Abstract
Leptosphaeria maculans is a fungal pathogen that causes heavy losses of Brassica napus crops. To develop novel means of plant protection it is necessary to understand the virulence mechanisms of pathogens. In this study we investigated a T-DNA insertion mutant of L. maculans that exhibited a hypovirulent phenotype (pHxt1ins). The mutant contains a single T-DNA insertion that affects expression of a single gene identified as a transmembrane hexose transporter (LmHxt1). This gene exhibited enhanced expression in the pHxt1ins mutant during in vitro cultivation. The excess of glucose in the cultivation medium lowered expression of LmHxt1 in the mutant line, suggesting that the gene might function as a sugar sensor. Lack of virulence of the pHxt1ins mutant in planta was observed using fluorescence microscopy in GFP labeled lines ruling out asymptomatic growth and showing inability to successfully colonize the apoplastic space of B. napus. On the other hand, in vitro growth did not differ between pHxt1ins and control lines. Interestingly, the mutant showed altered mycelium morphology and a change in conidial germination. Overall, our results suggest that the LmHxt1 gene is a novel important virulence factor for L. maculans. We also propose pHxt1ins mutant as a tool to study the role of sugar transporters in fungal metabolism and pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytopathogenic fungi are an important problem of agriculture causing yield losses and compromising the quality of food produced from infected plants. These fungi have various strategies for infecting plants and thriving in them while acquiring their nutrients. Plants have developed countermeasures to recognize the pathogens and activate defense cascades that typically involve hypersensitive cell death preventing further spread of the infection (Jones and Dangl 2006). Thus, a successful pathogen needs to find ways of avoiding recognition by the host.
During evolution, fungal pathogens have developed various molecules and structures to help them bypass the pathogen recognition mechanisms of plants and successfully colonize them. Generally, such molecules are called virulence factors (Peterson 1996; Cross 2008). Recently some membrane transporters have also been recognized as virulence factors, as they can change the nutrient acquisition strategy of the pathogen, making the pathogen undetectable by the host. This is done by hexose transporters (referred to as Hxt further in the text) that are responsible for sugar transport through the cell membrane. Such transporters sometimes also have a specific signaling function. So far, the sugar signaling function has been best described in yeasts, especially in Saccharomyces cerevisiae. The hexose transporters Snf3 and Rgt2 of S. cerevisiae are important for sensing the amount of glucose in the environment and regulation of the expression of the hexose transporter genes to be suited for the actual concentration of glucose (Özcan et al. 1996; Özcan and Johnston 1999). Similarly, the transporter Hxs1 in the yeast Hansenula polymorpha is needed to trigger the expression of Hxt1 transporter in the presence of a suitable sugar (Stasyk et al. 2008). In fungi there are also examples like UmHxt1 from U. maydis, a pathogen of corn (Wahl et al. 2010; Schuler et al. 2015), which has the dual role of both sugar transport and signaling. Candida albicans possesses a hexose transporter with a signaling function orthologous to Snf3 and Rgt2 from S. cerevisiae. This transporter is responsible for regulation of three other hexose transporter genes (Brown et al. 2006). Similarly, one of the hexose transporters of C. graminicola called CgHxt4 has been postulated to have a sugar signaling role because of its constitutive low expression during the whole infection cycle, low transport activity in CgHxt4-yeast expressing cells, and notable orthology to Snf3 and Rgt2 (Lingner et al. 2011). Some of the hexose transporters have also been described as important virulence factors, for example, the hexose transporters of U. maydis. One of these transporters called Srt1 enables the fungus to transport sucrose directly, replacing the normal mechanism of cleaving it to glucose and fructose, thus, circumventing the plant’s ability to recognize the pathogen by sensing a change in the glucose level in the apoplastic space (Wahl et al. 2010). Srt1 is also important for symptom formation, as its deletion caused absence of infection symptoms even during successful colonization of plant tissues. The other sugar transporter, UmHxt1, has particularly important roles during the pathogenic and saprophytic stages of the fungal life cycle where it has a sugar transporting and signaling role (Schuler et al. 2015). Similarly, the transporter Hxs1 from the pathogen Cryptococcus neoformans has been shown as vital for both fitness and virulence (Liu et al. 2013). Some hexose transporters seem to be most connected to different phases of fungal growth and virulence cycle, like in the case of CgHxt1 – 5 from Colletotrichum graminicola (Lingner et al. 2011) or ChHxt4 and 6 from Colletotrichum higginsianum (Yuan et al. 2021). CgHxt1 and CgHxt3 are expressed during the biotrophic phase of growth, while CgHxt5 is expressed during the necrotrophic phase. CgHxt4 is expressed in low amounts throughout the whole infection cycle.
The ascomycete Leptosphaeria maculans (anamorph Phoma lingam (Desmazières 1851)) is one of the major pathogens of Brassica plants, most notably the oilseed rape (Brassica napus). It has a hemibiotrophic life cycle which consists of three phases (Hammond and Lewis 1987). At first, the fungus establishes itself in the mesophyll of the plant asymptomatically, which is followed by a second phase of hyphal proliferation in the leaf and in the end by a third systemic phase. Behind the hyphal front, the mycelium enters a necrotrophic phase, invading and destroying plant cells, and causing leaf lesions and stem cankers, also known as ‘blackleg’ disease (West et al. 2001; Howlett 2004; Rouxel and Balesdent 2005). At the first stage, it is crucial for the fungus to avoid detection, so its spores can germinate and start mycelial growth. The growing mycelium requires a suitable carbon source such as sugars from the host plant cells. Like other pathogenic fungi, L. maculans utilizes hexose transporters to harvest various sugars (Voegele et al. 2001; Polidori et al. 2007; Lingner et al. 2011). These transporters are usually paired with invertases and other cleaving enzymes that cleave complex sugars, such as sucrose, into monosaccharides, which are subsequently transported into the pathogen cells by specific hexose transporters (Scholes et al. 1994).
In this study, we identify and characterize a T-DNA L. maculans mutant with a hypovirulent phenotype. The position of the single T-DNA insert was localized in the promoter of a hexose transporter gene entitled LmHxt1, thus the mutant was named pHxt1ins. This mutant shows a constitutively elevated expression of the LmHxt1 gene. By combining bioinformatics, in vitro characterization methods, and growth in planta, we present the first experimental evidence that a hexose transporter can be a virulence factor of L. maculans.
Materials and methods
Plant material
Fourteen-day-old Brassica napus cv. Columbus cotyledons were used for all experiments. Plants were grown under artificial LED light with light regime 14/10 h light/dark, light intensity of 150 μE.m2.s−1, temperature 20 °C during night and 24 °C during day. Growth substrate was perlite (Perlit Praha, Prague, Czechia), watering was performed with tap water until germination of plants, and Steiner solution (Steiner 1961) after germination.
