Abstract
Bread wheat (Triticum aestivum L.) is the most widely grown crop in the world. Rhizoctonia cerealis, the causal agent of wheat sharp eyespot disease, has 21 become epidemic in many countries. In the present study, we performed transcriptome analysis in wheat infected by R. cerealis at 0, 12, 30, 70, and 98 h post-infection using R. cerealis-resistant and -susceptible genotypes (CI12633 and ‘Yangmai15’, respectively). We used quantitative real-time PCR to validate the Illumina gene expression data, and identified new gene annotations for 23,654 unigenes in the RNA samples from the resistant and susceptible cultivars. Comparing the same inoculation times, we found that the number of DEGs (differentially-expressed genes) increased gradually before 70 h and declined at 98 h in the two RNA samples. Furthermore, the expression of resistance-associated genes occurred earlier in CI12633 than in ‘Yangmai15’, and higher mRNA expression levels were detected in CI12633; this suggests that timing and relative expression levels of these genes are important in the CI12633-R. cerealis interaction. Functional annotations associated with sharp eyespot resistance included genes involved in energy production and conversion, posttranslational modification, protein turnover, chaperones, secondary metabolite biosynthesis, transport and catabolism, and defense mechanisms. The results of pathway enrichment analysis showed that the DEGs participate in glutathione metabolism, glycerophospholipid metabolism, lysine degradation, plant-pathogen interaction, glyoxylate and dicarboxylate metabolism, and other resistance-associated metabolic pathways. Disease inoculation experiments and the validation of in vitro antifungal activity of the candidate genes showed that the genes were up- or down-regulated in the resistant genotype CI12633 30 h after inoculation compared to its control, which validated the results of the RNA-seq analysis. The results of our study will help to understand the molecular basis of the host response to R. cerealis infection in wheat, and will also enable the future genetic improvement of sharp eyespot resistance in wheat through the incorporation of novel resistance genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat (Triticum aestivum L.) is the third most important cereal crop in the world after rice and corn. Sharp eyespot, caused by the soil-borne fungus R. cerealis van der Hoeven, is an important disease that threatens wheat production worldwide (Lemańczyk et al. 2013), which results in severe reductions in both yield and grain quality.
Wheat sharp eyespot always occurred in temperate wheat growing regions such as China. In China, the wheat can be attacked by the sharp eyespot as early as when seedling stage in autumn, but the attack occurs more frequently in the next year spring and early summer as manifested by lesions on wheat basal leaf sheaths and stems.
However, strong resistance to R. cerealis infection is rare reported in wheat cultivars. Currently, pyramiding resistance genes/QTL is an environment-friendly and economically strategy for controlling this disease (Chen et al. 2013; Ying et al. 2015).
At present, few studies have focused on genetic resistance to sharp eyespot in wheat. Zhang Zengyan’s research group through microarray-based comparative transcriptomics on the wheat lines CI12633 and ‘Wenmai 6’ that are resistant and susceptible to sharp eyespot, respectively, identified many genes related to sharp eyespot resistance, such as plant caffeic acid 3-O-methyltransferase (COMT) ( Wang et al. 2018), the wheat ethylene response factor transcription factor PATHOGEN-INDUCED ERF1 (Zhu et al. 2014), the wheat AGC kinase gene TaAGC1 (Zhu et al. 2015), the cutinase-encoding gene RcCUT1 (Lu et al. 2018), the cinnamyl alcohol dehydrogenase gene (TaCAD12) ( Rong et al. 2016), eukaryotic type 2A protein phosphatase (PP2A) ( Zhu et al. 2018), the wheat calcium-dependent protein kinase TaCPK7-D (Wei et al. 2016), the wheat NB-LRR gene TaRCR1 (Zhu et al. 2017), the wheat R2R3-MYB transcription factor TaRIM1 (Shan et al. 2016), and the antimicrobial peptide DmAMP1W(Su et al. 2020). However, so far, no major genes have been reported to provide high resistance to sharp eyespot, which hinders the application of marker-assisted selection (MAS) technique in resistant breeding.
The difficulty in the genetic study of sharp eyespot resistance is that the resistance identification is mainly influenced by environmental factors, which retards accurate mapping of QTL/genes resistant to sharp eyespot. Some studies have shown that sharp eyespot resistance in wheat is a quantitative trait controlled by additive-effect mechanism of multiple genes (Chen et al. 2013; Wu et al. 2017). However, due to the complex of the genetic basis of sharp eyespot resistance, less stable resistance QTL/genes for marker assisted breeding are available.
Many studies proved that changes in gene expression profiles can help to identify the biochemical processes involved in pathogen infection and colonization (Cui et al. 2019). Comparative transcriptome analysis has been an important tool for discovering the genes involved in complex traits in wheat. RNA sequencing (RNA-seq) is a highly effective technique that can be used to identify single-nucleotide polymorphisms (SNPs) in transcribed genes, and is particularly effective in non-model plant species for which genomic resources may be unavailable (Chepelev et al. 2009; O'Neil et al. 2013; Patterson et al. 2019). The high sensitivity, quantitative nature, and single-base resolution of RNA-seq greatly promotes our understanding of gene expression patterns in certain metabolic pathways (Wang et al. 2009). RNA-seq has been widely used to quantify genome-wide changes in gene expression. Gene expression difference can be calculated directly by counting the numbers of reads that map to a gene, and can also be used to identify new transcript-derived fragments (TDFs) (Que et al. 2014). Comparative transcriptome analyses performed in plants exposed to pathogen infection have enabled the identification of many stress-response genes from species such as Arabidopsis thaliana, Oryza sativa, Zea mays, and Triticum aestivum, and the mechanisms underlying pathogen resistance were also explored.
