Abstract
Since the establishment of a free economic zone and a simultaneous increase in the population of the QwaQwa region, aquatic systems of the area were expected to undergo an increase in metal contamination. The aims of this study were to assess the quality of sediments from the Metsi-Matsho, Namahadi and Elands Rivers of the QwaQwa region, and to investigate their impact on the survival and reproduction of the collembolan Folsomia candida. Due to the lack of freshwater sediment quality guidelines in South Africa, metal contents of the sediments were analysed and critically interpreted using the national action list for the screening of dredged sediment proposed for marine disposal, and international sediment quality guidelines (SQGs). In the laboratory, F. candida was exposed to 0, 50, 75 and 100% of the sediment samples for 28 days at 20 °C. Sediments were variably contaminated with Cr, with the Namahadi River middle site having the highest concentration (569 mg.kg−1, dry weight) followed by the lower site on the Elands River (529 mg.kg−1, dry weight), both above the recommended prohibition level of 500 mg Cr.kg−1. According to the consensus-based SQGs, only the concentrations of Cr and Ni (upper reach of Metsi-Matsho River) were higher than probable effect concentration, denoting that harmful effects of those metals are likely to occur. Of the two endpoints assessed, reproduction was the most sensitive with the upper reach of the Metsi-Matsho River (half maximal effective concentration, EC50 = 24% sediment) being the most deleterious site to the reproduction of F. candida. Ecotoxicological bioassays using F. candida could be suitable complements to chemical analysis in the assessment of the ecological risks of freshwater sediments as this collembolan species is sensitive to sediments with variable ranges of metal contamination.
Similar content being viewed by others
Introduction
Water safety and quality are fundamental to human development and well-being (WHO, 2003). The water right is the right for humans to access water of adequate quality and in sufficient quantity to meet their basic needs (WHO, 2003; Benettayeb et al., 2022a, 2022b). However, the availability of sufficient and good-quality water resources in parts of Africa is often unlikely due to the lack of strict pollution regulations regarding the dumping of hazardous waste in the environment (Yabe et al., 2010). Predominant sources of water pollution are soil erosion, leaching from dumping sites, agriculture, industrialization, urbanization, and other activities that lead to waste discharge in rivers, lakes, and dams (Akhtar et al., 2021; Benettayeb et al., 2022a, 2022b, 2023).
Moreover, river sediments can be better indicators of the contamination status of a watercourse than water samples themselves (Binning & Baird, 2001; Ustaoğlu & Tepe, 2019). Once in the river water, the pollutants often diffuse from the water column and become adsorbed onto the sediments. These sediment-bound contaminants are often more excessive in magnitude than the contaminants in the water column (Daskalakis & O’Connor, 1995). From the sediments, they can be further mobilized or released depending on the changes to the natural state of the waterbody (De Groot et al., 1976; Benettayeb et al., 2022a, 2022b; Benettayeb & Haddou, 2023). Thus, waterbodies with degraded sediment quality often indicate deteriorated water quality.
Characterizing the extent of sediment (and water) contamination can be expensive and time-consuming. A number of sediment quality guidelines (SQGs) for freshwater sediments have been developed for different purposes including sediment quality criteria, sediment quality standards and designing monitoring programs (USEPA, 1997a; CCME, 2001). They are also useful in cataloguing contaminants of concern in freshwater ecosystems at local, regional or national levels (USEPA, 1997a, b). The SQGs used together with sediment (eco)toxicity tests represent a useful and complementary approach to evaluating the quality of freshwater sediments (Ju et al., 2022; Sahli et al., 2021). SQGs rely solely on chemical analyses of the sediment and should be complemented with data describing the effects of the sediment as a whole on organisms that might come in contact with it (Cesar et al., 2015a; Sahli et al., 2021). To better understand how concentrations of single and mixture of metals in freshwater sediments affect their toxicity, it can be helpful to use Folsomia candida, a terrestrial organism adapted to a moist environment. Folsomia candida is a cosmopolitan collembolan species that can be found in almost all biogeographical regions over the world (Bellinger et al., 2023), where it can reach high densities (Handschin, 1955). It prefers high organic matter like in compost or manure and has a significant influence on soil microbial ecology, decomposition of organic material and nutrient cycling, thus useful bioindicator (Culik & Zeppelini, 2003). Folsomia candida, which is a sensitive soil invertebrate dwelling in direct contact with the substrate in which it lives (Crouau et al., 1999) can be used to evaluate the ecotoxicity associated with land disposal of sediments (Cesar et al., 2015a, b; Vezzone et al., 2018). Here the approach is extended by adding different doses of artificial OECD (Organization for Economic Co-operation and Development) soil to sediments in order to find out whether these would affect the toxicity of metals. Moreover, at present, it is unknown whether information provided by F. candida toxicity tests may in certain extent corroborate the SQGs.
