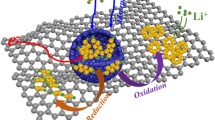
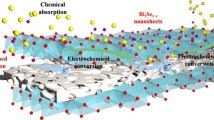
Abstract
Defect engineering techniques have gained significant attention worldwide as a promising strategy to amend the electronic and atomic arrangements of nanomaterials. By introducing defects such as dislocations or vacancies in polar materials, it is possible to create electrophilic adsorption sites that can effectively trap polysulfide species by lowering the energy transfer barrier for electrons. In this study, non-stoichiometric antimony selenide (Sb2Se2.2) nanocrystals embedded in a three-dimensional hollow microsphere composed of a reduced graphene oxide (rGO) matrix (H-Sb2Se2.2@rGO‒600) were synthesized by precisely controlling the heating conditions. Density functional theory (DFT) calculations revealed that thermally induced anionic Se-defects caused atomic disorder in the crystal structure, altering the electronic structure and in turn enhancing the adsorption strength of polysulfide through improved electrophilic coupling interactions between \(\mathrm{Sb}^{\delta+}-\mathrm S_x^{2-}\) and \(\mathrm{Li}^+-\mathrm{Se}^{\delta-}\). Lithium–sulfur (Li–S) batteries incorporating H-Sb2Se2.2@rGO‒600-coated separator and a typical sulfur electrode (≈2.0 mg cm–2) exhibited excellent high-rate capability, with a discharge rate of up to 4.0 C, and exceptional cycling stability. After 1300 continuous charge‒discharge cycles at 4.0 C, the cell showed a capacity retention of 90.4%, with an average capacity decay rate of only 0.007% per cycle. The impressive performance was maintained under more demanding cell conditions, such as high effective S content (66%), high S loading (6.0 mg cm–2), and a low electrolyte-to-sulfur ratio (4.3 µL mg–1). The Li–S cell demonstrates excellent cycling stability (120 cycles at 0.1 C) and maintains feasible rate performance up to 0.3 C.









Similar content being viewed by others
Data availability
The data are available from the corresponding author upon reasonable request.
References
Li Q, Li G, Fu C, Luo D, Fan J, Li L (2014) K+-doped Li1.2Mn0.54Co0.13Ni0.13O2: a novel cathode material with an enhanced cycling stability for lithium-ion batteries. ACS Appl Mater Interfaces 6(13):10330–10341. https://doi.org/10.1021/am5017649
Saroha R, Ahn J-H, Cho JS (2021) A short review on dissolved lithium polysulfide catholytes for advanced lithium-sulfur batteries. Korean J Chem Eng 38(1):461–474. https://doi.org/10.1007/s11814-020-0729-5
Jayasubramaniyan S, Lee H-W (2023) Directing battery chemistry using side-view operando optical microscopy. Korean J Chem Eng 40(1):488–496. https://doi.org/10.1007/s11814-022-1321-y
Kim JH, Kim BR, Im JS (2023) Optimization of the molecular weight range of coating pitch and its effect on graphite anodes for lithium-ion batteries. Korean J Chem Eng 40(1):2839–2846. https://doi.org/10.1007/s11814-023-1529-5
Saroha R, Oh JH, Seon YH, Kang YC, Lee JS, Cho JS (2021) Freestanding interlayers for Li-S batteries: design and synthesis of hierarchically porous N-doped C nanofibers comprising vanadium nitride quantum dots and MOF-derived hollow N-doped C nanocages. J Mater Chem A 9(19):11651–11664. https://doi.org/10.1039/D1TA01802G
Son D, Lim W-G, Le J (2023) A short review of the recent developments in functional separators for lithium-sulfur batteries. Korean J Chem Eng 40(1):473–487. https://doi.org/10.1007/s11814-022-1372-0
