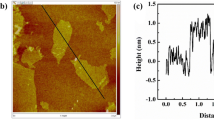
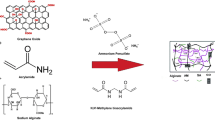
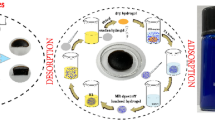
Abstract
When used to treat printing and dyeing effluents, the existing hydrogels have the following problems: limited mechanical strength, slow adsorption rate, and poor reusability. Therefore, a simple one-pot method was used to prepare poly (acrylic acid-2-acrylamide-2-methyl-1-propanesulfonic acid)-g-sodium alginate/graphene oxide (P(AA-AMPS)/SA-GO) composite hydrogel, which was analyzed by Fourier transform infrared, scanning electron microscope, confocal laser scanning microscope and Brunauer–Emmet–Teller method. The mechanical properties, swelling ratios and adsorption performance of the composite hydrogel were determined in detail. The results show that the composite hydrogel has a porous structure and excellent mechanical properties. The maximum elongation at break and tensile strength can reach 803% and 104.8 kPa, respectively. When the GO content is 1.2%, the swelling ratio and methylene blue (MB) adsorption capacity are the highest, 54.9 g/g and 486.5 mg/g, respectively. The external conditions have a significant effect on the adsorption performance of the hydrogel. When the adsorption conditions are as follows: the adsorption temperature is 25 ℃, the amount of hydrogel is 0.1 g, the pH is 8, and the initial concentration of MB is 500 mg/L, the highest removal efficiency of MB is 98.1% in 90 min. Coexisting ions can inhibit the removal of MB. The adsorption model fitting and kinetic analyses show that P(AA-AMPS)/SA-GO follows the Langmuir adsorption model and belongs to single-layer adsorption. The adsorption kinetic model study shows that the gel fits the quasi-second-order kinetic model, with chemical adsorption as the primary and physical adsorption as the auxiliary. The P(AA-AMPS)/SA-GO hydrogel has a remarkable adsorption effect on methylene blue wastewater and can be used as an efficient adsorbent for wastewater treatment.









Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
References
Crini G, Lichtfouse E (2019) Advantages and disadvantages of techniques used for wastewater treatment. Environ Chem Lett 17:145–155. https://doi.org/10.1007/s10311-018-0785-9
Mohsenpour SF, Hennige S, Willoughby N, Adeloye A, Gutierrez T (2021) Integrating micro-algae into wastewater treatment: a review. Sci Total Environ 752:142168. https://doi.org/10.1016/j.scitotenv.2020.142168
Rashid R, Shafiq I, Akhter P, Iqbal MJ, Hussain M (2021) A state-of-the-art review on wastewater treatment techniques: the effectiveness of adsorption method. Environ Sci Pollut Res 28:9050–9066. https://doi.org/10.1007/s11356-021-12395-x
Chai WS, Cheun JY, Kumar PS, Mubashir M, Majeed Z, Banat F, Show PL (2021) A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J Clean Prod 296:126589. https://doi.org/10.1016/j.jclepro.2021.126589
Velusamy S, Roy A, Sundaram S, Kumar Mallick T (2021) A review on heavy metal ions and containing dyes removal through graphene oxide-based adsorption strategies for textile wastewater treatment. Chem Rec 21:1570–1610. https://doi.org/10.1002/tcr.202000153
Yang J, Chen X, Zhang J, Wang Y, Wen H, Xie J (2021) Role of chitosan-based hydrogels in pollutants adsorption and freshwater harvesting: a critical review. Int J Biol Macromol 189:53–64. https://doi.org/10.1016/j.ijbiomac.2021.08.047
Han D, Zhao H, Gao L, Qin Z, Ma J, Han Y, Jiao T (2021) Preparation of carboxymethyl chitosan/phytic acid composite hydrogels for rapid dye adsorption in wastewater treatment. Colloids Surf A Physicochem Eng Asp 628:127355. https://doi.org/10.1016/j.colsurfa.2021.127355
Lü T, Ma R, Ke K, Zhang D, Qi D, Zhao H (2021) Synthesis of gallic acid functionalized magnetic hydrogel beads for enhanced synergistic reduction and adsorption of aqueous chromium. Chem Eng J 408:127327. https://doi.org/10.1016/j.colsurfa.2021.127355
Xie M, Zeng Y, Wu H, Wang S, Zhao J (2022) Multifunctional carboxymethyl chitosan/oxidized dextran/sodium alginate hydrogels as dressing for hemostasis and closure of infected wounds. Int J Biol Macromol 219:1337–1350. https://doi.org/10.1016/j.ijbiomac.2022.08.166
Yi J, Choe G, Park J, Lee JY (2020) Graphene oxide-incorporated hydrogels for biomedical applications. Polym J 52:823–837. https://doi.org/10.1038/s41428-020-0350-9
