Abstract
The molecular characterization of Boeremia strasseri (Moesz) Gruyter et Verkley (basionym Phoma strasseri Moesz) isolates from peppermint crops grown in south-eastern and central Poland was studied using the Random Amplified Polymorphic DNA (RAPD) -PCR technique. Tests were performed using randomly selected primers. DNA profiles obtained using five primers proved to be useful in determining the genetic variability among B. strasseri genotypes. Molecular analysis of four loci: (i) rDNA internal transcribed spacer region (ITS1, 5.8S, ITS2); (ii) LSU (partial large subunit DNA 28S); (iii) tub 2 (gene region of β-tubulin); (iv) act (gene region of gamma-actin) proved that the actin gene is the most suitable DNA barcode for the accurate and rapid identification B. strasseri species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Phoma Sacc. emend. Boerema et G.J. Bollen (Pleosporales) is a cosmopolitan taxon of coelomycetous fungi containing numerous plant pathogenic, saprobic and endophytic species associated with a wide range of hosts (Aveskamp et al. 2008). This phytopathologically significant fungal genus has always been considered one of the most difficult to identify (Aveskamp et al. 2008; Zhang et al. 2009). Initially, it comprised more than 3000 species (Monte et al. 1991). Such a large number of taxa described within Phoma was related to the use of nomenclature mainly based on the characteristics of the host plant and marginalization of micro-morphological properties (Aveskamp et al. 2008). Intensive work carried out by Dutch mycologists, who had studied the morphology of these fungi in artificial cultures since 1992 led to their division into nine sections (Boerema 1997). The results of 40 years of taxonomic research based on the properties of Phoma species were presented in an article on their identification, and, consequently, the number of species was reduced to 223 (Boerema 1997; Boerema et al. 2004). However, the developed taxonomic system seemed a little artificial to the above authors, thus they began molecular DNA analyses of the species within individual sections. These studies revealed the phylogenetic heterogeneity of the species in the Phoma sections, disproved the existing division of the genus into sections and caused the necessity to re-classify (de Gruyter et al. 2009, 2010, 2012; Aveskamp et al. 2010). The genus Phoma was divided into clades, raised to the rank of genera, that comprised species with similar levels of relationships (Aveskamp et al. 2010).
Boeremia strasseri (Moesz) Gruyter et Verkley (basionym Phoma strasseri Moesz), family Didymellaceae (Chen et al. 2015), has been reported as the causal agent of black stems and rhizome rot of peppermint (Mentha piperita L.) in the USA (Horner 1971), Japan (de Gruyter et al. 2002), India (Kalra et al. 2004), Hungary (Paizs and Naggy 1975), and is present in Poland since 2004 (Zimowska and Machowicz-Stefaniak 2005; Zimowska 2007). The disease causes yield losses up to 90% due to the fast degradation of stem and rhizome tissues. A very high activity of β-glucosidase allows B. strasseri to penetrate the host cell wall and tissues, followed by the action of other pectinolityc enzymes that break down pectin to low molecular weight compounds, which are the primary source of carbon for the pathogen (Zimowska 2012; Zimowska and Targoński 2015).
In this study we present the molecular characterization of B. strasseri isolates obtained from peppermint cultivated in various regions of Poland. We also attempted to evaluate the taxonomy of two isolates recovered from Melissa officianatis (lemon balm) and Leonurus. cardiaca (motherwort) using barcoding sequences. Many phylogenetic studies were based on internally transcribed spacers (ITS). However, in the case of Phoma sensu lato, ITS sequence analyses did not show significant nucleotide differences to infer relationships (Balmas et al. 2005). Considering the above, we have addressed other gene loci, i.e. LSU, β-tubulin and actin in data sets to enhance the phylogenetic analysis.