Saroha R, Oh JH, Lee JS, Kang YC, Jeong SM, Kang D-W, Cho C, Cho JS (2021) Hierarchically porous nanofibers comprising multiple core–shell Co3O4@graphitic carbon nanoparticles grafted within N-doped CNTs as functional interlayers for excellent Li–S batteries. Chem Eng J 426(1):130805. https://doi.org/10.1016/j.cej.2021.130805
Zhang Z, Luo D, Li G, Gao R, Li M, Li S, Zhao L, Dou H, Wen G, Sy S, Hu Y, Li J, Yu A, Chen Z (2020) Tantalum-based electrocatalyst for polysulfide catalysis and retention for high-performance lithium-sulfur batteries. Matter 3(1):920–934. https://doi.org/10.1016/j.matt.2020.06.002
Saroha R, Cho JS (2022) Nanofibers comprising interconnected chain-like hollow N‐doped C nanocages as 3D free‐standing cathodes for Li–S batteries with super‐high sulfur content and lean electrolyte/sulfur ratio. Small Methods 6(5):2200049. https://doi.org/10.1002/smtd.202200049
Saroha R, Heo J, Liu Y, Angulakshmi N, Lee Y, Cho K-K, Ahn H-J, Ahn J-H (2022) V2O3-decorated carbon nanofibers as a robust interlayer for long-lived, high-performance, room-temperature sodium–sulfur batteries. Chem Eng J 431(2):134205. https://doi.org/10.1016/j.cej.2021.134205
Chiochan P, Kaewruang S, Phattharasupakun N, Wutthiprom J, Maihom T, Limtrakul J, Nagarkar SS, Horike S, Sawangphruk M (2017) Chemical adsorption and physical confinement of polysulfides with the janus-faced interlayer for high-performance lithium-sulfur batteries. Sci Rep 7(1):17703. https://doi.org/10.1038/s41598-017-18108-0
Seon YH, Saroha R, Cho JS (2022) Hierarchically porous N-doped C nanofibers comprising TiO2 quantum dots and ZIF-8-derived hollow C nanocages as ultralight interlayer for stable Li–S batteries. Compos B Eng 237(1):109856. https://doi.org/10.1016/j.compositesb.2022.109856
Yuan H, Liu T, Liu Y, Nai J, Wang Y, Zhang W, Tao X (2019) A review of biomass materials for advanced lithium–sulfur batteries. Chem Sci 10(32):7484–7495. https://doi.org/10.1039/C9SC02743B
Saroha R, Seon YH, Jin B, Kang YC, Kang D-W, Jeong SM, Cho JS (2022) Self-supported hierarchically porous 3D carbon nanofiber network comprising Ni/Co/NiCo2O4 nanocrystals and hollow N-doped C nanocages as sulfur host for highly reversible Li–S batteries. Chem Eng J 446(2):137141. https://doi.org/10.1016/j.cej.2022.137141
Saroha R, Heo J, Li X, Angulakshmi N, Lee Y, Ahn H-J, Ahn J-H, Kim J-H (2022) Asymmetric separator integrated with ferroelectric-BaTiO3 and mesoporous-CNT for the reutilization of soluble polysulfide in lithium-sulfur batteries. J Alloys Compd 893(1):162272. https://doi.org/10.1016/j.jallcom.2021.162272
Jana M, Xu R, Cheng X-B, Yeon JS, Park JM, Huang J-Q, Zhang Q, Park H-S (2020) Rational design of two-dimensional nanomaterials for lithium–sulfur batteries. Energy Environ Sci 13(4):1049–1075. https://doi.org/10.1039/C9EE02049G
Saroha R, Ka HS, Cho JS (2022) A novel three-dimensional ordered mesoporous microspheres comprising N-doped graphitic carbon-coated FexP nanoparticles as multifunctional interlayers to suppress polysulfide crossover in Li–S batteries. Appl Surf Sci 612(1):155892. https://doi.org/10.1016/j.apsusc.2022.155892
Yang Y, Zhong Y, Shi Q, Wang Z, Sun K, Wang H (2018) Electrocatalysis in lithium sulfur batteries under lean electrolyte conditions. Angew Chem 130(47):15775–15778. https://doi.org/10.1002/anie.201808311
Choi JM, Saroha R, Kim JS, Jang MR, Cho JS (2023) Porous nanofibers comprising VN nanodots and densified N-doped CNTs as redox-active interlayers for Li–S batteries. J Power Sources 559(1):232632. https://doi.org/10.1016/j.jpowsour.2023.232632