The main goal of this research is to investigate the molecular and biochemical nature of sharp eyespot resistance in wheat. Two wheat genotypes that differ with respect to their sharp eyespot disease reaction, the resistant cultivar CI12633 and the susceptible cultivar ‘Yangmai15’, were inoculated with Rhizoctonia cerealis. The relative expression of several randomly-chosen genes was quantified from RNA-seq data and then confirmed in quantitative real-time PCR (qRT-PCR) assays. The selected differentially expressed genes (DEGs) and their chromosomal locations were compared in the two wheat genotypes (Table S6). We also compared the dynamics of changes in gene expression in CI12633 and ‘Yangmai15’ in order to identify the genes located in the chromosomal interval harboring the sharp eyespot resistance QTL QSe.jaas-2BS that are differentially expressed in the two cultivars following R. cerealis infection (Wu et al. 2007). The results of our study will provide a broader view of the genetics of sharp eyespot resistance in wheat cultivar CI12633. In addition, key genes conferring sharp eyespot resistance might be identified and applied for marker-assisted selection (MAS) in wheat breeding procedure.
Materials and methods
Plant and pathogen materials
Two wheat cultivars, CI12633 and ‘Yangmai15’, were used in this study. Several years of disease trials confirmed that CI12633 expresses stable resistance to sharp eyespot disease. ‘Yangmai15’, a high-yielding wheat cultivar grown in China along the middle and lower reaches of the Yangtze River, is susceptible to sharp eyespot. R0301, a highly virulent isolate of R. cerealis, was provided by Prof. Huaigu Chen (Jiangsu Academy of Agricultural Sciences, China).
Growth conditions and treatments
Wheat plants were grown under a 16 h light/8 h dark photoperiod at 25 °C/10 °C. At tillering, we inoculated each wheat plant at the second basal sheath with mature mycelia of R. cerealis using small toothpick fragments, and the control plants were mock inoculated using small toothpick fragments harboring only culture medium. Inoculated plants were maintained at 90% relative humidity, and the whole plants except for the roots, were sampled at 12, 30, 70 and 98 h post-inoculation (hpi). The 0 h were used for treated and controls. Each time point had 3 biological replicates for the two cultivars, and the total number of samples in this study was 54. At each time point the samples were harvested, immediately flash frozen in liquid nitrogen, and stored at -80 °C freezer prior to further study.
RNA extraction and qualification
Total RNA was extracted from the wheat tissue using Trizol Reagent (Life technologies, California, USA) as directed by the manufacturer. The integrity and concentration of each RNA sample was assessed using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). mRNA was purified from the total RNA samples using the NEBNext Poly (A) mRNA Magnetic Isolation Module (New England Biolabs, Ipswich, MA; product E7490).
Library preparation for transcriptome sequencing
cDNA libraries were constructed using the NEBNext Ultra RNA Library Prep Kit for Illumina (NEB; product number E7530) and the NEBNext Multiplex Oligos for Illumina (NEB: E7500) and directed by the manufacturer. In brief, the purified mRNA was fragmented into pieces of ~ 200 nt in length, which were then used as templates for the synthesis of first- and second-strand cDNA. The double-stranded cDNA preparations were subjected to end-repair/dA-tailing and adaptor ligation. The end-modified cDNA fragments were isolated using Agencourt AMPure XP beads (Beckman Coulter, Inc.) and enriched by PCR amplification. Finally, the cDNA libraries for all samples were pooled and sequenced on a single flow cell of an Illumina HiSeq™ 2500 sequencing instrument. The Sequencing depth was 10 Gb per sample.
Processes of reads manipulation
The Illumina adaptors were trimmed from the reads, and low-quality sequence reads were removed from the data sets. Raw sequences were transformed into clean reads after data processing, and the clean reads were then aligned to the reference genome. Only reads with no more than a single mismatch were further analyzed and annotated based on the reference genome of common wheat. The RNA-seq reads were aligned to the reference genome using Tophat2 software.
Differential gene expression analysis
Relative gene expression levels were calculated by the FPKM (fragments per kilobase of transcript per million fragments mapped) method using the following formula:
FPKM = cDNA Fragment/[Mapped Fragments(Millions) × Transcript Length(kb)].
For samples with biological replicates, differential gene expression analysis in the two groups was performed using the DESeq R package (1.10.1) (Hardcastle and Kelly 2010). The resulting p values were adjusted using the Benjamini-Hochberg (BH) procedure to decrease the false discovery rate (FDR) (Genovese et al. 2002). Genes with adjusted p-values < 0.05 calculated by DESeq were considered to be differentially expressed. Differential gene expression analysis was performed using BMKCloud (www.biocloud.net).
Construction and visualization of co-expression network
The DEGs were then used to perform the co-expression network analysis using the R package WGCNA (Langfelder and Horvath 2008). The co-expression network was visualized using the free software Cytoscape (Shannon et al. 2003). The expression data were clustered with Cluster 3.0 (de Hoon et al. 2004) and displayed using Java Treeview (Saldanha 2004).
Gene function annotation
Gene functions were annotated based on searches of the following databases: Nr (NCBI non-redundant protein sequences) (ftp://ftp.ncbi.nih.gov/blast/db), Nt (NCBI non-redundant nucleotide sequences) (https://www.ncbi.nlm.nih.gov/genome/), Pfam (collection of protein families)(http://pfam.xfam.org/), KOG/COG (Clusters of Orthologous Groups of proteins) (http://ftp.ncbi.nih.gov/pub/COG/KOG/kyva); Swiss-Prot, KO (KEGG Ortholog database) (http://www.genome.jp/kaas-bin/kaas_main); and GO (Gene Ontology) (http://systemsbiology.cau.edu.cn/agriGOv2/index.php).
GO enrichment analysis
The differentially expressed genes (DEGs) were subjected to Gene Ontology (GO) enrichment as implemented in the GOseq R package. The analysis is based on Wallenius’ noncentral hypergeometric distribution (Young et al. 2010) to adjust for gene length bias in the DEGs. GO enrichment analysis was performed using BMKCloud (www.biocloud.net).