Materials and methods
Fungal isolates
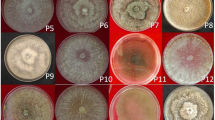
The material used for the study consisted of 22 isolates of B. strasseri, obtained from symptomatic peppermint (M. piperita) stems and rhizomes from earlier research on the health of peppermint in the south-eastern and central regions of Poland, and two Boeremia-like isolates collected from different hosts (Zimowska 2007, 2008). For comparative purposes, the reference isolate CBS 261.92 was retrieved from the culture collection of the CBS KNAW Fungal Biodiversity Centre, Utrecht (The Netherlands). Origin of the isolates is shown in Table 1. The Boeremia isolates have previously been used to study their morphological features following the methods described by Boerema et al. (2004). Some of these isolates were also examined for pathogenicity to peppermint stems and rhizomes (Zimowska and Machowicz-Stefaniak 2005; Zimowska 2012). Single spore cultures of these isolates were grown on 2% malt extract agar plates (MEA; Difco Laboratories, USA). at 22 °C in the dark for two weeks. Four replicates of each isolate were performed.
For phylogenetic analysis, sequences of 20 additional isolates of Boeremia spp. were retrieved from GenBank.
DNA extraction and analysis
Genomic DNA was extracted from fungal mycelium using the Invisorb Spin Plant Midi Kit (Stratec Molecular, Germany), according to the manufacturer’s protocol. DNA concentration was first estimated on 1.5% agarose gel and compared with GeneRuler™ DNA Ladder Plus (Thermo Scientific, USA) then measured using a UV-Vis NanoDrop 2000c/2000 spectrophotometer (Thermo Scientific). DNA samples were diluted to a concentration of 20 ng/μl and stored at 20 °C for future use.
Estimation of Boeremia strasseri genetic variability
Genetic variability of the studied isolates was investigated using randomly selected Random Amplified Polymorphic DNA (RAPD) primers (Operon Technologies, USA). A set of 30 primers were tested in five B. strasseri isolates, and five primers generated reproducible and detectable amplification products and were selected for further study (Table 2).
RAPD-PCR analyses were performed according to Williams et al. (1990) with some modification. The reaction volume was 20 μl and the reaction solution contained 1 x Taq buffer [750 mM Tris HCl, pH 8.8, 200 mM (NH4)2SO4, 0.1% Tween 20] (Thermo Scientific), 2.5 mM x MgCl2, 0.1 mM dNTP, 0.1 U polymerase Taq (Thermo Scientific), 0.2 μM primer, 20 ng/μl of genomic DNA and distilled water. The control reaction was run with water instead of DNA template. Amplification was performed using a DNA Engine Dyad Thermal Cycler (Biorad, USA), using the following program: initial denaturation for 3 min at 95 °C followed by 45 cycles consisting of 45 s of denaturation at 94 °C, 45 s of annealing at 37 °C, 45 s extension at 72 °C and a final extension at 72 °C for 10 min. Amplification products were separated by electrophoresis using a 1.5% agarose gel at 80 V for 1.5 h in 1 x TBE buffer containing 0.01% ethidium bromide. The products obtained were visualized under UV light, photographed and analyzed using BioGene (UK) software.
RAPD products were scored on photographs as present (1) or absent (0). Only bright and reproducible products were scored. Genetic pairwise similarities (SI-similarity index) between genotypes were evaluated according to Dice’s formula after Nei and Li (1979). Cluster analysis was conducted using the Unweighted Pair-Group Method with Arithmetic Mean (UPGMA) distance method implemented in the PAST program (Hammer et al. 2001). Clustering was verified by bootstrapping. Principal component analysis (PCA) was performed using PAST software (Hammer et al. 2001).
Sequence analysis of ITS region, LSU, β-tubulin, actin
Phylogenetic analyses were based on differences in nucleotide sequences of PCR-amplified fragments of the rDNA ITS region (ITS1, 5.8 rDNA gene, ITS2) and three other loci: LSU, β-tubulin (tub2), and actin (act). Two universal primers were used for each reaction: ITS1 and ITS4, the LSU region with LR0R and LR5, the tub2 region with Bt2a and Bt2b primers, and the act region with ACT-512F and ACT-783R, respectively (Table 3).