Yang SH, Choi JM, Saroha R, Cho SW, Kang YC, Cho JS (2023) Hollow porous carbon nanospheres containing polar cobalt sulfide (Co9S8) nanocrystals as electrocatalytic interlayers for the reutilization of polysulfide in lithium–sulfur batteries. J Colloid Interface Sci 645(1):33–44. https://doi.org/10.1016/j.jcis.2023.04.083
Liang Q, Wang S, Yao Y, Dong P, Song H (2023) Transition metal compounds family for Li–S batteries: the DFT-guide for suppressing polysulfides shuttle. Adv Funct Mater 33(32):2300825. https://doi.org/10.1002/adfm.202300825
Zhang P, Yue L, Liang Q, Gao H, Yan Q, Wang L (2023) A review of transition metal compounds as functional separators for lithium-sulfur batteries. ChemistrySelect 8(1):e202203352. https://doi.org/10.1002/slct.202203352
Liang X, Kwok CY, Lodi-Marzano F, Pang Q, Cuisinier M, Huang H, Hart HJ, Houtarde D, Kaup K, Sommer H, Brezesinski T, Janek J, Nazar LF (2016) Tuning transition metal oxide–sulfur interactions for long life lithium sulfur batteries: the Goldilocks principle. Adv Energy Mater 6(6):1501636. https://doi.org/10.1002/aenm.201501636
Chen Z, Lv W, Kang F, Li J (2019) Theoretical investigation of the electrochemical performance of transition metal nitrides for lithium–sulfur batteries. J Phys Chem C 123(41):25025–25030. https://doi.org/10.1021/acs.jpcc.9b04670
Chen L, Li X, Xu Y (2018) Recent advances of polar transition-metal sulfides host materials for advanced lithium–sulfur batteries. Funct Mater Lett 11(6):1840010. https://doi.org/10.1142/S1793604718400106
Zhang C, Cui L, Abdolhosseinzadeh S, Heier J (2020) Two-dimensional MXenes for lithium‐sulfur batteries. InfoMat 2(4):613–638. https://doi.org/10.1002/inf2.12080
Wang H, Deng N, Wang S, Wang X, Li Y, Zeng Q, Luo S, Cui X, Cheng B, Kang W (2022) Advanced preparation and application of transition metal selenides in lithium-sulfur batteries: a review. J Mater Chem A 10(44):23433–23466. https://doi.org/10.1039/D2TA05576G
Huang S, Huixiang E, Yang Y, Zhang Y, Ye M, Li CC (2021) Transition metal phosphides: new generation cathode host/separator modifier for Li–S batteries. J Mater Chem A 9(12):7458–7480. https://doi.org/10.1039/D0TA11919A
Manzeli S, Ovchinnikov D, Pasquier D, Yazyev OV, Kis A (2017) 2D transition metal dichalcogenides. Nat Rev Mater 2(8):17033. https://doi.org/10.1038/natrevmats.2017.33
Wang M, Fan L, Wu X, Qiu Y, Guan B, Wang Y, Zhang N, Sun K (2019) Metallic NiSe2 nanoarrays towards ultralong life and fast Li2S oxidation kinetics of Li–S batteries. J Mater Chem A 7(25):15302–15308. https://doi.org/10.1039/C9TA03361K
Yang T, Zhou J, Song TT, Shen L, Feng YP, Yang M (2020) High-throughput identification of exfoliable two-dimensional materials with active basal planes for hydrogen evolution. ACS Energy Lett 5(7):2313–2321. https://doi.org/10.1021/acsenergylett.0c00957
Sun Y, Zhang X, Mao B, Cao M (2016) Controllable selenium vacancy engineering in basal planes of mechanically exfoliated WSe2 monolayer nanosheets for efficient electrocatalytic hydrogen evolution. Chem Commun 52(99):14266–14269. https://doi.org/10.1039/C6CC07832J
Gong X, Li R, Chen H, He C, Gao Za, Xie H (2023) (111)-Oriented crystalline plane MnO loaded by biomass carbon separator to facilitate sulfur redox kinetics in lithium–sulfur batteries. Arab J Chem 16(6):104752. https://doi.org/10.1016/j.arabjc.2023.104752
Wen J, Pei Y, Liu L, Su D, Yang M, Wang Q, Zhang W, Dai J, Feng Y, Wu T, Wang X (2021) Fully encapsulated Sb2Se3/Sb/C nanofibers: towards high-rate, ultralong-lifespan lithium-ion batteries. J Alloys Compd 874(1):159961. https://doi.org/10.1016/j.jallcom.2021.159961
