA, Mookerjee PK, Norton JA, Peterson RF (1997) Emamectin benzoate: A novel avermectin derivative for control of lepidopterous pests. In: Proceedings of the 3rd International Workshop on Management of Diamondback Moth and Other Crucifer Pests. MARDI, Kuala Lumpur, Malaysia, pp 1–7
Jones M, Paine TD (2015) Effect of chipping and solarization on emergence and boring activity of a recently introduced ambrosia beetle (Euwallacea sp., Coleoptera: Curculionidae: Scolytinae) in Southern California. J Econ Entomol 108:1852–1859. https://doi.org/10.1093/jee/tov169
Jones M, Paine TD (2018) Potential pesticides for control of a recently introduced ambrosia beetle (Euwallacea sp.) in southern California. J Pest Sci 91:237–246. https://doi.org/10.1007/s10340-017-0866-8
Jones M, Kabashima J, Eskalen A, Dimson M, Mayorquin JS, Carrillo JD, Hanlon CC, Paine TD (2017) Evaluations of insecticides and fungicides for reducing Attack rates of a new invasive ambrosia beetle (Euwallacea sp., Coleoptera: Curculionidae: Scolytinae) in infested landscape trees in California. J Econ Entomol 110:1611–1618. https://doi.org/10.1093/jee/tox163
Kiss M, Sörös C, Gutermuth Á, Ittzés A, Szabó Á (2023) Avermectin trunk injections: a promising approach for managing the walnut husk fly (Rhagoletis completa). Horticulturae 9:655
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47:583–621. https://doi.org/10.1080/01621459.1952.10483441
Li L, Zhu T, Song Y, Feng L, Kear PJ, Riseh RS, Sitohy M, Datla R, Ren M (2022) Salicylic acid fights against Fusarium wilt by inhibiting target of rapamycin signaling pathway in Fusarium oxysporum. J Adv Res 39:1–13. https://doi.org/10.1016/j.jare.2021.10.014
Lynch SC, Carrillo JD, Stouthamer R, Eskalen A (2018) Severity of Fusarium Dieback–Shot Hole Borers analyzed. From The Grove: Winter, pp 46–50. https://ucanr.edu/sites/eskalenlab/files/296160.pdf. Accessed 6 June 2023
Lynch SC, Eskalen A, Gilbert GS (2021) Host evolutionary relationships explain tree mortality caused by a generalist pest–pathogen complex. Evol Appl 14:1083–1094. https://doi.org/10.1111/eva.13182
Mayorquin JS, Carrillo JD, Twizeyimana M, Peacock BB, Sugino KY, Na F, Wang DH, Kabashima JN, Eskalen A (2018) Chemical management of invasive shot hole borer and fusarium dieback in California sycamore (Platanus racemosa) in Southern California. Plant Dis 102:1307–1315. https://doi.org/10.1094/PDIS-10-17-1569-RE
Mendel Z, Protasov A, Sharon M, Zveibil A, Yehuda SB, O’Donnell K, Rabaglia R, Wysoki M, Freeman S (2012) An Asian ambrosia beetle Euwallacea fornicatus and its novel symbiotic fungus fusarium sp. pose a serious threat to the Israeli avocado industry. Phytoparasitica 40:235–238. https://doi.org/10.1007/s12600-012-0223-7
Mendel Z, Protasov A, Maoz Y, Maymon M, Miller G, Elazar M, Freeman S (2017) The role of Euwallacea nr. fornicatus (Coleoptera: Scolytinae) in the wilt syndrome of avocado trees in Israel. Phytoparasitica 45:341–359. https://doi.org/10.1007/s12600-017-0598-6
Nel WJ, Slippers B, Wingfield MJ, Yilmaz N, Hurley BP (2023) Efficacy of commercially available entomopathogenic agents against the Polyphagous Shot Hole Borer in South Africa. Insects 14:361. https://doi.org/10.3390/insects14040361
Paap T, de Beer ZW, Migliorini D, Nel WJ, Wingfield MJ (2018) The polyphagous shot hole borer (PSHB) and its fungal symbiont Fusarium euwallaceae: a new invasion in South Africa. Australas Plant Pathol 47:231–237. https://doi.org/10.1007/s13313-018-0545-0
Paap T, Wingfield MJ, de Beer ZW, Roets F (2020) Lessons from a major pest invasion: the polyphagous shot hole borer in South Africa. S Afr J Sci 116:8757. https://doi.org/10.17159/sajs.2020/8757
PanAf (n.d.) PSHB products. https://www.panafricanfarms.co.za/pshb-products.html. Accessed 6 June 2023
Parthiban M (1992) Studies on Euwallacea fornicatus (Eichhoff) (Coleoptera: Scolytidae), the shot hole borer of tea. Dissertation, Bharathiar University Coimbatore District, India