Fungal strains and growth conditions in vitro
Two strains of L. maculans were used in this study; a wild-type strain (WT) JN2 (Balesdent et al. 2001), kindly provided by Thierry Rouxel (INRAE, France), and a JN2-derived mutant strain labeled pHxt1ins, which was generated by Agrobacterium tumefaciens mediated T-DNA insertional mutagenesis (Gardiner and Howlett 2004). The strain contains an RNAi insert for silencing the auxin gene LmIAD1.2. Sporulation of L. maculans was performed according to Ansan-Melayah et al. (1995). Conidia were washed once with distilled water after harvesting, diluted to 108 conidia/ml, and stored at -20 °C for a maximum period of 12 months.
To enable pathogen growth visualization in planta, L. maculans WT strain and the pHxt1ins line were transformed with a pSULPHgfp construct (Sesma and Osbourn 2004) which carries a gene responsible for GFP expression. Transformation was performed according to the protocol by Šašek et al. (Šašek et al. 2012). For liquid culture growth tests fungi were cultivated in 100 ml spore suspension in concentration of 105 in 250 ml Erlenmeyer flasks for 6 days in liquid Gamborg B5 medium with vitamins (Duchefa, G0210, Haarlem, The Netherlands), supplemented with 6% (w/v) sucrose and buffered with 10 mM MES (pH 6.8). At 6 dpi, fresh weight of fungal mycelium was measured. For growth tests on agar medium, L. maculans conidia were applied as a 10 µl droplet (conidial concentration 108 conidia/ml) on a 10% V8 juice agar plates and grown for 10 days in a cultivation box (temperature 26 °C). Radial growth was measured at 3, 5, 7 and 10 dpi.
Preparation and characterization of silenced LmIAD1.2 L. maculans lines
A construct to perform RNAi gene silencing targeting the gene LmIAD1.2 was prepared. A 500-bp region of the target gene LmIAD1.2 was amplified from the cDNA using a proof-reading Pfu polymerase (Thermo Fisher Scientific, Waltham, MA, United States) and attB1- and attB2-tailed primers. The primers for LmIAD1.2 (Leontovyčová et al. 2020) were as follows: F: GGGGACAAGTTTGTACAAAAAAGCAGGCTACAGTTTGACCGTGTTCTC, R: GGGGACCACTTTGTACAAGAAAGCTGGGTCTTCGTGCCCAGATTGAC, the fragment was cloned into a pDONR-Zeo vector (Invitrogen, Carlsbad, CA, United States) using BP clonase (Invitrogen), then recombined by LR clonase (Invitrogen) in two opposing orientations in pHYGGS (Fox et al. 2008), resulting in a final gene silencing vector. The lack of mutations was confirmed by sequencing using two primer pairs: AGCAAGGTAAGTGAACGAC and GGACACAGGCAATGAGG (pair 1), and ACCTCATTGCCTGTGTCC and GACACCAACGATCTTATATCCAG (pair 2). The final vector was used for Agrobacterium tumefaciens (strain LBA4404)—mediated DNA delivery into conidia of L. maculans strain JN2. Transformants were subjected to three rounds of selection on hygromycin (50 μg/ml). In parallel, a control non-transformed line JN2-WT was subjected to all rounds of the cefotaxime exposure used to clear out Agrobacterium. At least 10 mutant fungal lines were generated and analyzed, assessing the level of target transcripts by qPCR, growth and virulence, and comparing to the wild-type line to minimize the unlikely effects of ectopic integrations of T-DNA.
Bioinformatic analysis
For identification of LmHxt1 orthologues in L. maculans and other fungi and yeasts blastX algorithm (https://www.ncbi.nlm.nih.gov/) was used. For modeling and prediction of transmembrane (TM) domains three different programs were used to reach a consensus (TMpred (Hofman 1993), HMMTOP (Tusnady and Simon 2001), Phobius (Käll et al. 2004)). Multiple sequence alignment was performed using Clustal Omega (Sievers et al. 2011).
Inoculation tests
Inoculation tests were performed on cotyledons of 14-day old plants by using infiltration method with conidia suspension, puncture inoculation of conidial suspension (Šašek et al. 2012), or with a suspension of mature mycelium. For the infiltration method, conidial suspension (105 conidia/ml) was infiltrated into apoplastic space with a needleless syringe. For the puncture inoculation, the conidial suspension (107 conidia/ml) was applied as a 10 μl droplet on a cotyledon that was either punctured with a needle or mechanically damaged with tweezers. Distilled water was used as mock control. For the infection with mature mycelium, L. maculans GFP-labeled strains of WT and pHxt1ins mutant were pre-grown on 10% V8 juice agar plates for 7 days in a cultivation box (temperature 26 °C). Mycelium was aseptically removed from the agar surface and put into pre-weighted tubes containing 1 g of ceramic beads, 100—200 mg of fresh mycelium was mixed with 1 ml of distilled water in the tubes. The content of the tube was homogenized in MPbio Fastprep-24 (MP Biomedicals, Irvine, CA, USA) (s = 6.5 m/s; t = 40 s). The homogenous suspension was diluted to concentrations 50 mg/ml and 10 mg/ml and used for infiltration.
After development of infection symptoms (10 dpi) leaves were detached and scanned. Relative lesion area was determined by image analysis using APS Assess 2.0 software (American Phytopathological Society, St. Paul, MN, United States). At least 10 plants were analyzed per variant.
Mycelium growth assay with different hexoses
To study the growth of L. maculans in the presence of different sugars as carbon sources, conidia of JN2-GFP and pHxt1ins-GFP were grown in sterile Gamborg B5 medium with vitamins (Duchefa) supplemented with a range of concentrations (0.0006 – 6% (w/v) or no sugar as control) of hexose sugars (glucose, fructose, sucrose, maltose, mannose or galactose) and 10 mM MES (pH 6.8), at the final concentration of 2500 conidia per well of a black 96-well plate (Nunc®). Covered plates were sealed with Parafilm and incubated in darkness at 26 °C for 5 days. GFP-associated fluorescence was measured at 5 dpi using a Tecan F200 fluorescence reader (Tecan, Männedorf, Switzerland) equipped with a 485/20 nm excitation filter and 535/25 nm emission filter, 16 individual wells for each variant. Alternatively, conidia of both isolates were grown in sterile Gamborg B5 medium with vitamins (Duchefa) supplemented with 6% (w/v) sucrose and 10 mM MES (pH 6.8) at the final concentration of 103 and 105 conidia per well of a black 96-well plate (Nunc®). Fluorescence was measured every 24 h during 7 days.