KEGG pathway enrichment analysis
The Kyoto Encyclopedia of Genes and Genomes (KEGG) (Kanehisa et al. 2008) database is a resource for integrating genomic data with biological and chemical information to interpret large datasets derived from genome sequencing and other high-throughput analytical and ‘omics’ technologies (http://www.genome.jp/kegg/). We used KOBAS (Mao et al. 2005) software to test for statistical enrichment of the DEGs detected in our study in KEGG pathways. KEGG pathway enrichment analysis was performed using BMKCloud (www.biocloud.net).
Validating RNA-seq data using qRT-PCR
We used real-time quantitative RT-PCR (qRT-PCR) to validate the expression of selected DEGs that was calculated from the RNA-seq data. Two reference genes and eight annotated DEGs were selected for qRT-PCR gene expression analysis. The qRT-PCR results were normalized using geometric averaging followed by the comparative Ct method (2−△△Ct) to quantify relative gene expression (Vandesompele et al. 2002). The correlations between expression levels determined from qRT-PCR and RNA-seq data for each pairwise comparison were determined with Minitab 15. The names and sequences of all primers used are listed in Table S5.
Results
Construction of RNA-seq libraries and high-throughput nucleotide sequencing
Total RNA was extracted from wheat plants sampled at 0, 12, 30, 70 and 98 h after inoculation with R. cerealis, and their corresponding controls (0 h, 12 h, 30 h, 70 h, 98 h, 0 h CK, 12 h CK, 30 h CK, 70 h CK, and 98 h CK) were subjected to RNA-seq analysis. High-throughput next-generation Illumina sequencing of the six libraries yielded 33.13–52.41 million (M) 100-bp paired-end reads from each (Table 1). Following stringent quality filtering, 648.99 Gb of clean read data remained, with a Q30 quality score percentage of ≥ 93.42%. The Illumina reads were mapped to the Chinese spring wheat reference genome sequence. The percentages of reads that mapped to the reference genome were similar among the 21 RNA-seq libraries (93.3%-94.23%), and 83.3%-88.8% of the reads aligned perfectly to the reference genome with no mismatched bases (International Wheat Genome Sequencing et al. 2018).
We assembled all of the clean RNA-seq reads into transcripts which were compared to the reference gene models. In total, 130,839 known genes were identified; the number of transcripts between 300 and 1,000 bp was 35,448, and 89,907 were longer than 1,000 bp. We annotated all known and newly-predicted genes using searches against five public protein databases (Nr, Nt, Pfam, KOG/COG and GO), and we identified genes that were differentially expressed in each pair of RNA samples using the criteria given in the Methods section. Genes with FPKM values < 0.3 in the 54 samples were filtered out (Table S1). Finally, 18,433 DEGs were chosen for further analysis.
Expression profiles of the DEGs in response to R. cerealis infection
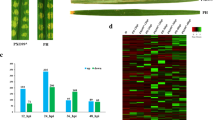
We performed gene expression comparisons between the R. cerealis-infected and control samples in the two cultivars (Table S2). When we compared the 12 h control and inoculated libraries, 68 DEGs were identified in CI12633, with 31 being up-regulated and 37 down-regulated, and in ‘Yangmai15’, only 20 DEGs were identified, of which 14 were up-regulated and 6 down-regulated (Fig. 1). When the 30 h control and inoculated libraries were compared, we found 59 DEGs in CI12633 (37 up-regulated, 22 down-regulated), and 26 DEGs in ‘Yangmai15’, (14 up-regulated, 12 down-regulated). In total, 193 DEGs were identified in the comparison of the 70 h control and inoculated libraries of CI12633, of which 102 were up-regulated and 91 were down-regulated in the inoculated library compared to the control. Also, there were 86 DEGs identified in the 70 h inoculated library compared with the 70 h control in ‘Yangmai15’, of which 75 were up-regulated and 11 were down-regulated in the inoculated compared to the control samples. In the 98 h comparison, the number of DEGs was down, with 130 DEGs identified in CI12633 (71 up-regulated and 59 down-regulated), but only 37 DEGs were identified in ‘Yangmai15’ (21 up-regulated and 16 down-regulated). Of all the pairwise comparisons, we found the largest number of DEGs in the 70 h comparison. These results show that the resistance response was the most intense ~ 3 days after R. cerealis infection. Briefly, we found that differential expression of certain genes was induced earlier in the sharp eyespot resistant cultivar CI12633 (12 h) than in the susceptible cultivar ‘Yangmai15’ (30 h).
Co-expression network analysis identified sharp eyespot resistance-related DEGs
Genes related to sharp eyespot resistance in the non-redundant DEGs were identified in a weighted gene co-expression network analysis (WGCNA). In the WGCNA, all of the genes clustered into five modules (black, blue, green, brown, and turquoise) (Fig. 2a). The black, green, and blue modules showed significant correlations with the resistant cultivar CI12633 inoculated with the sharp eyespot pathogen, while the brown and turquoise modules were correlated with ‘Yangmai15’ (Fig. 2b-f). In the turquoise module, all genes in CI12633 were up-regulated and genes in ‘Yangmai15’ were down-regulated (Fig. 2f). We performed KEGG enrichment analysis to identify the pathways that were enriched in each module that was correlated with the sharp eyespot-infected samples. Two pathways, including phenylpropanoid biosynthesis (ko00940) and plant-pathogen interaction (ko04626), were enriched in all sharp eyespot-infected samples. Therefore, these two pathways probably play critical roles in sharp eyespot resistance in wheat.
