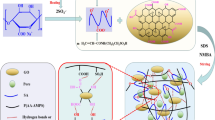
Abstract
Dyes are among the main pollutants in the textile wastewaters, which, due to their complex molecular structure, are often poisonous, carcinogenic, and environmentally perilous. Therefore, the main objective of this study is to evaluate the removal efficiency of textile dye (tested on methylene blue, MB) from aqueous medium using graphene oxide (GO)-containing hydrogels due to their high surface area and ability to adsorb a large amount of water and water-soluble species. Therefore, a series of GO-based nanocomposites based on acrylamide and sodium alginate crosslinked by methylene bis-acrylamide (MBA) have been prepared to be used and evaluated as the starter. The effect of different parameters such as initial dye concentration, temperature, and contact time on the removal of MB is investigated as well as determination of the adsorption isotherm of MB on GO/hydrogel using the Langmuir model. Various concentrations, temperatures, and contact time have also been investigated on GO/hydrogels. The chemical structure of the GO/hydrogel is determined by scanning electron microscopy, FTIR, and X-ray diffraction. The results show that the GO/hydrogel can absorb MB by up to 95.76% efficiency in removal of MB from an aqueous media.

The efficiency of removal of textile colorants from aqueous medium with graphene oxide containing hydrogels examined.









Similar content being viewed by others
References
Mane VS, Mall ID, Srivastava VC (2007) Use of bagasse fly ash as an adsorbent for the removal of brilliant green dye from aqueous solution. Dyes Pigments 73(3):269–278
Xiao X, Zhang F, Feng Z, Deng S, Wang Y (2015) Adsorptive removal and kinetics of methylene blue from aqueous solution using NiO/MCM-41 composite. Physica E: Low-dimensional Systems and Nanostructures 65:4–12
Donia AM, Atia AA, Al-amrani WA, El-Nahas AM (2009) Effect of structural properties of acid dyes on their adsorption behaviour from aqueous solutions by amine modified silica. J Hazard Mater 161(2):1544–1550
Deng JH, Zhang XR, Zeng GM, Gong JL, Niu QY, Liang J (2013) Simultaneous removal of cd (II) and ionic dyes from aqueous solution using magnetic graphene oxide nanocomposite as an adsorbent. Chem Eng J 226:189–200
Houa H, Zhou R, Wua P, Wu L (2012) Removal of Congo red dye from aqueous solution with hydroxyapatite/chitosan composite. Chem Eng J 211:336–342
Wu Z, Zhong H, Yuan X, Wang H, Wang L, Chen X, Zeng G, Wu Y (2014) Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res 67:330–344
Li Y, Du Q, Liu T, Sun J, Wang Y, Wu S, Wang Z, Xia Y, Xia L (2013) Methylene blue adsorption on graphene oxide/calcium alginate. Carbohydr Polym 95(1):501–507
Hamidi M, Azadi A, Rafiei P (2008) Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev 60(15):1638–1649
Chang C, Duan B, Cai J, Zhang L (2010) Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur Polym J 46:92–100
Richter A, Paschew G, Klatt S, Lienig J, Arndt K-F, Adler H-JP (2008) Review on hydrogel-based pH sensors and microsensors. Sensors 8:561–581
Dittmeyer R, Höllein V, Daub K (2001) Membrane reactors for hydrogenation and dehydrogenation processes based on supported palladium. J Mol Catal A Chem 173:135–184
Yang J, Xu C, Kopečková P, Kopeček J (2006) Hybrid hydrogels self-assembled from HPMA copolymers containing peptide grafts. Macromol Biosci 6:201–209
Malkoch M, Vestberg R, Gupta N, Mespouille L, Dubois P, Mason AF (2006) Synthesis of well-defined hydrogel networks using click chemistry. Chem Commun:2774–2776
Lee KY, Bouhadir KH, Mooney DJ (2000) Degradation behavior of covalently cross-linked poly (aldehyde guluronate) hydrogels. Macromolecules 33:97–101
Kataoka T, Kidowaki M, Zhao C, Minamikawa H, Shimizu T, Ito K (2006) Local and network structure of thermoreversible polyrotaxane hydrogels based on poly (ethylene glycol) and methylated α-cyclodextrins. J Phys Chem B 110:24377–24383
Peppas NA (1997) Hydrogels and drug delivery. Curr Opin Colloid Interface Sci 2:531–537
Spanoudaki A, Fragiadakis D, Vartzeli-Nikaki K, Pissis P, Hernandez JCR, Pradas MM (2006) Nanostructured and nanocomposite hydrogels for biomedical applications. Surface Chemistry in Biomedical and Environmental Science (Nato Science Series II):229–240
Lester CL, Smith SM, Colson CD, Guymon CA (2003) Physical properties of hydrogels synthesized from lyotropic liquid crystalline templates. Chem Mater 15:3376–3384
Xu J, Li X, Sun F (2010) Cyclodextrin-containing hydrogels for contact lenses as a platform for drug incorporation and release. Acta Biomater 6:486–493
Yoshida R, Uchida K, Kaneko Y, Sakai K, Kikuchi A, Sakurai Y (1995) Comb-type grafted hydrogels with rapid deswelling response to temperature changes. Nature 374:240–242
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. J Chem Eng 156:2–10
Zohuriaan-Mehr MJ, Kabiri K (2008) Superabsorbent polymer materials: a review. Iran Polym J 17:451–477
Y. Li, Y. Q. Du, T. Liu, J. Sun, Y. Wang, S. Wu, Z. Wang, Y. Xia, L. Xia L., Methylene blue adsorption on graphene oxide/calcium alginate. Carbohydr Polym, 2013, 95 (1): 501–507
He F, Fan J, Ma D, Zhang L, Leung C, Chan HL (2010) The attachment of Fe3O4 nanoparticles to graphene oxide by covalent bonding. Carbon 48(11):3139–3144
Li Y, Qiuju D, Liu T, Peng X, Wang J, Sun J, Wang Y, Wu S, Wang Z, Xia Y, Xi L (2013) Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem Eng Res Des 91(2):361–368
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fallah, S., Mamaghani, H.R., Yegani, R. et al. Use of graphene substrates for wastewater treatment of textile industries. Adv Compos Hybrid Mater 3, 187–193 (2020). https://doi.org/10.1007/s42114-020-00146-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42114-020-00146-4