PCR reaction was performed in a total volume of 25 μl according to Sambrook and Russel (2001). PCR products with fluorescent dye addition were separated electrophoretically in a 1.5% agarose gel at 80 V for 1.5 h in 1X TBE buffer containing 0.01% ethidium bromide and visualized under UV light. After checking and determining the size of the resulting PCR product, it was subjected to purification using a low melting point temperature agarose gel. Sequencing was conducted by the Genomed S.A. (Poland) using PCR primers and a Big Dye® Terminator Cycle Sequencing Kit V. 3.1 (Applied Biosystems, Life Technologies, USA) and separated on a 3730XL DNA Analyzer capillary sequencer. Sequences from each primer combination were used to obtain consensus sequences with BioEdit v. 7.2.5 (Hall 1999). Nucleotide sequences were analysed with sequences retrieved form GenBank using BLAST software (http://www.ncbi.nlm.nih.gov/BLAST/) (Altschul et al. 1997). The sequence of native isolates were compared with reference strains of other Boeremia spp. downloaded from GenBank (https://www.ncbi.nlm.nih.gov/) (Table 4). Phylogenetic analysis was carried out with the “one clik” tool using the Phylogeny.fr program (http://www.phylogeny.fr). Sequences were aligned to each other using the MUSCLE (v 3.7), program and the sequence regions containing gaps or mismatched ends with the Gblocks (v 0.91b) program. A phylogenetic tree was constructed by using the PhyloML bootstrap (v 3.0) with an HKY85 algorithm of the maximum likelihood of substitution model and presented grafically using TreeDyn (v 198.3) gamma shape parameter 88.402.
Results
Of the 30 RAPD primers only five, i.e. OPA-13, OPL-07, OPR-15, OPU-05 and OPV-10, were useful for genetic similarity determination among the studied isolates of B. strasseri.
RAPD analysis amplified 58 fragments based on five selected primers, 43 of which (74.14%) were polymorphic, while seven fragments were monomorphic. The number of amplified DNA fragments per primer ranged from nine (OPU-05) to 14 (OPR-15 and OPL-07) with sizes ranging between 324 and 2300 bp (Table 2). Of the 58 amplification products, eight were specific for single isolates. The highest number of specific products was identified in isolates Lc722 and M1532 (three specific products each) recovered form L. cardiaca and M. officinalis, respectively.
Genetic similarity matrices were produced based on RAPD markers using the Sørensen-Dice coefficient. The genetic similarity between isolates ranged from 0.370 to 1.00. Isolates F 126 and F 1023, F 126 and ML 125, F 289 and ML 134, F 1023 and Ml 125, F 1023 and ML 175, Ml 125 and ML 175 as well as a group of three isolates (F 365, F 742 and F 743) showed 100% similarity. The average similarity of the analyzed isolates was 0.820. Isolates collected from L. cardiaca and M. officinalis showed the lowest similarity to other isolates, with values of 0.459 and 0.508, respectively. Cluster analysis using the UPGMA method divided isolates into two main groups A and B (Fig. 1). Group A comprised isolates from peppermint that were distributed into three subgroups, regardless of the plant organ and place of origin. Two isolates collected from lemon balm and motherwort were grouped in cluster B.
The relationships between the 25 analyzed native isolates were determined based on principal component analysis (PCA) (Fig. 2), which gave results comparable to those obtained by UPGMA clustering. Boeremia spp. isolates formed two distinct groups, that corresponded to UPGMA clusters A and B. For the RAPD data, the first three principal components explained 70.5% of the total variation, with PC-1, PC-2 and PC-3 accounting for 40.8%, 16.4% and 13.3% of the total variation, respectively.