Global patterns of differential gene expression in response to Rhizoctonia cerealis infection. a, the DEGs were analyzed using a weighted gene co-expression network analysis (WGCNA). In panels b-f, the upper parts of the figures are heatmaps showing the relative gene expression levels among the modules that were most significantly correlated with the R. cerealis infected samples. The bottom parts of the figures show the classification of sharp eyespot responsive genes in the ‘biological process’ GO category in each module
Gene ontology enrichment analysis of the DEGs
We performed gene ontology (GO) analysis of the DEGs for all three major ontologies (‘biological process’, ‘cellular component’, and ‘molecular function’) (Fig. 3). At 12 h after infection, 68 DEGs were annotated in CI12633 versus 20 DEGs in ‘Yangmai15’, no common DEGs between the two cultivars were identified when the control DEGs were removed. At 30 h after infection, 59 DEGs were annotated in CI12633 versus 26 DEGs in ‘Yangmai15’, and no common DEGs remained between the two cultivars when the control DEGs were removed. At 70 h, 193 DEGs were annotated in CI12633 versus 86 DEGs in ‘Yangmai15’, and one common DEG remained when the control DEGs were removed, which is annotated as a new gene. At 98 h, 130 DEGs were annotated in CI12633 versus 37 in ‘Yangmai15’, and one DEG left after the control DEGs were removed, which was annotated as a chlorophyll A-B binding protein.
Differentially expressed genes (DEGs) in the two wheat cultivars at 12, 30, 70, and 98 h after infection with the sharp eyespot disease pathogen Rhizoctonia cerealis. a, the DEGs at 12 h after R. cerealis infection. G13 is the DEGs in ‘Yangmai15’ (susceptible) and G44 is the DEGs in CI12633 (resistant) at 12 h after R. cerealis infection compared with the corresponding controls. b, the DEGs at 30 h after R. cerealis infection. G21 is ‘Yangmai15’ and G50 is ‘CI12633’ at 30 h after R. cerealis infection compared with the controls;. c, the DEGs at 70 h after R. cerealis infection. G27 is ‘Yangmai15’ and G54 is CI12633 at 70 h after R. cerealis infection compared with the controls; d, the DEGs at 98 h after R. cerealis infection. G31 is ‘Yangmai15’ and G56 is CI12633 at 98 h after R. cerealis infection compared with the controls. The number of genes in the top GO terms in the ‘biological process’, ‘cellular component’, and ‘molecular function’ ontologies are shown on the right
In the annotated DEGs from the CI12633 versus ‘Yangmai15’ comparisons, there were more significantly enriched GO terms in the ‘biological process’ category compared to ‘cellular component’ and ‘molecular function’ (Fig. 4). ‘Defense response’ (GO:0,006,952) was a significantly enriched GO term, which implies that defense genes are associated with sharp eyespot resistance. GO terms related to biotic stress were also significantly enriched; examples are ‘response to hydrogen peroxide catabolic process’ (GO:0,042,744), ‘response to chitin catabolic process’ (GO:0,006,032), and ‘response to abscisic acid’ (GO:0,009,737). Genes associated with the defense response in the term ‘callose deposition in cell wall’ (GO:0,052,544) were also enriched.
Functional annotation of the DEGs identified in wheat plants in response to R. cerealis infection by gene ontology (GO) analysis. a, (top) Venn diagram showing the numbers of DEGs in CI12633 from four time points after R. cerealis infection. Genes that were differentially expressed in both DEG sets are represented by overlapping segments. Treatment vs. control at 12 h (G44), 30 h (G50), 70 h (G54), and 98 h (G56) after infection with R. cerealis. (bottom) KEGG enrichment analysis of the DEGs. Each circle represents a KEGG pathway, the y-axis shows the pathway name, and the x-axis shows the enrichment factor, which compares the ratio of genes annotated to a pathway among the DEGs to the ratio of genes annotated to that pathway among all genes. The larger the enrichment factor, the more significant is the enrichment of DEGs in the pathway. The q values are represented by the color of the circle, and smaller q values indicate more reliable enrichment significances of the DEGs in the pathway; the size of each circle represents the number of genes enriched in the pathways. b, Venn and KEGG analyses of the DEGs in ‘Yangmai 15’. G13, G21, G27, and G31 represent the DEGs at 12, 30, 70, and 98 h after R. cerealis infection compared with the respective uninoculated controls
In CI12633, the same DEG, which was annotated as having pyrophosphatase activity (GeneID: Triticum_aestivum_newGene_94921, the sequence was in Table S6), was detected in both the 12 h and 30 h comparisons (Fig. 4). Another DEG, which encodes a predicted protein (GeneID: TraesCS1A02G015100), was also found in both the 30 h and 70 h comparisons. There were three DEGs (GeneID: TraesCS5A02G139900, predicted protein; GeneID: TraesCS6A02G301800, trehalose biosynthetic process; GeneID: Triticum_aestivum_newGene_106079, predicted protein) were common to the 70 h and 98 h comparisons. There were one and two DEGs shared between the 30 h and 98 h (GeneID: TraesCS2A02G110300, cellular component) and 12 h and 98 h (GeneID: TraesCS4A02G234100 and TraesCS4D02G079900, dioxygenase activity) comparisons, respectively. KEGG pathway analysis showed that “steroid biosynthesis”, “glyoxylate and dicarboxylate metabolism”, and “plant-pathogen interaction” were significantly enriched in wheat plants infected with the sharp eyespot pathogen.
Gene expression patterns and functional analysis of DEGs in the resistant cultivar CI12633
The unigenes were functionally annotated by BLAST searches against the GO, COG, KOG, KEGG, and eggnog databases. Based on their expression profiles, co-expression clustering grouped 299 DEGs from CI12633 into nine clusters (Fig. 5). Forty-three genes were classified into cluster1 (K1), and expression showed gradual up-regulation from 12 to 98 h, indicating that the expression of these genes responded to R. cerealis infection. Most of them participate in pathways such as “phenylpropanoid biosynthesis”, “glutathione metabolism”, and “glycerophospholipid metabolism” (Fig. S1). Compared with K1, the genes in K2 also showed gradual up-regulation, but expression was down-regulated at 70 h and then returned to normal levels. Genes belonging to cluster 6 (K6) were persistently up-regulated, and transcription was stimulated by sharp eyespot disease. Compared with K6, genes in K8 was also showed up-regulated expression, although at a relatively higher level, in response to R. cerealis infection. Most of the genes were enriched in “glutathione metabolism”, “terpenoid backbone biosynthesis”, and “thiamine metabolism”. Genes in cluster 5 were also up-regulated and reached their peak expression level, but transcription then declined at 70 h. Cluster 9 (K9) contained more genes (128); the expression levels of these genes were relatively unchanged by R. cerealis infection, and most were enriched in “endocytosis”, “glycerophospholipid metabolism”, and “alpha-linolenic acid metabolism”. Expression of genes in cluster 7 was repressed at 12 h, but mRNA levels recovered after 30 h.