Srikaew M, Jumpapaeng P, Suwanakood P, Kaiyasuan C, Promarak V, Saengsuwan S (2023) Rapid synthesis and optimization of UV-photopolymerized cassava starch-based superabsorbent hydrogels as a biodegradable, low-cost, and effective adsorbent for MB removal. J Ind Eng Chem 118:53–69. https://doi.org/10.1016/j.jiec.2022.10.045
Patra BR, Nanda S, Dalai AK, Meda V (2021) Taguchi-based process optimization for activation of agro-food waste biochar and performance test for dye adsorption. Chemosphere 285:131531. https://doi.org/10.1016/j.chemosphere.2021.131531
Wang X, Zheng Y, Zong L, Zhang C, Ren X, Ding Y, Zhou Y (2022) Porous biochar composite hydrogel for effective removal of low-concentration methylene blue from wastewater. J Polym Res 29:1–15. https://doi.org/10.1007/s10965-022-03295-w
Zhu L, Liu Y, Wang F, He T, Tang Y, Yang J (2018) Preparation and the swelling properties of sodium alginate graft poly (acrylic acid-co-2-acrylamide-2-methyl propane sulfonic acid)/graphene oxide hydrogel composite. Adv Polym Technol 37:2885–2893. https://doi.org/10.1002/adv.21960
Meng Y, Ye L (2017) Synthesis and swelling property of superabsorbent starch grafted with acrylic acid/2-acrylamido-2-methyl-1-propanesulfonic acid. J Sci Food Agric 97:3831–3840. https://doi.org/10.1002/jsfa.8247
Mok CF, Ching YC, Muhamad F et al (2020) Adsorption of dyes using poly (vinyl alcohol)(PVA) and PVA-based polymer composite adsorbents: a review. J Polym Environ 28:775–793. https://doi.org/10.1007/s10924-020-01656-4
Jiang C, Wang X, Hou B, Hao C, Li X, Wu J (2020) Construction of a lignosulfonate–lysine hydrogel for the adsorption of heavy metal ions. J Agric Food Chem 68:3050–3060. https://doi.org/10.1021/acs.jafc.9b07540
Mok CF, Ching YC, Abu MF, Osman NA, Hai ND, Che Hassan CR (2020) Adsorption of dyes using poly (vinyl alcohol)(PVA) and PVA-based polymer composite adsorbents: a review. J Polym Environ 28:775–793. https://doi.org/10.1007/s10924-020-01656-4
Lei C, Bian Y, Zhi F, Hou X, Lv C, Hu Q (2022) Enhanced adsorption capacity of cellulose hydrogel based on corn stalk for pollutants removal and mechanism exploration. J Clean Prod 375:134130. https://doi.org/10.1016/j.jclepro.2022.134130
Duman O, Polat TG, Diker CÖ, Tunç S (2020) Agar/κ-carrageenan composite hydrogel adsorbent for the removal of methylene blue from water. Int J Biol Macromol 160:823–835. https://doi.org/10.1016/j.ijbiomac.2020.05.191
Alharby NF, Almutairi RS, Mohamed NA (2021) Adsorption behavior of methylene blue dye by novel crosslinked O-CM-Chitosan hydrogel in aqueous solution: kinetics, isotherm and thermodynamics. Polymers 13:3659. https://doi.org/10.3390/polym13213659
Junlapong K, Maijan P, Chaibundit C, Chantarak S (2020) Effective adsorption of methylene blue by biodegradable superabsorbent cassava starch-based hydrogel. Int J Biol Macromol 158:258–264. https://doi.org/10.1016/j.ijbiomac.2020.04.247
Wan X, Rong Z, Zhu K, Wu Y (2022) Chitosan-based dual network composite hydrogel for efficient adsorption of methylene blue dye. Int J Biol Macromol 222:725–735. https://doi.org/10.1016/j.ijbiomac.2022.09.21310.1016/j.ijbiomac.2022.09.213
Li Y, Liu SJ, Chen FM, Zuo JE (2019) High-strength apatite/attapulgite/alginate composite hydrogel for effective adsorption of methylene blue from aqueous solution. J Chem Eng Data 64:5469–5477. https://doi.org/10.1021/acs.jced.9b00616
Funding
This work was supported by the Dongying scientific development fund (DJ2021021) (DJ2021018).
Author information
Authors and Affiliations
Contributions
XW, contributed to the conception of the study; YZ, performed the experiment and wrote the manuscript; LZ, performed the data analyses.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare there is no conflicts of interest regarding the publication of this paper.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
The paper has not been published elsewhere, and it has not been submitted simultaneously for publication elsewhere. The authors agree to publication in the journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, Y., Zong, L. & Wang, X. Graphene oxide enhanced hydrogel as an adsorbent for effective removal of methylene blue. Polym. Bull. 81, 4407–4426 (2024). https://doi.org/10.1007/s00289-023-04920-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04920-4