Gene expression analysis
Total RNA was extracted from 150 mg of either frozen fungal mycelium or infected plant material using a Spectrum Plant Total RNA Kit (Sigma–Aldrich, St. Louis, MO, United States) and treated with a DNA-free Kit (Ambion, Austin, TX, United States). Extracted total RNA (1 µg) was used for reverse transcription to cDNA using M-MLV RNase H– Point Mutant reverse transcriptase (Promega Corp., Fitchburg, WI, United States) with anchored oligo dT21 primer (Metabion, Martinsried, Germany). Gene expression was analyzed by qPCR using LightCycler 480 SYBR Green I Master kit and a LightCycler 480 machine (Roche, Basel, Switzerland). The PCR conditions were set as follows: denaturation 95 °C for 10 min, followed by 45 cycles of 95 °C for 10 s, 55 °C for 20 s (annealing), and 72 °C for 20 s (elongation), followed by a melting curve analysis. Relative expression was calculated with efficiency correction and normalization to the housekeeping gene LmTubulin (primers by Trdá et al. (Trdá et al. 2017)). All values were normalized to WT expression level. Primers were designed using PerlPrimer v1.1.21 (Marshall 2004). Primer sequences can be found in Table 1.
To analyze expression of LmHxt1 in planta during infection, discs of 6 mm diameter were cut from cotyledons of at least 6 independent plants infected by either the WT strain or pHxt1ins line, and sampled at 3, 5 and 7 dpi. Relative expression was calculated with efficiency correction and normalized to the housekeeping gene LmITS1 (Persson et al. 2009).
TAIL-PCR
To locate the site of T-DNA fragment incorporation and verify the number of insertions into L. maculans genome we used the TAIL-PCR method according to Liu et al. (1995). Genomic DNA was extracted from 100 mg of liquid culture grown mycelium (Gamborg B5 medium) from transformed lines, cultivated for 7 days in dark with temperature of 26 °C and continuous shaking at 130 RPM. Extraction was done using Jena Animal and Fungi DNA Preparation—Solution Kit (Jena Bioscience, Jena, Germany). Extracted gDNA was used as a template in the primary reaction of TAIL-PCR. For secondary and tertiary reactions, the product of the previous reaction was diluted 50-times and 1 μl of the diluted reaction was used for the next reaction. The combination of primers and reaction temperatures can be found in Table 2. Primer sequences can be found in Table 3. Amplification was performed using DreamTaq Green PCR Master Mix (2X) (Thermofisher Scientific, Waltham, MA, USA). Amplified products were separated in 1% agarose gel electrophoresis. Reaction products were purified using High Pure PCR Cleanup Micro Kit (Roche, Basel, Switzerland) and sent for sequencing (GATC, Eurofins genomics, Konstanz, Germany). Acquired sequences were then compared to the annotated genome of L. maculans JN3 also known as v23.1.3 using the NCBI database as both JN2 and JN3 come from the same parental cross and should be near isogenic lines (Balesdent et al. 2001).
Microscopy
For live in planta growth visualization, cotyledons infected with GFP labeled WT strain and mutant pHxt1ins line (10 dpi) were detached from the B. napus plants and immediately microscoped using a confocal microscope Zeiss LSM 880 with Airyscan with HXP120 V Illuminator/HAL 100 (Argon ion laser 488 nm excitation) with a Plan-Apochromat 5x/0.16 M27 objective. Additionally, mycelium growth of non-GFP strains was visualized by trypan blue staining. Infected cotyledons (10 dpi) were detached from the B. napus plants and put into 6-well plates. Leaves were bleached by mixture of ethanol and glacial acetic acid (1:3, v/v) overnight, and rehydrated by a solution with a decreasing amount of ethanol (70%, 50%, 25%) and distilled water. After rehydration samples were stained with trypan blue (10 ml lactic acid, 10 ml glycerol, 10 ml water, 10 g phenol, 40 mg trypan blue) for 2 h after which the staining solution was removed and changed for 50% glycerol. These samples were then microscoped using a Leica DM5000B microscope with a HC PL FLUOTAR 10x/0,30 objective using the bright field setting. Dynamics of mycelium growth in infected cotyledons was evaluated using GFP-labeled strains and observed using a Zeiss AxioImager ApoTome2 microscope upon 50 × total magnification (EC PLAN_NEOFLUOR 5x/0,16 M27 objective using GFP filter (excitation: 488 nm, emission: 509 nm)). Samples of infected cotyledons were analyzed at 3, 5, 7 and 10 dpi. Final images were processed using FiJi software (Schindelin et al. 2012).
Phylogenetic analysis
Phylogenetic analysis was based on the comparison of the amino acid sequence of the LmHxt1 transporter from L. maculans to other known sequences of hexose transporters from fungi and yeasts. Sequences were acquired from GenBank and UniProt databases. Multiple alignment was done using MUSCLE (Edgar 2004). Phylogenetic analysis was done using the maximum likelihood method in MEGA X software. Bootstrap step was set to 1000 replicates to support inferred clades (Felsenstein 1985). The tree is drawn in a manner where branch lengths reflect evolutionary distances in the phylogenetic tree. Evolutionary distances were computed using the WAG model (Whelan and Goldman 2001) which represents units of the number of amino acid substitutions per site. The rate variation between sites was chosen as uniform. Overall, 102 amino acid sequences were used for this phylogenetic analysis.
Statistical analysis
All experiments were performed in at least 3 independent biological repeats. The exact number of replicates, technical repetitions, and the statistical tests applied are stated in figure legends. Data was processed using GraphPad Prism 8 software.
Results
T-DNA insertion mutant pHxt1ins shows decreased virulence
A set of T-DNA insertion mutants carrying an RNAi-gene silencing construct was generated to investigate the ability of L. maculans to produce phytohormones within the frame of an independent study (Leontovyčová et al. 2020).
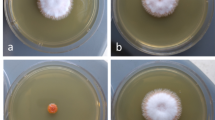
The set of 10 mutant lines was screened for the auxin content and for altered virulence on B. napus cotyledons. One strain displayed a hypovirulent phenotype (Fig. 1a, b). This L. maculans line was referred to as pHxt1ins. No infection symptoms were observed on cotyledons at 10 dpi after syringe infiltration with the pHxt1ins conidia. This particular phenotype remained robust even when conidia concentration used for infection of B. napus plants was increased 100-fold (Fig. S1). To investigate whether the phenotype was not caused by asymptomatic growth of mycelium, we generated a GFP-labeled line derived from the pHxt1ins (pHxt1ins-GFP). Again, the pHxt1ins-GFP displayed significantly reduced growth in the apoplast compared to the WT-GFP (Fig. 1c). This reduced apoplastic growth was further confirmed in the initial pHxt1ins mutant line in planta analysis by staining the fixed mycelium in infected cotyledons with trypan blue (Fig. S2). Other methods of conidia inoculation were also tested (conidia infiltration into cotyledons via syringe, puncture inoculation, mechanical wounding) but none were effective (Fig. S3), showing overall highly impaired lesion formation and apoplastic growth of the pHxt1ins mutant. Interestingly, some mycelium development and respectful disease symptoms (Fig. 1d,e) were detected for the pHxt1ins line while cotyledons were infected with mature mycelium. Still, the mutant strain remained significantly less successful than the WT strain.