Expression profiles of the DEGs shown in the sharp eyespot-resistant wheat cultivar CI12633 by co-expression clustering Nine major clusters (K1-K9) were identified based on DEG expression over the course of the experiment. The time course of R. cerealis infection (0, 12, 30, 70, and 98 h) is shown on the x-axis, and the relative gene expression levels are shown on the y-axis
DEGs that play roles in phytohormone signaling
We analyzed hormone signal transduction pathways in an attempt to identify DEGs associated with hormonal responses in wheat plants infected with sharp eyespot disease (Fig. S2). One gene known to be involved in tryptophan metabolism that encodes an ARF (auxin response factor) was significantly up-regulated in CI12633 at 30 h compared with the control. One DEG involved in zeatin biosynthesis was down-regulated in CI12633 at 70 h. However, there were no DEGs in the gibberellin, brassinosteroid, and abscisic acid pathways. In the susceptible cultivar ‘Yangmai15’, only one DEG belonging to the CTR1 (serine/threonine-protein kinase) group was up-regulated at 70 h. One DEG encoding COI1 (coronatine-insensitive protein 1) was significantly up-regulated in CI12633 at 98 h. In salicylic acid signaling, five DEGs belonging to the PR-1 (pathogenesis-related protein 1) family were up-regulated in ‘Yangmai15’ at 70 h, and one DEG was up-regulated in CI12633 at 98 h. In brief, the resistant cultivar CI12633 had more types of phytohormone-related DEGs than did the susceptible cultivar ‘Yangmai15’, and the response time for expression of the genes was initiated earlier than in ‘Yangmai15’.
DEGs involved in plant-fungal interactions
We also analyzed DEGs in the plant-pathogen interaction pathways, especially the pathway related to fungi (Fig. S3). We found that nine DEGs encoding PR1 (pathogenesis-related protein 1) proteins were significantly up-regulated in ‘Yangmai15’ at 70 h, resulting in defense-related gene induction. Furthermore, one DEG encoding MEKK1 (mitogen-activated protein kinase 1) was down-regulated during R. cerealis infection in CI12633 at 70 h. However, there was one down-regulated gene in ‘Yangmai15’ at 70 h that encodes calmodulin and induces the hypersensitive response (HR) and cell wall reinforcement. One disease resistance protein1 (RPM1) DEG was strongly down-regulated in CI12633 at 98 h. In the susceptible cultivar ‘Yangmai15’, most plant-pathogen interaction genes were expressed at 70 h at higher levels than in CI12633, indicating that the resistance of CI12633 to R. cerealis may come from other pathways.
Transcription factors related to host responses to R. cerealis infection
Genome-scale screening and molecular genetic studies, mostly in Arabidopsis (Arabidopsis thaliana), have identified a group of genes encoding various transcription factors (TFs) that are regulated by biotic and abiotic stresses (Vidhyasekaran 2016). In this study, we identified 13,827 TFs belonging to 20 families in the DEGs (Fig. S4). The AP2/ERF-ERF family was the predominant class of TFs. In wheat infected by R. cerealis compared to the same time control, 34 TF or PK (protein kinase) DEGs were found between the two cultivars (Table S3). Interestingly, more DEGs were up-regulated in the resistant cultivar CI12633 (8) than in susceptible ‘Yangmai15’ (26). Also, more DEGs showed increases in the rate of expression after 70 h in CI12633 when infected by R. cerealis.
DEGs present on chromosome 2B
Based on our previous study (Wu et al. 2017), we searched the genome sequence (available online: http://plants.ensembl.org/Triticum_aestivum/Info/Index) to find the distances between the SNP marker loci RAC875_c730_234 and RAC875_c16697_1502 on wheat chromosome 2B. We identified a chromosomal region of 344,346,772 bp, within which the resistance QTL QSe.jaas-2BS is located. In this interval, we identified eight DEGs, seven of which were previously annotated and one of which was annotated in this study as a new gene (Table S4). Using BLAST searches, we identified DEGs that are highly homologous to known genes for glycogen phosphorylase (gene ID: TraesCS3D02G359200), a zinc finger SWIM domain-containing protein (gene ID: Triticum_aestivum_newGene_81221), malate synthase (gene ID: TraesCS2B02G364000), and isocitrate lyase (gene ID: TraesCS2B02G244600). Four of the DEGs showed no homology to any previously-identified wheat genes (gene IDs: TraesCS2B02G298300, TraesCS2B02G040500, TraesCS2B02G232800 and TraesCS2B02G217900).
Validation of the expression differences in the DEGs
In order to validate the expression profiles using Illumina sequencing, the expression levels of genes closely associated with the sharp eyespot disease response, including TraesCS4D02G010100, Triticum_aestivum_newGene_86977, Triticum_aestivum_newGene_34844, TraesCS7B02G483400, TraesCS1A02G025500, TraesCS1B02G148000, TraesCS2B02G119100, TraesCS2D02G101400, TraesCS4B02G216700, TraesCS2B02G298300, TraesCS2B02G040500, and TraesCS2B02G364000, were further analyzed by qRT-PCR. The results showed that the trends in the relative changes in expression of these selected genes based on qRT-PCR were similar to those determined using Illumina sequencing data (Fig. 6). However, the fold-changes in the expression levels of the genes that respond to R. cerealis infection detected by qRT-PCR had some difference with those detected by Illumina sequencing.