Dong Y, Feng Y, Deng J, He P, Ma J (2020) Electrospun Sb2Se3@C nanofibers with excellent lithium storage properties. Chin Chem Lett 31(3):909–914. https://doi.org/10.1016/j.cclet.2019.11.039
Li C, Song H, Mao C, Peng H, Li G (2019) A novel MoS2 nanosheets-decorated Sb@Sb2S3@C tubular composites as anode material for high performance lithium ion battery. J Alloys Compd 786(1):169–176. https://doi.org/10.1016/j.jallcom.2019.01.315
Fan G, Wang Z, Ren H, Liu Y, Fan R (2021) Dielectric dispersion of copper/rutile cermets: dielectric resonance, relaxation, and plasma oscillation. Scr Mater 190(1):1–6. https://doi.org/10.1016/j.scriptamat.2020.08.027
Fan G, Wang Z, Sun K, Liu Y, Fan R (2021) Doped ceramics of indium oxides for negative permittivity materials in MHz-kHz frequency regions. J Mater Sci Technol 61(1):125–131. https://doi.org/10.1016/j.jmst.2020.06.013
Saroha R, Panwar AK (2017) Effect of in situ pyrolysis of acetylene (C2H2) gas as a carbon source on the electrochemical performance of LiFePO4 for rechargeable lithium-ion batteries. J Phys D: Appl Phys 50(25):255501. https://doi.org/10.1088/1361-6463/aa708c
Deng Z, Chen D, Tang F, Ren J, Muscat AJ (2009) Synthesis and purple-blue emission of antimony trioxide single-crystalline nanobelts with elliptical cross section. Nano Res 2(2):151–160. https://doi.org/10.1007/s12274-009-9014-y
Pattini F, Rampino S, Mezzadri F, Calestani D, Spaggiari G, Sidoli M, Delmonte D, Sala A, Gilioli E, Mazzer M (2020) Role of the substrates in the ribbon orientation of Sb2Se3 films grown by low-temperature pulsed electron deposition. Sol Energy Mater Sol Cells 218(1):110724. https://doi.org/10.1016/j.solmat.2020.110724
Yin L-C, Liang J, Zhou G-M, Li F, Saito R, Cheng H-M (2016) Understanding the interactions between lithium polysulfides and N-doped graphene using density functional theory calculations. Nano Energy 25(1):203–210. https://doi.org/10.1016/j.nanoen.2016.04.053
Zhang B, Wu J, Gu J, Li S, Yan T, Gao X-P (2021) The fundamental understanding of lithium polysulfides in ether-based electrolyte for lithium–sulfur batteries. ACS Energy Lett 6(2):537–546. https://doi.org/10.1021/acsenergylett.0c02527
Wang L, Hu Z, Wan X, Hua W, Li H, Yang QH, Wang W (2022) Li2S4 anchoring governs the catalytic sulfur reduction on defective SmMn2O5 in lithium–sulfur battery. Adv Energy Mater 12(20):2200340. https://doi.org/10.1002/aenm.202200340
Lim D-H, Wilcox J (2011) DFT-based study on oxygen adsorption on defective graphene-supported pt nanoparticles. J Phys Chem C 115(46):22742–22747. https://doi.org/10.1021/jp205244m
Zhang L, Xia Z (2011) Mechanisms of oxygen reduction reaction on nitrogen-doped graphene for fuel cells. J Phys Chem C 115(22):11170–11176. https://doi.org/10.1021/jp201991j
Li X, Paier J, Sauer J, Mirabella F, Zaki E, Ivars-Barceló F, Shaikhutdinov S, Freund H-J (2018) Surface termination of Fe3O4 (111) films studied by CO adsorption revisited. J Phys Chem B 122(2):527–533. https://doi.org/10.1021/acs.jpcb.7b04228
Zhang J, Li W, Wang J, Pu X, Zhang G, Wang S, Wang N, Li X (2023) Engineering p-band center of oxygen boosting H+ intercalation in δ‐MnO2 for aqueous zinc ion batteries. Angew Chem Int Ed 62(8):e202215654. https://doi.org/10.1002/anie.202215654
Martini I, Chevallay E, Fedosseev V, Hessler C, Neupert H, Nistor V, Taborelli M (2015) Surface characterization at CERN of photocathodes for photoinjector applications. IPAC2015, Richmond, VA, USA (May)