Peyton LR, Gallagher S, Hashemzadeh M (2015) Triazole antifungals: a review. Drugs Today 51:05–718
Scandiani MM, Ruberti DS, Giorda LM, Pioli RN, Luque AG, Bottai H, Ivancovich JJ, Aoki T, O’Donnell K (2011) Comparison of inoculation methods for characterizing relative aggressiveness of two soybean sudden-death syndrome pathogens, Fusarium virguliforme and F. tucumaniae. Trop Plant Pathol 36:133–140. https://doi.org/10.1590/S1982-56762011000300001
Schnabel G, Fernández-Ortuño D, Bridges WC, Hudson SB (2012) Multiyear evaluation of an orange peel oil-based spray additive for managing insect pests and brown rot of nectarine. Plant Health Prog 13:12. https://doi.org/10.1094/PHP-2012-0617-01-RS
Schuler H, Witkowski R, van de Vossenberg B, Hoppe B, Mittelbach M, Bukovinszki T, Schwembacher S, van de Meulengraaf B, Lange U, Rode S, Andriolo A (2023) Recent invasion and eradication of two members of the Euwallacea fornicatus species complex (Coleoptera: Curculionidae: Scolytinae) from tropical greenhouses in Europe. Biol Invasions 25:299–307. https://doi.org/10.1007/s10530-022-02929-w
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52:591–611. https://doi.org/10.1093/biomet/52.3-4.591
Six DL (2012) Ecological and evolutionary determinants of bark beetle—fungus symbioses. Insects 3:339–366. https://doi.org/10.3390/insects3010339
Tukey JW (1949) Comparing individual means in the analysis of variance. Biometrics 99–114. https://doi.org/10.2307/3001913
Twiddy D, Fell S, de Beer ZW, Fourie G (2021) Screening for susceptibility of macadamia to Euwallacea fornicatus and its fungal symbiont Fusarium euwallaceae. Plant Dis 105:739–742. https://doi.org/10.1094/PDIS-07-20-1555-SC
Umeda C, Eskalen A, Paine TD (2016) Polyphagous shot hole borer and Fusarium dieback in California. In: Paine TD, Lieutier F (eds) Insects and Diseases of mediterranean forest systems. Springer, Switzerland, pp 757–767. https://doi.org/10.1007/978-3-319-24744-1_26
United States Environmental Protection Agency (2006) Reregistration eligibility decision (RED) for propiconazole. https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_PC-122101_18-Jul-06.pdf. Accessed 6 June 2023
van Rooyen E, Paap T, Beer WD, Townsend G, Fell S, Nel WJ, Morgan S, Hill M, Gonzalez A, Roets F (2021) The polyphagous shot hole borer beetle: current status of a perfect invader in South Africa. S Afr J Sci 117:1–10. https://doi.org/10.17159/sajs.2021/9736
Acknowledgements
The authors thank Lourensford Fruit Company for financial support for this study and Lourensford Estate for providing the space and equipment to conduct experiments. We thank Koos Jordaan, Pieter Steyn, Evan Kortjé and Casper Geldenhuys for their willingness to help while conducting experiments. Vergelegen Estate is acknowledged for partial funding of equipment used in experiments.
Funding
Open access funding provided by Stellenbosch University. Lourensford Fruit Company funded this project.
Open access funding provided by Stellenbosch University.
Author information
Authors and Affiliations
Contributions
All authors conceived and designed research. Elise Roberts conducted experiments. Trudy Paap and Francois Roets supervised experiments. All authors analysed data. Elise Roberts prepared the first manuscript draft. All authors contributed to the writing of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was not needed for this project.
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roberts, E., Paap, T. & Roets, F. Chemical control of the polyphagous shot hole borer beetle (PSHB, Euwallacea fornicatus) and Fusarium euwallaceae in American sweetgum (Liquidambar styraciflua). J Plant Pathol 106, 457–468 (2024). https://doi.org/10.1007/s42161-023-01583-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-023-01583-y