Comparison of changes in the relative expression levels in 12 selected DEGs in the two wheat cultivars CI12633 and’Yangmai15’ as determined by RNA-seq and qRT-PCR. The left vertical axis shows the FPKM values determined from the RNA-seq data (blue line), and right vertical axis shows the relative expression level as determined by qRT-PCR (orange line)
Discussion
Plant hormone signal transduction related to sharp eyespot disease resistance
Phytohormones such as gibberellic acid (GA), auxin (IAA), cytokinin (CK), brassinosteroids (BR), abscisic acid (ABA), ethylene (ET), jasmonic acid (JA), and salicylic acid (SA) are small dual-function signaling molecules that act not only on endogenous developmental processes, but also transduce environmental stimuli to multiple hormone-responsive genetic pathways that allow plants to adapt to adverse environmental conditions (Ha et al. 2014). Nakasita et al. showed that BR can enhance resistance to a broad range of diseases in tobacco and also induce resistance in rice to rice blast and bacterial blight diseases caused by Magnaporthe grisea and Xanthomonas oryzae pv. oryzae, respectively (Nakashita et al. 2003). Similar results occurred in our study. Ten DEGs enriched in plant hormone signal transduction, six of which belonging to the salicylic acid signaling pathway were up-regulated, and all of them encode members of the “cysteine-rich secretory protein family”. Salicylic acid is an endogenous growth regulator that is important in plant defense. The role of SA in plant innate immunity is well documented in dicotyledonous plants, where it is required for the establishment of basal pathogen resistance and also for the induction of systemic acquired resistance (SAR), a physiological response conferring resistance to many diverse pathogens. Sorahinobar et al. found that SA applied as a soil drench induces antioxidant defense responses in plants and may also induce SAR, which then contributes to F. graminearum resistance (Sorahinobar et al. 2015). Therefore, we hypothesized that SA could play a significant role in the wheat response to sharp eyespot disease. We also identified genes involved in IAA, CK, ET, and JA regulation. Four DEGs identified in this study are predicted to encode four key proteins, which are the “threonine-protein kinase CTR1”, “plant hormone signal transduction”, “two-component response regulator” and “berberine bridge enzyme-like” proteins. This indicates that these phytohormones potentially play an important role in sharp eyespot resistance in wheat.
The phenylpropanoid biosynthesis pathway
Phenylpropanoids have a range of important functions in plants, including their roles as structural components, protectants against biotic and abiotic stresses. Lignin, an important phenylpropanoid compound, is mainly deposited in plant secondary cell walls, and may provide strength to prevent direct penetration by fungal pathogens. A group of phenylpropanoid pathway genes have been shown to be highly expressed in response to biotic and abiotic stresses, which results in increases in the accumulation of pathway enzymes as well as the corresponding enzymatic activities (Bhuiyan et al. 2007; Zhao et al. 2009). The disruption of flux through the phenylpropanoid pathway could explain some of the resistance mechanisms, because a range of antimicrobial compounds such as phenolic phytoalexins, stilbenes, and coumarins are synthesized by this pathway (Yu et al. 2000). In wheat, the TaCOMT-3D gene contributes to both the disease resistance response and stem strength in adult plants infected with R. cerealis, possibly by increasing the synthesis and accumulation of lignin (especially syringyl monolignol) (Wang et al. 2018). The phenylpropanoid pathway is a major focus of our study because of its role in the synthesis of defense-related compounds as well as its involvement in countering the actions of pathogens to weaken the cell wall. In this study, the four genes TraesCS5D02G488800, TraesCS4A02G430100, TraesCS3A02G510900, and TraesCS3D02G080700 encoding enzymes in the phenylpropanoid biosynthesis pathway were all up-regulated. The above results show that the phenylpropanoid biosynthesis pathway is probably an essential player in the response of wheat plants to sharp eyespot disease.
Transcription factor and protein kinase genes induced in wheat plants infected with R. cerealis
Transcription factor proteins play vital roles in plant responses to biotic and abiotic stresses (Pandey and Somssich 2009). Members of several transcription factor gene families, including WRKY, ERF, MYB, bHLH, bZIP, and NAC, have been reported to play crucial roles in plant defense and stress responses. In the present study, we identified 34 TF genes that were differentially expressed in wheat plants in response to infection by the sharp eyespot pathogen. Among these TFs, the largest family is NAC, indicating that NAC family plays critical role in the response of wheat plants to sharp eyespot disease. NAC TFs belong to one of the largest families of plant transcriptional regulators, and it has been suggested that NAC family members play important roles in regulating the transcriptional reprogramming that is associated with plant stress responses (Christiansen and Gregersen 2014). In our study, four NAC TF genes (TraesCS2B02G119000, TraesCS2B02G119100, TraesCS2D02G101400, and TraesCS7A02G305200) showed significant up-regulation after pathogen infection, indicating that these genes may play key roles in determining sharp eyespot resistance in wheat.
MYB TFs have been characterized with a MYB DNA-binding domain. The N-terminal MYB domain consists of approximately 52 amino acid residues that form a helix-turn-helix (HTH) structure that intercalates into the major groove of double-stranded DNA. A previous study identified and functionally characterized a MYB TF gene, TaRIM1, which is up-regulated after R. cerealis infection (Shan et al. 2016). In this study, on the contrary, the expression of TraesCS5B02G278000, a MYB-related gene, was down-regulated in the resistant wheat cultivar CI12633.
Protein kinases (PKs) are enzymes that catalyze the transfer of phosphate groups to other proteins, and phosphorylation then functions to regulate protein activities (Dardick et al. 2007). PKs are one of the largest protein families in plants, and they play critical roles in many aspects of plant growth and development, including developmental patterning, signal transduction, defense responses, and disease resistance (Shiu and Bleecker 2001). The largest class of PKs is the RLK/Pelle family, and within this family the largest subclass is the leucine-rich repeat receptor-like kinases (LRR-RLKs), which are likely to be ancestral to the RLK/Pelle family in plants (Gish and Clark 2011). As can be expected from their large numbers, LRR-RLKs participate in a range of diverse physiological functions that include pathogen recognition, disease resistance, and epidermal patterning (Gomez-Gomez and Boller 2000). In this study, we detected 15 RLK/Pelle-related DEGs, and most of these genes showed up-regulated expression. The high transcript levels of these genes may be key factors in sharp eyespot resistance.