Sequence similarity to the reference B. strasseri isolate CBS 261.92 was 99% for the ITS region, 99% for the LSU region. M 1532 isolate showed 98% similarity to the tub2 region, Lc 722 isolate 97%, while the similarity for the act region was 96%.
The phylogenetic tree, based on the ITS sequences of 24 tested isolates and other selected Boeremia reference strains showed the clustering of all isolates into two main clades. The first clad was divided into two sub-clades. The first one included 22 native isolates of B. strasseri, and reference strains of B. strasseri, two reference strains of Boeremia diversispora (Bubák) Aveskamp, Gruyter et Verkley and Boeremia crinicola (Siemaszko) Aveskamp, Gruyter et Verkley, respectively, while the reference strain of B. divertispora was in a separate branch. The second clad included one reference strain of Boeremia exigua (Desm.) Aveskamp, Gruyter et Verkley, Boeremia foveata (Foister) Aveskamp, Gruyter et Verkley and the native isolates M 1532 and Lc 722 (Fig. 3a). As a result of the phylogenetic analysis on the LSU region two clades were obtained, one of which grouped only native isolates, while the other included reference strains of Boeremia spp. (Fig. 3b).
The phylogenetic tree constructed with tub2 sequences of 24 native isolates and reference strains of Boeremia spp. grouped these isolates into two main clades, the first of which was divided into five sub-clades, characterized by smaller or larger sequences variability. The one of them included two reference strains of B. crinicola, while the second grouped 15 native isolates and two reference strains of B. strasseri. The second clad was divided on tree sub-clades. The first one grouped in the individual tree reference strains of B. diversispora, second included tree reference strains of B. foveata, while the third included two native isolates Lc 722 and M 1532 and reference B. exigua strains (Fig. 3c).
The phylogenetic analysis of the act region produced four clades. The first one included 22 native isolates and two reference strains of B. strasseri, the second grouped two native isolates Lc 722 and M 1532 with Boeremia exigua var. exigua (Desm.) Aveskamp, Gruyter et Verkley and B. foveata reference strains, while B. foveata was included in the individual sub-clades The third clad grouped reference strains of B. crinicola, while the fourth included only reference strains of B. diversispora (Fig. 3d).
Disscusion
The identification of Phoma species based on in vitro morphological characteristics is rather difficult and depends on the fungal strain, its place of origin and the host plants. Since this resulted in discrepancies and misidentifications, a multifaceted approach including rapid molecular tools, is desirable, to enable accurate identification. PCR-based RAPD is one of the most common techniques to estimate genetic diversity among Phoma isolates (Zhuo et al. 2005; Rai and Tiwari 2014).
Balmas et al. (2005) used the RAPD method to characterize Italian isolates of Phoma tracheiphila (Petri) L.A Kantsch. et Gikaschvili. They found that the analyzed isolates were genetically identical. On the other hand, Zhuo et al. (2005) found a significant genetic variation among Didymella macrostoma (Mont.) Q. Chen et L. Cai (Basionym Phoma macrostoma Mont.) isolates based on RAPD markers. Tiwari et al. (2013) showed that Allophoma tropica (R. Schneid. et Boerema) Q. Chen et L. Cai (basionym Phoma tropica R. Schneid. et Boerema) isolates collected from various host plants and environmental conditions were genetically differentiated. Our study analyzed 22 isolates of B. strasseri, obtained from different parts of plants growing under different environmental conditions, as well as two B. exigua var. exigua isolates from various hosts. The results showed that the isolates from peppermint were highly similar and grouped together on the dendrogram. In contrast, the isolates from lemon balm and motherwort were genetically different and formed a separate cluster. Tiwari et al. (2013) suggested that the genetic diversity observed among P. tropica species might be due to difference in environmental conditions or different host types from which the species were isolated. Our study confirmed that host differences significantly influenced the genetic diversity of the analyzed isolates.