Park J-S, Kang YC (2019) Uniquely structured sb nanoparticle-embedded carbon/reduced graphene oxide composite shell with empty voids for high performance sodium-ion storage. Chem Eng J 373(1):227–237. https://doi.org/10.1016/j.cej.2019.05.036
Bodenes L, Darwiche A, Monconduit L, Martinez H (2015) The solid electrolyte interphase a key parameter of the high performance of Sb in sodium-ion batteries: comparative X-ray photoelectron spectroscopy study of Sb/Na-ion and Sb/Li-ion batteries. J Power Sources 273(1):14–24. https://doi.org/10.1016/j.jpowsour.2014.09.037
Whittles TJ, Veal TD, Savory CN, Welch AW, de Souza Lucas FW, Gibbon JT, Birkett M, Potter RJ, Scanlon DO, Zakutayev A, Dhanak VR (2017) Core levels, band alignments, and valence-band states in CuSbS2 for solar cell applications. ACS Appl Mater Interfaces 9(48):41916–41926. https://doi.org/10.1021/acsami.7b14208
Petit E, Riga J, Caudano R (1991) Surface and interface XPS characterization of the oxide layer grown on antimony under UV laser irradiation. Surf Sci 251–252(1):529–534. https://doi.org/10.1016/0039-6028(91)91049-4
Tang X, Van Welzenis R, Van Setten F, Bosch A (1986) Oxidation of the InSb surface at room temperature. Semicond Sci Technol 1(6):355. https://doi.org/10.1088/0268-1242/1/6/004
Seon YH, Kang YC, Cho JS (2021) One-dimensional porous nanostructure composed of few-layered MoSe2 nanosheets and highly densified-entangled-N-doped CNTs as anodes for na ion batteries. Chem Eng J 425(1):129051. https://doi.org/10.1016/j.cej.2021.129051
Lee JS, Saroha R, Cho JS (2022) Porous microspheres comprising CoSe2 nanorods coated with N-doped graphitic C and polydopamine-derived C as anodes for long-lived Na-ion batteries. Nano-Micro Lett 14(1):113. https://doi.org/10.1007/s40820-022-00855-z
Lee JS, Park J-S, Baek KW, Saroha R, Yang SH, Kang YC, Cho JS (2023) Coral-like porous microspheres comprising polydopamine-derived N-doped C-coated MoSe2 nanosheets composited with graphitic carbon as anodes for high-rate sodium-and potassium-ion batteries. Chem Eng J 456(1):141118. https://doi.org/10.1016/j.cej.2022.141118
Lee JS, Jo MS, Saroha R, Jung DS, Seon YH, Lee JS, Kang YC, Kang D-W, Cho JS (2020) Hierarchically well-developed porous graphene nanofibers comprising N‐doped graphitic C‐coated cobalt oxide hollow nanospheres as anodes for high‐rate Li‐ion batteries. Small 16(32):2002213. https://doi.org/10.1002/smll.202002213
Lee JS, Saroha R, Oh SH, Shin DH, Jeong SM, Kim JK, Cho JS (2021) Rational design of perforated bimetallic (Ni, Mo) sulfides/N-doped graphitic carbon composite microspheres as anode materials for superior Na‐ion batteries. Small Methods 5(9):2100195. https://doi.org/10.1002/smtd.202100195
Kim CS, Saroha R, Choi HH, Oh JH, Park GD, Kang D-W, Cho JS (2023) High-performance cathode promoted by reduced graphene oxide nanofibers with well-defined interconnected meso-/micro pores for rechargeable Li-Se batteries. J Ind Eng Chem 121(1):489–498. https://doi.org/10.1016/j.jiec.2023.02.004
Xie P, Zhang Z, Liu K, Qian L, Dang F, Liu Y, Fan R, Wang X, Dou S (2017) C/SiO2 meta-composite: overcoming the λ/a relationship limitation in metamaterials. Carbon 125(1):1–8. https://doi.org/10.1016/j.carbon.2017.09.021
Hao Y, Leng Z, Yu C, Xie P, Meng S, Zhou L, Li Y, Liang G, Li X, Liu C (2023) Ultra-lightweight hollow bowl-like carbon as microwave absorber owning broad band and low filler loading. Carbon 212(1):118156. https://doi.org/10.1016/j.carbon.2023.118156