Characterization of hypovirulent phenotype of the L. maculans pHxt1ins mutant on Brassica napus. a, b Necrotic lesion development at 10 dpi on cotyledons of Brassica napus cv. Columbus infected by infiltration with a needleless syringe with a conidial suspension. a Representative cotyledons showing difference in virulence between WT and pHxt1ins mutant. b Symptom severity quantification based on the ratio of leaf-to-lesion area relative to WT strain. Data represent mean values ± SE of 5 independent biological repetitions. Asterisks indicate statistically significant difference between the mutant and WT strains (***, P < 0.001), t-test, n = 76 for WT and n = 71 for pHxt1ins mutant; each repetition included 10–23 plants. c Colonization of apoplastic space of cotyledons infected with GFP-labeled WT and mutant pHxt1ins strain. The GFP signal of mycelium, bright field view of surrounding tissue and merged image of both are shown. Scale bars 100 µm; experiment repeated 3 times, 1 representative repetition is shown. d Representative pictures of cotyledons infected with mature mycelium of either the WT or pHxt1ins mutant strain; mycelium concentration was 50 mg/ml, scale bars 5000 µm. e Infection quantification in cotyledons infected with mature mycelium based on the ratio of leaf to lesion area normalized to WT strain. Statistically different variants are labeled by different letters – one-way ANOVA with Tukey’s post hoc test, p < 0.05; n = 61 for WT and pHxt1ins 10 mg/ml, n = 63 for WT 50 mg/ml and n = 46 for pHxt1ins 50 mg/ml; each repetition included 11–31 plants
Aside from observing endpoint infection symptoms (at 10 dpi), we studied the progress of infection in time for the mutant pHxt1ins line and the WT strain (Fig. S4). Using GFP labeled strains we observed the infection spreading throughout the cotyledon from the initial spot of infection. While the WT strain started to show infection symptoms at 7 dpi and the mycelium spread within the leaf tissues until it caused necrotic lesions at 10 dpi, the pHxt1ins mutant did not show growth of mycelium outside the inoculation site.
Localization of T-DNA insert in the genome of L. maculans and change of expression in surrounding genes
As mentioned above, the pHxt1ins strain was generated as a part of an auxin production study in L. maculans. The mutant line was created by using a T-DNA insertion for RNAi silencing the L. maculans auxin production pathway gene IAD1.2 (indole-3-acetaldehyde dehydrogenase) (Leontovyčová et al. 2020). Among 10 tested insertion lines with a silenced LmIAD1.2 gene (Fig. S5a) only one showed a hypovirulent phenotype (Fig. S5b). No correlation was observed between virulence and the level of silencing. We thus confirmed that the observed phenotype was a result of the position of a T-DNA insert and not a direct effect of the insert itself. Next, we investigated which genes could have been influenced by the position of the insertion in L. maculans genome. To localize the insertion, we used the TAIL-PCR method and sequencing of an acquired product (Fig. S6). Sequence alignment revealed that the insert was incorporated between the genes LEMA_P020240.1 and LEMA_P020250.1 somewhere near or inside the promoter region of the LEMA_P020240.1 (the insert is about 400 bases from the start of the coding sequence of the LEMA_P020240.1 gene). Four genes LEMA_P020220.1, LEMA_P020230.1, LEMA_P020240.1, LEMA_P020250.1 (Fig. 2a) surrounding the insertion site were chosen as potentially putatively affected, although, aside from LEMA_P020240.1 all were oriented towards the insert by their terminator sequence. TAIL-PCR and final product sequencing was repeated three times with the same output confirming that the position of the single T-DNA insert is only in the promoter zone of LmHxt1, so the mutant line was named pHxt1ins. Next, expression of the genes situated close to the insert was compared between the WT strain and the pHxt1ins mutant strain during in vitro cultivation in liquid Gamborg medium supplemented with sucrose in flasks. Only the expression of LEMA_P020240.1 was induced 6 times compared to WT (Fig. 2b). Based on this and the orientation towards the insert (as the insert is incorporated near the promoter sequence as shown in Fig. 2a), the gene LEMA_P020240.1 was chosen as the gene of interest potentially responsible for the observed hypovirulent phenotype of pHxt1ins. Next, we investigated the expression of LEMA_P020240.1 in planta during the infection cycle of the pathogen at 3 different time points (3, 5 and 7 dpi). The expression remained stable throughout the infection for both the WT and the mutant pHxt1ins line (Fig. S7), with the only statistically significant difference between the WT and mutant being at 5 dpi, where the mutant had about 43% higher expression. According to the NCBI database, the protein encoded by this gene was annotated as a possible hexose transporter. Three other neighboring genes (LEMA_P020220.1, LEMA_P020230.1 and LEMA_P020250.1) were only predicted in the NCBI database as putative proteins with unknown function.
T-DNA insert localization in the genome of pHxt1ins and expression of genes in its proximity. a Map showing the position of the T-DNA insert in L. maculans genome; scale bar 1000 bp. b In vitro expression levels of LmHxt1 and the surrounding genes in 10-day old L. maculans mycelium of the WT strain and pHxt1ins mutant grown in liquid Gamborg medium supplemented with sucrose in flasks. Gene expression is normalized to housekeeping gene LmTubulin and reported to expression in the WT strain. Asterisks indicate statistically significant differences between the mutant and WT strain (***, P < 0.001), t-test, n = 15 (5 technical replicates in each biological repetition)
LmHxt1 phylogenetic analysis and protein topology modeling
To understand the possible function of the protein encoded by the LEMA_P020240.1 gene and whether it could possibly be a hexose transporter, we performed a phylogenetic analysis based on the predicted amino acid sequence. BlastX showed that the predicted protein encoded by the LEMA_P020240.1 gene has about 62 – 33% identity with other fungal putative hexose transporters. We thus name the LEMA_P020240.1 gene as LmHxt1 further in the text. Based on sequence similarities we found 20 potential hexose transporters in L. maculans (the closest being LEMA_P025430.1 we named LmHxt2 with an identity of 62%) (Fig. S8). We generated a phylogenetic tree with similar sequences from L. maculans and 102 other fungal and yeast hexose transporters, to see which cluster LmHxt1 transporter belongs to, and what are its closest orthologues. Phylogenetic analysis showed that LmHxt1 clusters close with several fungal and yeast hexose transporters (Fig. S8), in particular with CgHxt4 from the fungus C. graminicola (Lingner et al. 2011), high and low affinity glucose transporters Snf3 and Rgt2 from the yeast S. cerevisiae (Özcan et al. 1996), and glucose transporter Rco3 from fungus N. crassa (Madi et al. 1997) (clustering shown in Fig. 3a). All of these proteins have a described sugar signaling function, pointing to a possible sugar sensing function in LmHxt1. However, most other close orthologues are still functionally uncharacterized hexose transporters (Fig. 3a and S8).