Comparison of the ITS region sequence, which is widely used in taxonomy and molecular phylogeny (Balmas et al. 2005; Aveskamp et al. 2009; Rai and Tiwari 2014), showed insignificant nucleotide differences. For this reason, it is difficult to clearly confirm the affiliation of the tested isolates to any given species. Similar correlations were found by Badillo-Vargas et al. (2008) who studied Phoma species. They observed that nucleotide sequences in the ITS region of Phoma putaminum Speg. and Didymella macrostoma (Mont.) Q. Chen et L. Cai [basionym Phoma macrostoma var. incolorata (A.S. Horne) Boerema et Dorenb.)] matched the sequence of closely related species. In another study distinct morphological variants of Phoma exigua could not be distinguished from one another based on the molecular analysis of their ITS regions, (Abeln et al. 2002).
Our results confirmed the usefulness of the actin barcode in species determination. The same locus had previously been addressed while identification techniques were developed, proving highly reliable for deep-level phylogeny (Voigt et al. 2001; Aveskamp et al. 2009). and useful for discriminating closely related Phoma taxa (Aveskamp et al. 2009).
The present study, which is based on DNA phylogeny, showed that morphological characterization on standardized media is not reliable for identification at the species level. In fact, two fungal isolates from L. cardiaca and M. officinalis that were classified as B. strasseri based on their morphological characteristics, showed greater similarity to B. exigua var. exigua than to B. strasseri at the DNA level.
Concluding, to truly elucidate the taxonomy of Phoma-like genera, a concerted global effort is required not only for reclassifying previously described species, but also for providing GenBank with new sequences of isolates from countries that have been largely neglected or undersampled by mycologists and plant pathologists.
References
Abeln ECA, Stax AM, de Gruyter J, van der As HA (2002) Genetic differentiation of Phoma exigua varieties by means of AFLP fingerprints. Mycol Res 106:419–427
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Aveskamp MM, de Gruyter J, Crous PW (2008) Biology and recent developments in the systematics of Phoma, a complex genus of major quarantine significance. Fungal Divers 31:1–18
Aveskamp MM, Woudenberg JHC, de Gruyter J, Turco E, Groenewald JZ, Crous PW (2009) Development of taxon-specific sequence characterized amplified region (SCAR) markers based on actin sequences and DNA amplification fingerprinting (DAF): a case study in the Phoma exigua species complex. Mol Plant Pathol 10:403–414
Aveskamp MM, de Gruyter J, Woudenberg JHC, Varkley GJM, Crous PW (2010) Highlights of the Didymellaceae: a polyphasic approach to characterise Phoma and related pleosporalean genera. Stud Mycol 65:1–60
Badillo-Vargas IS, Rivera-Vargas LI, Calle-Bellido J (2008) Morphological, pathogenic and molecular characterization of Phoma spp. isolated from onion field soils in Puerto Rico. Journal of Agriculture-University of Puerto Rico 92:73–86
Balmas V, Scherm B, Ghignone S, Salem AOM, Cacciola SO, Migheli Q (2005) Characterisation of Phoma tracheiphila by RAPD-PCR, microsatellite-primed PCR and ITS rDNA sequencing and development of specific primers for in planta PCR detection. Eur J Plant Pathol 111:235–247
Boerema GH (1997) Contributions towards a monograph of Phoma (Coelomycetes) – V. Subdivision of the genus in sections. Mycotaxon 64:321–333
Boerema GH, de Gruyter J, Noordeloos ME, Hamers MEC (2004) Phoma Identification Manual. Differentiation of Specific and Infra-specific Taxa in Culture. CABI Publishing, Wallingford
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91:553–556
Chen Q, Zhang K, Zhang GZ, Cai L (2015) A polyphasic approach to characterize two novel species of Phoma (Didymellaceae) from China. Phytotaxa 197:267–281
De Gruyter J, Boerema GH, van der Aa HA (2002) Contributions towards a monograph of Phoma (Coelomycetes) VI – 2. Section Phyllostictoides: outline of its taxa. Persoonia 18:1–53