Xie P, Li H, He B, Dang F, Lin J, Fan R, Hou C, Liu H, Zhang J, Ma Y (2018) Bio-gel derived nickel/carbon nanocomposites with enhanced microwave absorption. J Mater Chem C 6(32):8812–8822. https://doi.org/10.1039/C8TC02127A
Kim CS, Lee JS, Saroha R, Park YB, Kang YC, Kang D-W, Jeong SM, Cho JS (2022) Porous nitrogen-doped graphene nanofibers comprising metal organic framework-derived hollow and ultrafine layered double metal oxide nanocrystals as high-performance anodes for lithium-ion batteries. J Power Sources 523(1):231030. https://doi.org/10.1016/j.jpowsour.2022.231030
Lee JS, Saroha R, Oh JH, Cho C, Jin B, Kang D-W, Cho JS (2022) Camphene-derived hollow and porous nanofibers decorated with hollow NiO nanospheres and graphitic carbon as anodes for efficient lithium-ion storage. J Ind Eng Chem 114(1):276–287. https://doi.org/10.1016/j.jiec.2022.07.017
Lee JS, Ka HS, Saroha R, Kang YC, Kang D-W, Cho JS (2023) Three-dimensional hierarchically porous micro sponge-ball comprising anatase TiO2 nanodots and nitrogen-doped graphitic carbon as anodes for ultra-stable lithium-ion batteries. J Energy Storage 66(1):107396. https://doi.org/10.1016/j.est.2023.107396
Oh SH, Park SM, Kang D-W, Kang YC, Cho JS (2020) Fibrous network of highly integrated carbon nanotubes/MoO3 composite bundles anchored with MoO3 nanoplates for superior lithium ion battery anodes. J Ind Eng Chem 83(1):438–448. https://doi.org/10.1016/j.jiec.2019.12.017
Saroha R, Choi HH, Cho JS (2023) Boosting redox kinetics using rationally engineered cathodic interlayers comprising porous rGO–CNT framework microspheres with NiSe2-core@N-doped graphitic carbon shell nanocrystals for stable Li–S batteries. Chem Eng J 473:145391. https://doi.org/10.1016/j.cej.2023.145391
Kim CS, Saroha R, Cho JS (2023) N-doped graphene nanofibers with porous channel comprising FeS nanocrystals and intertwined N-doped CNTs as efficient interlayers for Li-S batteries. Int J Energy Res 2023:3610577. https://doi.org/10.1155/2023/3610577
Chung S-H, Luo L, Manthiram A (2018) TiS2–Polysulfide hybrid cathode with high sulfur loading and low electrolyte consumption for lithium–sulfur batteries. ACS Energy Lett 3(3):568–573. https://doi.org/10.1021/acsenergylett.7b01321
Funding
This work was supported by the National Research Foundation of Korea (NRF) and funded by the Korean Government (MSIP) (grant numbers No. RS-2023-00217581 and NRF-2021R1I1A3057700). This work was partly supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government (MOTIE) (20224000000070, Human Resource Training for Smart Energy New Industry Cluster). This research was supported by the “Regional Innovation Strategy (RIS)” through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2021RIS-001).
Author information
Authors and Affiliations
Contributions
Rakesh Saroha—designed the idea, performed experiments, and prepared the initial blueprint. Dong Yun Shin—DFT calculations and analysis. Jae Seob Lee and Sung Woo Cho—performed experiments and data accumulation. Dong-Hee Lim—DFT analysis, review and editing. Jung Sang Cho—supervision, writing, review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saroha, R., Shin, D.Y., Lee, J.S. et al. Theoretically endured defect-engineered antimony selenide nanocrystals grafted within three-dimensional reduced graphene oxide hollow microspheres with large open cavities as polysulfide barrier for robust sulfur kinetics. Adv Compos Hybrid Mater 7, 93 (2024). https://doi.org/10.1007/s42114-024-00892-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42114-024-00892-9