Phylogenetic and topology analysis of LmHxt1 a Section of the maximum likelihood phylogenetic tree of LmHxt1 protein sequence and other known fungal and yeast hexose transporter protein sequences (GenBank and Uniprot database). The tree was generated using the WAG model. Bootstrap value of each branch is indicated by the number above. The transporter LmHxt1 is shown in the red bracket, other notable hexose transporters are shown in the black bracket. b Topology model of LmHxt1 transmembrane domains (TM) showing 12 TM domains characteristic for MFS transporters. Letters pointed out by arrows show conserved motifs in amino acid sequence typical for sugar transporters (number by the motif shows position in the sequence). Dark background indicates a substitution of an amino acid in the motif
LmHxt1 topology analysis revealed that LmHxt1 belongs to the major facilitator superfamily (MFS), a family of transmembrane proteins that facilitate transport of small molecules across the cell membrane. Modeling of transmembrane domains of LmHxt1 showed a consensus of 12 transmembrane helices with a long intracellular loop between helix 6 and 7 and cytosolic N and C-terminal (Fig. 3b), all of which are characteristic for the MFS. Analysis of the sequence using PROSITE also suggested that LmHxt1 belongs to the MFS family and contains motifs present in sugar transporters. Alignment of LmHxt1 against sequences of several characterized sugar transporters showed the presence of conserved motifs typical for sugar transporters (Doege et al. 2000), namely [GRR] and [R-GRR] in loops 2 and 7, [PETPR] in loop 6 and [PETKG] in the C-terminus of the amino acid sequence (Fig. 3b and S9). A rather high number of asparagine- and glutamine-rich regions in the C-terminus of LmHxt1 might be linked to protein–protein interactions that might be a part of a signaling cascade.
In vitro growth and changes of conidia germination
On top of the observed hypovirulent phenotype in planta we investigated the influence of the mutation in the LmHxt1 promoter region at the general growth and mycelium morphology during in vitro cultivation.
First, we characterized the growth of the mutant in axenic liquid culture. Dry weight of mycelium did not differ between WT and the pHxt1ins mutant after 6 days of cultivation in liquid Gamborg medium supplemented with sucrose in flasks (Fig. 4a). Interestingly, during cultivation in flasks on a rotary shaker, the WT grew into round spheres with short strands of mycelium hanging from the surface, while in the mutant strain the mycelial spheres did not form any strands and aggregated together instead (Fig. 4b). Secondly, radial growth on agar medium was assessed. Both WT and the pHxt1ins mutant grew similarly on agarized V8 medium for 9 days (Fig. 4c). However, even though the rate of radial growth was similar for both strains, we observed a certain change in the morphology of the mycelium, as the mutant grew more aerial mycelium compared to the WT (Fig. 4e).
In vitro growth characterization of pHxt1ins mutant. a Biomass production (dry weight) in WT and pHxt1ins mutant after 6 days of cultivation of the mycelium in liquid Gamborg medium supplemented with sucrose in flasks. Bars represent mean values ± SE of 3 independent biological repetitions (each including 3 technical repetitions). b Phenotype of 6-day old mycelium of WT and pHxt1ins mutant grown in liquid Gamborg medium supplemented with sucrose in flasks. c Colony diameters of WT strain and pHxt1ins mutant cultivated on agarized V8 medium for 3—9 days. Bars represent mean values ± SE of 3 independent biological repetitions (each including 4 technical repetitions). d Percentage of germinated conidia on Gamborg supplemented with sucrose agar plate media of L. maculans WT and mutant pHxt1ins. Data represent the number of germinated single colonies out of 100 applied conidia (4 days of cultivation). Bars represent mean values ± SE of 3 independent biological repetitions. Asterisks indicate statistically significant difference between the WT and mutant strain (***, P < 0.001), t-test, n = 15. e Mycelium grown on V8 agar plates for 9 days from a drop of conidial suspension with concentration 108 conidia/ml
Given the fact that conidial infection did not lead to lesion development but inoculation with mature mycelium caused infection, we investigated whether the mutant is affected in conidial germination. We compared the germination of conidia for WT and pHxt1ins mutant on agarized Gamborg medium supplemented with sucrose and observed 43% decrease in germination in the mutant strain (Fig. 4d). This germination rate was coherent with an in vitro growth assay using two different conidia concentrations (Fig. S10), showing that the delay in mycelium growth for the mutant line in comparison to WT could be evened out by increasing the conidia concentration to 105 conidia/ml. On the contrary, an increase of conidia concentration for the mutant did not result in catching up with WT line in planta, suggesting that not only the germination delay but also the early development in planta is affected in pHxt1ins (Fig. S1).
Hexose uptake in L. maculans mycelium and effect of glucose on expression of LmHxt1
To test whether the affected gene might function as a hexose transporter, we investigated the capacity of L. maculans to grow on different sugar sources. L. maculans WT and pHxt1ins were cultivated in vitro in a 96-well plate in Gamborg liquid medium supplemented with different sugars as a carbon source. The chosen sugars were glucose, sucrose, fructose, mannose, galactose and maltose as they are the most common sugars fungi utilize (Kowalczyk et al. 2014). A clear preference for glucose and sucrose was observed for both tested strains (Fig. 5a). Interestingly, no significant difference in mycelium growth was observed between strains growing on the same sugar, regardless of the sugar type.
Comparison of in vitro growth of WT and mutant pHxt1ins strain in liquid Gamborg medium supplemented with different sugars and relative expression levels of LmHxt1 in the presence and absence of glucose. a Relative fluorescence of GFP labeled WT and pHxt1ins strains was measured in a 96-well plate at 5 dpi. Data shown in the graph represent mean values ± SE of 3 independent biological repetitions. Difference between variants is shown by different letters – one-way ANOVA with Tukey’s post hoc test, p < 0.05, n = 24. b Relative expression of LmHxt1 in presence or absence of glucose. Mycelium was pre-cultivated for 7 days in liquid Gamborg medium supplemented with sucrose. After 7 days the medium was changed for Gamborg medium containing either glucose or no sugar, gene expression was measured 24 h after medium change. Gene expression is normalized to housekeeping gene LmTubulin and reported to the expression in WT after medium change. Asterisks indicate statistically significant differences between the mutant and WT strain (**, P < 0.01), t-test, n = 4. Experiment was repeated three times with similar results; data from one representative repetition are shown
As glucose appeared to be the second best preferred sugar and best preferred monosaccharide for L. maculans growth, we investigated how the expression of LmHxt1 would change depending on the glucose availability in the medium. After 7 days of cultivation in Gamborg medium supplemented with sucrose the cultivation medium was changed for fresh medium either supplemented or not with glucose as a sole carbon source. The presence or absence of glucose for 24 h did not affect LmHxt1 expression in WT (Fig. 5b). Interestingly, we detected a 30-fold increase of expression of LmHxt1 in the absence of glucose in the pHxt1ins. This significant change in expression of LmHxt1 in response to glucose availability strongly supports our claim that the LmHxt1 works as a sugar sensor.