Potential solutions for sharp eyespot resistance breeding
In this research, a number of genes and major metabolic pathways were identified to associate with R. cerealis-induced resistance, which is helpful for the understanding of the genetic basis of sharp eyespot resistance in wheat. For example, DEGs related to SA pathway, phenylpropanoid biosynthesis pathway, and NAC TSs should be paid more attention to, all of which were related to basal pathogen resistance, systemic acquired resistance or plant stress responses. In previous study, we identified 5 QTLs resistant to sharp eyespot based on a RIL population from the cross between resistant cultivar CI12633 and susceptible cultivar Yangmai 9 (Wu et al. 2017). In our next research, we will focus on the functional analysis of DEGs associated with these QTL sites, especially the major QTL QSe.jaas-2BS. Overexpression of some pathogenesis-related protein deriving genes or blocking of the pathways associated with disease infection by gene edition are both potential schemes to improve the resistance to wheat sharp eyespot.
Conclusions
The results of our study showed that next-generation RNA-seq technology is an effective way to identify genes that express in response to R. cerealis infection in wheat. We identified a number of genes and major metabolic pathways that are associated with resistance to R. cerealis infection and disease development, which will help to understand the genetic basis of sharp eyespot resistance, and provided potential molecular solutions in sharp eyespot resistance breeding.
Data availability statement
The data presented in this study are available on request from the corresponding author.
Abbreviations
- DEGs:
-
Differentially expressed genes
- COMT:
-
Caffeic acid 3-O-methyltransferase
- RNA-seq:
-
RNA sequencing
- TDFs:
-
Transcript-derived fragments
- qRT-PCR:
-
Quantitative real-time PCR
- WGCNA:
-
Weighted gene co-expression network analysis
- GO:
-
Gene ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- TF:
-
Transcription factor
References
Bhuiyan NH, Liu W, Liu G, Selvaraj G, Wei Y, King J (2007) Transcriptional regulation of genes involved in the pathways of biosynthesis and supply of methyl units in response to powdery mildew attack and abiotic stresses in wheat. Plant Mol Biol 64(3):305–318
Chen J, Li GH, Du ZY, Quan W, Zhang HY, Che MZ, Wang Z, Zhang ZJ (2013) Mapping of QTL conferring resistance to sharp eyespot (Rhizoctonia cerealis) in bread wheat at the adult plant growth stage. Theor Appl Genet 126(11):2865–2878
Chepelev I, Wei G, Tang Q, Zhao K (2009) Detection of single nucleotide variations in expressed exons of the human genome using RNA-Seq. Nucleic Acids Res 37(16):e106
Christiansen MW, Gregersen PL (2014) Members of the barley NAC transcription factor gene family show differential co-regulation with senescence-associated genes during senescence of flag leaves. J Exp Bot 65(14):4009–4022
Cui L, Yang G, Yan J, Pan Y, Nie X (2019) Genome-wide identification, expression profiles and regulatory network of MAPK cascade gene family in barley. BMC Genomics 20(1):750
Dardick C, Chen J, Richter T, Ouyang S, Ronald P (2007) The rice kinase database. A phylogenomic database for the rice kinome. Plant Physiol 143(2):579–586
de Hoon MJ, Imoto S, Nolan J, Miyano S (2004) Open source clustering software. Bioinformatics 20:1453–1454
Genovese CR, Lazar NA, Nichols T (2002) Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15(4):870–878
Gomez-Gomez L, Boller T (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5(6):1003–1011
Gish LA, Clark SE (2011) The RLK/Pelle family of kinases. Plant J 66(1):117–127
Ha CV, Leyva-Gonzalez MA, Osakabe Y, Tran UT, Nishiyama R, Watanabe Y, Tanaka M, Seki M, Yamaguchi S, Dong NV (2014) Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc Natl Acad Sci U S A 111(2):851–856
Hardcastle TJ, Kelly KA (2010) baySeq: empirical Bayesian methods for identifying differential expression in sequence count data. BMC Bioinformatics 11:422
International Wheat Genome Sequencing C, investigators IRp, Appels R, et al (2018) Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 361:1–13. https://doi.org/10.1126/science.aar7191
Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T et al (2008) KEGG for linking genomes to life and the environment. Nucleic Acids Res 36:D480-484
Langfelder P, Horvath S (2008) WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9:559. https://doi.org/10.1186/1471-2105-9-559
Lemańczyk G, Kwaśna H (2013) Effects of sharp eyespot (Rhizoctonia cerealis) on yield and grain quality of winter wheat. Eur J Plant Pathol 135:187–2013
Lu L, Rong W, Massart S, Zhang Z (2018) Genome-Wide Identification and Expression Analysis of Cutinase Gene Family in Rhizoctonia cerealis and Functional Study of an Active Cutinase RcCUT1 in the Fungal-Wheat Interaction. Front Microbiol 9:1813
Mao X, Cai T, Olyarchuk JG, Wei L (2005) Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 21(19):3787–3793
Nakashita H, Yasuda M, Nitta T, Asami T, Fujioka S, Arai Y, Sekimata K, Takatsuto S, Yamaguchi I, Yoshida S (2003) Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J 33(5):887–898
O’Neil ST, Emrich SJ (2013) Assessing De Novo transcriptome assembly metrics for consistency and utility. BMC Genomics 14:465