De Gruyter J, Aveskamp MM, Woudenberg JHC, Verkley GJM, Groenewald JZ, Crous PW (2009) Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycol Res 113:508–519
De Gruyter J, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW (2010) Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 102:1066–1081
De Gruyter J, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW (2012) Redisposition of Phoma-like anamorphs in Pleosporales. Stud Mycol 75:1–36
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Appl Environ Microbiol 61:1323–1330
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hammer Q, Harper DAT, Ryan PD (2001) Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol Electron 4:1–9
Horner CE (1971) Rhizome and stem rot of peppermint caused by Phoma strasseri. Plant Diseases Reports 5:814–816
Kalra A, Singh HB, Pandey R, Samad A, Patra NK, Kumar S (2004) Diseases in mint: causal organisms, distribution and control measures. Journal of Herbs, Spices & Medicinal Plants 11:71–91
Monte E, Bridge PD, Sutton BC (1991) An integrated approach to Phoma systematics. Mycopathologia 115:89–103
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms restriction endonucleases. Proceedings National Academy of Sciences USA 76:5269–5273
Paizs L, Naggy F (1975) Phoma strasseri: A new pathogen of mint in Hungary. Herba Hungarica 14:65–75
Rai MK, Tiwari VV (2014) Advances in taxonomy of genus Phoma: Polyphyletic nature and role of phenotypic traits and molecular systematics. Indian J Microbiol 54:123–128
Sambrook J, Russel DW (2001) Molecular Cloning: A Laboratory Manual, 3rd edn. Cold Spring Laboratory, New York
Tiwari VV, Gade AK, Rai M (2013) A study of phylogenetic variations among Indian Phoma tropica species by RAPD-PCR and ITS-rDNA sequencing. Indian J Biotechnol 12:187–194
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4239–4246
Voigt K, Malgorzata J, Wöstemeyer J (2001) Strain typing of Polish Leptosphaeria maculans isolates supports at the genomic level the multispecies concept of aggressive and non-aggressive strains. Microbiol Res 156:169–177
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphism amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetic. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols. Academic Press, San Diego, pp 315–322
Zhuo L, Bailey KL, Chen CY, Keri M (2005) Molecular and genetic analyses of geographic variation in isolates of Phoma macrostoma used for biological weed control. Mycologia 6:612–620
Zhang Y, Schoch CL, Fournier J, Crous PW, de Gruyter J, Woudenberg JH, Hirayama K, Tanaka K, Pointing SB, Spatafora JW, Hyde KD (2009) Multi-locus phylogeny of the Pleosporales: a taxonomic, ecological and evolutionary reevaluation. Stud Mycol 64:85–102
Zimowska B, Machowicz-Stefaniak Z (2005) Characteristics of Phoma strasseri isolates not reported in Poland pathogen of peppermint (Mentha piperita L.) Acta Agrobot 58:151–162
Zimowska B (2007) Fungi colonizing and damaging different parts of peppermint (Mentha piperita L.) cultivated in south-eastern Poland. Herba Polonica 53:97–105
Zimowska B (2008) Biodiversity of fungi colonizing and damaging selected parts of motherwort (Leonurus cardiaca L.) Herba Polonica 54:30–40
Zimowska B (2012) Pathogenicity and ultrastructural studies of the mode of penetration by Phoma strasseri in peppermint stems and rhizomes. Pol J Microbiol 61:273–279
Zimowska B, Targoński Z (2015) Pectinolytic activity of Boeremia strasseri, the causal agent of black stem and rhizomes rot of peppermint. Acta Scientiarum Polonorum Hortorum Cultus 14:19–28
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zimowska, B., Król, E.D., Furmańczyk, A. et al. Molecular characterization of Boeremia strasseri the causal agent of black stems and rhizomes rot of peppermint. J Plant Pathol 100, 13–24 (2018). https://doi.org/10.1007/s42161-018-0003-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-018-0003-4