Discussion
Change in LmHxt1 gene expression is associated with the a loss of virulence in L. maculans
Our data strongly suggest that the loss of virulence in the L. maculans mutant strain pHxt1ins is linked to the expression upregulation of the LmHxt1 gene in the pathogen. The LmHxt1 overexpressing mutant pHxt1ins has lost its ability to colonize leaf tissue and cause infection (Fig. 1c). Another similar case was described for L. maculans gene LmSNF1 (Sucrose Nonfermenting 1), which is important for carbon catabolite repression in sugar metabolism (Feng et al. 2014). A knockout of SNF1 led to a loss of virulence due to attenuated expression of 11 cell wall degrading enzymes and a pathogenicity related gene LopB (Idnurm and Howlett 2003). Similar observations were made for genes responsible for sugar metabolism in U. maydis, where knockout of UmHxt1 gene, that encodes a hexose transporter, caused a loss of pathogenicity (Schuler et al. 2015). Also in U. maydis a deletion of a sucrose specific sugar transporter Srt1 caused a loss of virulence. However, unlike our pHxt1ins mutant strain the U. maydis deletion mutant was still able to successfully colonize the plant (Wahl et al. 2010). This shows that hexose transporters are important for pathogen virulence and changes in their expression have various effects on the pathogens’ ability to proliferate in planta upon infection. It seems to be linked to different expression levels of the Hxt genes throughout the life cycle of the fungus, as was shown in a study on Botrytis cinerea (Dulermo et al. 2009). In this study different levels of Hxt genes expression were associated with different developmental phases of the fungus: some genes were only expressed in dormant spores, others in germinating spores, and some were expressed exclusively during infection. Based on the germination and mycelium development tests, we concluded that the pHxt1ins mutant is indeed impaired in conidia germination, an early crucial stage of growth, that might define the observed further inability to successfully grow in planta. This supports the claim that the LmHxt1 gene is important at the early development of the fungus.
Overexpression of LmHxt1 does not affect mycelial biomass production in vitro but results in altered morphology of mycelium and impaired conidia germination
During in vitro cultivation, the pHxt1ins mutant did not differ from WT in biomass production or radial mycelium growth (Fig. 4a, 4c). However, we have observed a change in the morphology of the mycelium in both liquid culture and on agarized medium (Fig. 4b, 4e). We found this change of morphology particularly interesting, as to the best of our knowledge, a change in expression of a Hxt gene has never been linked with changes in mycelium morphology. It is, however, still unclear whether such phenotypic difference is linked to the loss in virulence or it is a separate phenomenon. For both the mutant and the WT we have observed a preference for sucrose and glucose as a carbon source (Fig. 5a). As the latter is a disaccharide, it is unknown whether L. maculans cleaves it into monosaccharides before uptake and how this can affect the growth rate. In the tested conditions, regardless of the sugar carbon source, we did not detect a significant growth difference between WT and pHxt1ins mutant. However, as L. maculans possesses several hexose transporters, they might overlap in function. Hexose transporters are of particular importance in conidia germination (MacCabe et al. 2003; Doehlemann et al. 2005; Pereira et al. 2013; Forment et al. 2014). Our data show that pHxt1ins conidia germination rate is significantly lower in vitro (Fig. 4d). A change in sugar uptake or sugar signaling can have a strong impact on fungal fitness since it is an energy dependent process. It is however unlikely to be the single cause of the observed virulence loss. Indeed, once plants were inoculated with the 100-fold higher concentration of pHxt1ins conidia, the hypovirulent phenotype remained pronounced (Fig. S1). This suggests the loss of virulence is a more complex phenomenon than just a result of lower conidia germination rate and requires further investigation.
LmHxt1 is a possible sugar sensor membrane protein
Many hexose transporters also possess a sugar signaling function. For example, Snf3 and Rgt2 in yeasts S. cerevisiae are low and high glucose level sensor membrane proteins respectively (Özcan et al. 1996). Lingner et al. described several characteristics shared between possible sugar sensors (Lingner et al. 2011). One of the most common is the gene downregulation in the presence of sugar. This is clearly the case of LmHxt1 in the presence of glucose, as we detected about a fivefold decrease in its expression in the presence of glucose in the pHxt1ins strain (Fig. 5b). This effect could not be observed in the WT background due to low expression of the LmHxt1 gene. Downregulation of the transporter expression levels in response to the glucose level in the environment surrounding the fungus was observed for other fungal hexose transporter proteins with a sensor function, such as CgHxt4 in C. graminicola (Lingner et al. 2011), UmHxt1 in U. maydis (Schuler et al. 2015) or Rco3 in Neurospora crassa (Madi et al. 1997). A constitutive overexpression of LmHxt1 in the mutant together with its decrease as a response to the glucose/sucrose withdrawal from the cultivation medium suggests that the sugar-related signaling is being misregulated in the pHxt1ins mutant. This could lead to the observed delay in early mycelium development, and also to a loss of pathogenicity in the pHxt1ins mutant. Similar results were reported by Schuler et al. (Schuler et al. 2015). In this work, authors connected an altered expression of the UmHxt1 to a loss of pathogenicity, although the fungal development remained the same. Another key point to claim LmHxt1 as a possible sugar sensor is the length and amino acid composition of the C terminus of the protein. A longer C terminus was observed in this type of sugar sensing transporters (Marshall-Carlson et al. 1990; Madi et al. 1997). Asparagine and glutamine rich regions outside the TM domain are needed for certain protein–protein interactions that can be tied to the sensor function (Michelitsch and Weissman 2000; Okazawa 2007; Lingner et al. 2011). The C terminus of LmHxt1 showed strong similarities with the C terminus of Rco3 (Fig. 3b), which has dual sensor-transporter function (Okazawa 2007). Throughout the infection cycle, an expression of transporters with a sugar sensing ability usually remains quite low and stable (Lingner et al. 2011). In our experiments, LmHxt1 expression also remained low and stable at various time points during the infection process both in WT strain and pHxt1ins line (Fig. S7). Lastly, a phylogenetic analysis of amino acid structure of LmHxt1 along with 102 different fungal and yeast sugar transporters showed a rather close phylogenetic proximity to 3 notable hexose transporters with a sugar sensing capability (Fig. 3 and S8), the already mentioned CgHxt4 (Lingner et al. 2011), Rgt2 and Snf3 (Özcan et al. 1996; Özcan and Johnston 1999). Taken together, our data strongly suggest the newly described LmHxt1 gene might function as a sugar sensor. This is supported by the LmHxt1 expression profile in response to glucose, and also based on the predicted structure of LmHxt1 and its placement within the phylogenetic tree of known sugar sensors/transporters. We describe a L. maculans mutant pHxt1ins, which has a constitutively overexpressed LmHxt1 and a particular mycelium morphology while cultivated in vitro, without biomass production being affected. Notably, the mutant was strongly impaired in growth in planta, and almost unable to colonize tissues. The described pHxt1ins mutant strain provides an interesting tool for further research in the field of sugar transporters and their role in fungal life or virulence.