Pandey SP, Somssich IE (2009) The role of WRKY transcription factors in plant immunity. Plant Physiol 150(4):1648–1655
Patterson J, Carpenter EJ, Zhu Z, An D, Liang X, Geng C, Drmanac R, Wong GK (2019) Impact of sequencing depth and technology on de novo RNA-Seq assembly. BMC Genomics 20(1):604
Que Y, Su Y, Guo J, Wu Q, Xu L (2014) A global view of transcriptome dynamics during Sporisorium scitamineum challenge in sugarcane by RNA-Seq. PLoS ONE 9(8):e106476
Rong W, Luo M, Shan T, Wei X, Du L, Xu H, Zhang Z (2016) A Wheat Cinnamyl Alcohol Dehydrogenase TaCAD12 Contributes to Host Resistance to the Sharp Eyespot Disease. Front Plant Sci 7:1723
Saldanha AJ (2004) Java Treeview–extensible visualization of microarray data. Bioinformatics 20:3246–3248
Shan T, Rong W, Xu H, Du L, Liu X, Zhang Z (2016) The wheat R2R3-MYB transcription factor TaRIM1 participates in resistance response against the pathogen Rhizoctonia cerealis infection through regulating defense genes. Sci Rep 6:28777
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res 13(11):2498–2504
Shiu SH, Bleecker AB (2001) Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci U S A 98(19):10763–10768
Sorahinobar M, Niknam V, Ebrahimzadeh H, Soltanloo H, Behmanesh M, Enferadi ST (2015) Central Role of Salicylic Acid in Resistance of Wheat Against Fusarium graminearum. J Plant Growth Regul 12(6):1–15
Su Q, Wang K, Zhang Z (2020) Ecotopic Expression of the Antimicrobial Peptide DmAMP1W Improves Resistance of Transgenic Wheat to Two Diseases: Sharp Eyespot and Common Root Rot. Int J Mol Sci 21(2)
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3(7)
Vidhyasekaran P (2016) Molecular Manipulation of Transcription Factors, the Master Regulators of PAMP-Triggered Signaling Systems. Springer, Berlin/Heidelberg, Germany
Wang M, Zhu X, Wang K, Lu C, Luo M, Shan T, Zhang Z (2018) A wheat caffeic acid 3-O-methyltransferase TaCOMT-3D positively contributes to both resistance to sharp eyespot disease and stem mechanical strength. Sci Rep 8(1):6543
Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10(1):57–63
Wei X, Shen F, Hong Y, Rong W, Du L, Liu X, Xu H, Ma L, Zhang Z (2016) The wheat calcium-dependent protein kinase TaCPK7-D positively regulates host resistance to sharp eyespot disease. Mol Plant Pathol 17(8):1252–1264
Wu X, Cheng K, Zhao R, Zang S, Bie T, Jiang Z, Wu R, Gao D, Zhang B (2017) Quantitative trait loci responsible for sharp eyespot resistance in common wheat CI12633. Sci Rep 7(1):11799
Ying L, Qiao-Feng Z, Bi-Sheng F, Shi-Bin C, Yan-Jie J, Zhi-Liang Z, Yuan-Yu D, Ji-Zhong W, Ting-Bo D (2015) Genetic Diversity of Wheat Germplasm Resistant to Sharp Eyespot and Genotyping of Resistance Loci Using SSR Markers. Acta Agron Sin 41(11):1671
Young MD, Wakefield MJ, Smyth GK, Oshlack A (2010) Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol 11(2):R14. https://doi.org/10.1186/gb-2010-11-2-r14
Yu O, Jung W, Shi J, Croes RA, Fader GM, McGonigle B, Odell JT (2000) Production of the isoflavones genistein and daidzein in non-legume dicot and monocot tissues. Plant Physiol 124(2):781–794
Zhao J, Buchwaldt L, Rimmer SR, Sharpe A, McGregor L, Bekkaoui D, Hegedus D (2009) Patterns of differential gene expression in Brassica napus cultivars infected with Sclerotinia sclerotiorum. Mol Plant Pathol 10(5):635–649
Zhu X, Lu C, Du L, Ye X, Liu X, Coules A, Zhang Z (2017) The wheat NB-LRR gene TaRCR1 is required for host defence response to the necrotrophic fungal pathogen Rhizoctonia cerealis. Plant Biotechnol J 15(6):674–687
Zhu X, Qi L, Liu X, Cai S, Xu H, Huang R, Li J, Wei X, Zhang Z (2014) The wheat ethylene response factor transcription factor pathogen-induced ERF1 mediates host responses to both the necrotrophic pathogen Rhizoctonia cerealis and freezing stresses. Plant Physiol 164(3):1499–1514
Zhu X, Wang Y, Su Z, Lv L, Zhang Z (2018) Silencing of the Wheat Protein Phosphatase 2A Catalytic Subunit TaPP2Ac Enhances Host Resistance to the Necrotrophic Pathogen Rhizoctonia cerealis. Front Plant Sci 9:1437
Zhu X, Yang K, Wei X, Zhang Q, Rong W, Du L, Ye X, Qi L, Zhang Z (2015) The wheat AGC kinase TaAGC1 is a positive contributor to host resistance to the necrotrophic pathogen Rhizoctonia cerealis. J Exp Bot 66(21):6591–6603
Funding
This work was supported by Jiangsu Agriculture Science and Technology Innovation fund (CX(20)3009) and Natural Science Foundation of Jiangsu Province (BK20211117), National Key Research and Development Program of China (2016YFC0101600), and National Key Research and Development Program of China, the China Agricultural Research System (CARS-03-03B). The funders had no role in study design, data collection and analysis, data interpretation, or in writing of the manuscript.
Author information
Authors and Affiliations
Contributions
WXJ and WJC designed the study; LL, ZX and ZBQ performed the experiments; JW and LM provided additional resources and suggestions; GDR and LCB provided guidance throughout the entire study; WXJ wrote and revised the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, X., Wang, J., Li, L. et al. Analysis of gene expression changes in wheat in response to Rhizoctonia cerealis infection using RNA-Seq. J Plant Pathol 104, 1383–1396 (2022). https://doi.org/10.1007/s42161-022-01170-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-022-01170-7