Data availability
RAW data supporting the publication, as well as the samples and sequences of plasmids are available from the corresponding author upon reasonable request.
References
Ansan-Melayah D, Balesdent M-H, Buee M, Rouxel T (1995) Genetic characterization of AvrLm1, the first avirulence gene of Leptosphaeria maculans. Phytopathology 85:1525–1529
Balesdent M-H, Attard A, Ansan-Melayah D, Delourme R, Renard M, Rouxel T (2001) Genetic control and host range of avirulence toward Brassica napus cultivars Quinta and Jet Neuf in Leptosphaeria maculans. Phytopathology 91:70–76. https://doi.org/10.1094/PHYTO.2001.91.1.70
Brown V, Sexton JA, Johnston M (2006) A Glucose Sensor in Candida albicans. Eukaryot Cell 5:1726–1737. https://doi.org/10.1128/EC.00186-06
Cross AS (2008) What is a virulence factor? Crit Care 12:1–2. https://doi.org/10.1186/cc7127
Desmazières M (1851) Dix-neuvième notice sur les plantes cryptogames, récemment découvertes en France. Ann Sci Nat Bot 3:296–330
Doege H, Schürmann A, Bahrenberg G, Brauers A, Joost H-G (2000) GLUT8, a novel member of the sugar transport facilitator family with glucose transport activity. J Biol Chem 275:16275–16280. https://doi.org/10.1074/jbc.275.21.16275
Doehlemann G, Molitor F, Hahn M (2005) Molecular and functional characterization of a fructose specific transporter from the gray mold fungus Botrytis cinerea. Fungal Genet Biol 42:601–610. https://doi.org/10.1016/j.fgb.2005.03.001
Dulermo T, Rascle C, Chinnici G, Gout E, Bligny R, Cotton P (2009) Dynamic carbon transfer during pathogenesis of sunflower by the necrotrophic fungus Botrytis cinerea: from plant hexoses to mannitol. New Phytol 183:1149–1162. https://doi.org/10.1111/j.1469-8137.2009.02890.x
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. https://doi.org/10.1093/nar/gkh340
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
Feng J, Zhang H, Strelkov SE, Hwang SF (2014) The LmSNF1 gene is required for pathogenicity in the canola blackleg pathogen Leptosphaeria maculans. PLoS ONE 9:e92503. https://doi.org/10.1371/journal.pone.0092503
Forment JV, Flipphi M, Ventura L, González R, Ramón D, MacCabe AP (2014) High-affinity glucose transport in Aspergillus nidulans is mediated by the products of two related but differentially expressed genes. PLoS ONE 9:e94662. https://doi.org/10.1371/journal.pone.0094662
Fox EM, Gardiner DM, Keller NP, Howlett BJ (2008) A Zn (II) 2Cys6 DNA binding protein regulates the sirodesmin PL biosynthetic gene cluster in Leptosphaeria maculans. Fungal Genet Biol 45:671–682. https://doi.org/10.1016/j.fgb.2007.10.005
Gardiner DM, Howlett BJ (2004) Negative selection using thymidine kinase increases the efficiency of recovery of transformants with targeted genes in the filamentous fungus Leptosphaeria maculans. Curr Genet 45:249–255. https://doi.org/10.1007/s00294-004-0488-6
Hammond KE, Lewis B (1987) The establishment of systemic infection in leaves of oilseed rape by Leptosphaeria maculans. Plant Pathol 36:135–147. https://doi.org/10.1111/j.1365-3059.1987.tb02213.x
Hofman K (1993) TMbase: a database of membrane spanning protein segments. Biol Chem Hoppe-Seyler 374:166
Howlett BJ (2004) Current knowledge of the interaction between Brassica napus and Leptosphaeria maculans. Can J Plant Path 26:245–252. https://doi.org/10.1080/07060660409507141
Idnurm A, Howlett BJ (2003) Analysis of loss of pathogenicity mutants reveals that repeat-induced point mutations can occur in the Dothideomycete Leptosphaeria maculans. Fungal Genet Biol 39:31–37. https://doi.org/10.1016/S1087-1845(02)00588-1
Jones JD, Dangl JL (2006) The plant immune system. Nature 444:323–329. https://doi.org/10.1038/nature05286
Käll L, Krogh A, Sonnhammer EL (2004) A combined transmembrane topology and signal peptide prediction method. J Mol Biol 338:1027–1036. https://doi.org/10.1016/j.jmb.2004.03.016
Kowalczyk JE, Benoit I, de Vries RP (2014) Regulation of plant biomass utilization in Aspergillus. Adv Appl Microbiol 88:31–56. https://doi.org/10.1016/B978-0-12-800260-5.00002-4
Leontovyčová H, Trdá L, Dobrev PI, Šašek V, Gay E, Balesdent MH et al (2020) Auxin biosynthesis in the phytopathogenic fungus Leptosphaeria maculans is associated with enhanced transcription of indole-3-pyruvate decarboxylase LmIPDC2 and tryptophan aminotransferase LmTAM1. Res Microbiol 171:174–184. https://doi.org/10.1016/j.resmic.2020.05.001
Lingner U, Münch S, Deising HB, Sauer N (2011) Hexose transporters of a hemibiotrophic plant pathogen: functional variations and regulatory differences at different stages of infection. J Biol Chem 286:20913–20922. https://doi.org/10.1074/jbc.M110.213678
Liu Y, Mitsukawa N, Oosumi T, Whittier RF (1995) Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J 8:457–463. https://doi.org/10.1046/j.1365-313X.1995.08030457.x
Liu TB, Wang Y, Baker GM, Fahmy H, Jiang L, Xue C (2013) The glucose sensor-like protein Hxs1 is a high-affinity glucose transporter and required for virulence in Cryptococcus neoformans. PLoS ONE 8:e64239. https://doi.org/10.1371/journal.pone.0064239
MacCabe AP, Miro P, Ventura L, Ramon D (2003) Glucose uptake in germinating Aspergillus nidulans conidia: involvement of the creA and sorA genes. Microbiology 149:2129–2136. https://doi.org/10.1099/mic.0.26349-0
Madi L, McBride SA, Bailey LA, Ebbole DJ (1997) Rco-3, a gene involved in glucose transport and conidiation in Neurospora crassa. Genetics 146:499–508. https://doi.org/10.1093/genetics/146.2.499
Marshall OJ (2004) PerlPrimer: cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics 20:2471–2472. https://doi.org/10.1093/bioinformatics/bth254
Marshall-Carlson L, Celenza J, Laurent B, Carlson M (1990) Mutational analysis of the SNF3 glucose transporter of Saccharomyces cerevisiae. Mol Cell Biol 10:1105–1115. https://doi.org/10.1128/mcb.10.3.1105-1115.1990
Michelitsch MD, Weissman JS (2000) A census of glutamine/asparagine-rich regions: implications for their conserved function and the prediction of novel prions. Proc Natl Acad Sci 97:11910–11915. https://doi.org/10.1073/pnas.97.22.11910
Okazawa H (2007) “Glutamine/Asparagine-Rich Regions in Proteins and Polyglutamine Diseases”, in Protein Misfolding, Aggregation, and Conformational Diseases. Springer. https://doi.org/10.1007/978-0-387-36534-3_22
Özcan S, Johnston M (1999) Function and Regulation of Yeast Hexose Transporters. Microbiol Mol Biol Rev 63:554–569. https://doi.org/10.1128/MMBR.63.3.554-569.1999
Özcan S, Dover J, Rosenwald AG, Wölfl S, Johnston M (1996) Two glucose transporters in Saccharomyces cerevisiae are glucose sensors that generate a signal for induction of gene expression. Proc Natl Acad Sci 93:12428–12432. https://doi.org/10.1073/pnas.93.22.12428
Pereira MF, de Araújo Dos Santos CM, de Fernandez E, de Queiroz MV, Bazzolli DMS (2013) Beginning to understand the role of sugar carriers in Colletotrichum lindemuthianum: the function of the gene mfs1. J Microbiol 51: 70–81. https://doi.org/10.1007/s12275-013-2393-5
Persson M, Staal J, Oide S, Dixelius C (2009) Layers of defense responses to Leptosphaeria maculans below the RLM1-and camalexin-dependent resistances. New Phytol 182:470–482. https://doi.org/10.1111/j.1469-8137.2009.02763.x
Peterson JW (1996) Bacterial pathogenesis. In Medical Microbiology, 4th ed. University of Texas Medical Branch at Galveston
Polidori E, Ceccaroli P, Saltarelli R, Guescini M, Menotta M, Agostini D et al (2007) Hexose uptake in the plant symbiotic ascomycete Tuber borchii Vittadini: biochemical features and expression pattern of the transporter TBHXT1. Fungal Genet Biol 44:187–198. https://doi.org/10.1016/j.fgb.2006.08.001
Rouxel T, Balesdent MH (2005) The stem canker (blackleg) fungus, Leptosphaeria maculans, enters the genomic era. Mol Plant Pathol 6:225–241. https://doi.org/10.1111/j.1364-3703.2005.00282.x
Šašek V, Nováková M, Dobrev PI, Valentová O, Burketová L (2012) β-aminobutyric acid protects Brassica napus plants from infection by Leptosphaeria maculans. Resistance induction or a direct antifungal effect? Euro J Plant Pathol 133:279–289. https://doi.org/10.1007/s10658-011-9897-9
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Scholes J, Lee P, Horton P, Lewis D (1994) Invertase: understanding changes in the photosynthetic and carbohydrate metabolism of barley leaves infected with powdery mildew. New Phytol 126:213–222. https://doi.org/10.1111/j.1469-8137.1994.tb03939.x
Schuler D, Wahl R, Wippel K, Vranes M, Münsterkötter M, Sauer N et al (2015) Hxt1, a monosaccharide transporter and sensor required for virulence of the maize pathogen Ustilago maydis. New Phytol 206:1086–1100. https://doi.org/10.1111/nph.13314
Sesma A, Osbourn AE (2004) The rice leaf blast pathogen undergoes developmental processes typical of root-infecting fungi. Nature 431:582–586. https://doi.org/10.1038/nature02880
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W et al (2011) Fast, scalable generation of high‐quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. https://doi.org/10.1038/msb.2011.75
Stasyk OG, Maidan MM, Stasyk OV, Dijck PV, Thevelein JM, Sibirny AA (2008) Identification of Hexose Transporter-Like Sensor HXS1 and Functional Hexose Transporter HXT1 in the Methylotrophic Yeast Hansenula polymorpha. Eukaryot Cell 7:735–746. https://doi.org/10.1128/EC.00028-08
Steiner AA (1961) A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 15:134–154. https://doi.org/10.1007/BF01347224
Trdá L, Barešová M, Šašek V, Nováková M, Zahajská L, Dobrev PI et al (2017) Cytokinin metabolism of pathogenic fungus Leptosphaeria maculans involves isopentenyltransferase, adenosine kinase and cytokinin oxidase/dehydrogenase. Front Microbiol 8:1374. https://doi.org/10.3389/fmicb.2017.01374
Tusnady GE, Simon I (2001) The HMMTOP transmembrane topology prediction server. Bioinformatics 17:849–850. https://doi.org/10.1093/bioinformatics/17.9.849
Voegele RT, Struck C, Hahn M, Mendgen K (2001) The role of haustoria in sugar supply during infection of broad bean by the rust fungus Uromyces fabae. Proc Natl Acad Sci 98:8133–8138. https://doi.org/10.1073/pnas.131186798
Wahl R, Wippel K, Goos S, Kämper J, Sauer N (2010) A novel high-affinity sucrose transporter is required for virulence of the plant pathogen Ustilago maydis. PLoS Biol 8:e1000303. https://doi.org/10.1371/journal.pbio.1000303
West JS, Kharbanda P, Barbetti M, Fitt BD (2001) Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia. Canada and Europe Plant Pathology 50:10–27. https://doi.org/10.1046/j.1365-3059.2001.00546.x
Whelan S, Goldman N (2001) A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol Biol Evol 18:691–699. https://doi.org/10.1093/oxfordjournals.molbev.a003851
Yuan Q, Yan Y, Sohail MA, Liu H, Huang J, Hsiang T et al (2021) A Novel Hexose Transporter ChHxt6 Is Required for Hexose Uptake and Virulence in Colletotrichum higginsianum. Int J Mol Sci 22:5963. https://doi.org/10.3390/ijms22115963
Acknowledgements
The work was supported by the European Regional Development Fund-Project "Centre for Experimental Plant Biology" (No. CZ.02.1.01/0.0/0.0/16_019/0000738) and NAZV grant from Ministry of Agriculture of the Czech Republic no. QK1710397. HL was supported by the PPPLZ fellowship provided by The Academy of Sciences of the Czech Republic no. L200382151. IEB Imaging Facility was supported by project of Ministry of Education, Youth and Sports “National Infrastructure for Biological and Medical Imaging (Czech-BioImaging – LM2018129)".
Funding
Open access publishing supported by the National Technical Library in Prague. European Regional Development Fund, CZ.02.1.01/0.0/0.0/16_019/0000738, Lenka Burketová, Národní Agentura pro Zemědělský Výzkum, QK1710397, Lenka Burketová, Akademie Věd České Republiky, L200382151, Hana Leontovyčová, Ministerstvo Školství, Mládeže a Tělovýchovy, LM2018129.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that there is no known conflict of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stehlík, D., Trdá, L., Leontovyčová, H. et al. Upregulation of LmHxt1 gene is associated with reduced virulence of Leptosphaeria maculans on Brassica napus. J Plant Pathol (2024). https://doi.org/10.1007/s42161-023-01568-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42161-023-01568-x