Various studies on the toxicity of metal-contaminated sediments with nematodes, earthworms and enchytraeids showed decreased survival, growth and reproduction (Cesar et al., 2014, 2015a, b; Heininger et al., 2007; Höss et al., 1997, 2010; Tietjen & Lee, 1984; Traunspurger et al., 1997; Vezzone et al., 2018). Fewer studies have been conducted with Collembola and freshwater sediments (Cesar et al., 2015a, b; Vezzone et al., 2018). In metal-contaminated sediments, an increase in the reproduction and sometimes mortality and habitat avoidance of F. candida is known to occur (Cesar et al., 2015a, b; Vezzone et al., 2018).
A limited number of studies conducted in the QwaQwa region have focused on river and dam water quality (Motholo, 2014) and on the pressure exerted by wastewater treatment plants (WWTPs) on the terrestrial (Mosolloane et al., 2019) and the aquatic environments (Moloi et al., 2020). There is a knowledge gap on river sediment in the region, especially regarding their levels of metal contamination and the potential effects thereof on sediment-dwelling organisms. This region is generally plagued by poor service delivery, indiscriminate garbage dumps, and domestic, agricultural, and industrial effluents flowing unabated into major waterways.
The aims of the present study were therefore to (1) assess the quality of three river sediments from the QwaQwa region of the eastern Free State of South Africa using SQGs for 8 metals and (2) to conduct ecotoxicity assessments of the effects associated with the disposal of river sediments in OECD soil to F. candida using survival and reproduction as endpoints. To this end, the quality of freshwater sediments was assessed using SQGs for 8 metals, and F. candida toxicity tests using gradients of sediment doses from different reaches of three rivers from the region. River sediments from various locations were sampled and metal analyzed for 8 key metals of interest (As, Cd, Cr, Cu, Hg, Ni, Pb and Zn), and exposed the collembolan F. candida with gradients of sediment doses. This study was carried out within the QwaQwa region of South Africa between December 2020 and March 2021.
Materials and methods
Study area
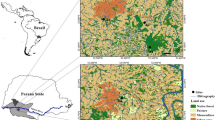
The study area lies within the QwaQwa region. It included upper and middle reaches of the Metsi-Matsho and Namahadi Rivers, as well as the Elands River which is formed by the merging of both the Metsi-Matsho and Namahadi Rivers. The QwaQwa region forms part of the Maluti-a-Phofung Municipality and is found in the summer-rainfall part of South Africa where summers stretch from 1 November to 31 March (Moeletsi & Walker, 2012). Most precipitations fall in January with an average of 184 mm. January is also the warmest month of the year with an average temperature of 17.8 °C, while July is the coldest with a temperature averaging 6.7 °C. The study area rises from 1700 to 2000 m above sea level (see Fig. 1).
Sediment samples collection
Between December 2020 and March 2021, sediment sampling occurred at the sites presented in Fig. 1 and Table 1. Site selection was governed by the longitudinal presentation of the river reaches (upper, middle and lower) and site accessibility. As shown in Fig. 1, the Elands River represented the joint lower reach of both the Metsi-Matsho and the Namahadi Rivers. Both rivers had independent sampling points representing the middle and upper reaches. Amongst the five chosen sites, Metsi-Matsho upper was located immediately below the Metsi-Matsho Dam. The others were located at different elevations along the selected rivers with no nearby artificial impoundment. The sediment samples were collected, following the USEPA (2007) standard method, from the riverbed at a depth of 5–15 cm using a shovel. The samples were stored temporarily in clean polyethene bags and transported to the laboratory where they were stored at 4 °C until further processing.
Preparation of the exposure treatments
Before carrying out the bioassay, the sediment samples were air-dried and sieved through a 2 mm sieve. The OECD artificial soil (OECD, 2004) was used as a control soil. It was made from 10% sphagnum peat (air-dried and sieved to 2 mm), 20% kaolin clay, and 70% sand. The pH of the artificial soil was 5.5. The sediment samples were thoroughly mixed with the OECD artificial soil to make up four concentrations (in %) of each of the sediment samples, namely: 0 (OECD soil only), 50, 75 and 100% (sediment soil only).
Folsomia candida exposures
The present study made use of the springtail species F. candida as the experimental organism. Folsomia candida was readily available from a laboratory culture maintained on a moist plaster of Paris substrate and fed baker’s yeast twice a week. Folsomia candida is a standard test species used for the testing of terrestrial habitats (ISO, 1999). The standard F. candida toxicity test described in ISO-guideline 11,267 was used as the exposure protocol (ISO, 1999). Cohorts of ten specimens (aged ten to twelve days old) were exposed to 20 g of the sediment treatments and placed in a climate control chamber (Labcon low-temperature incubator) at 20 °C in darkness for 28 days. During the course of the experiment, F. candida was fed 5 mg per treatment of granulated dry baker’s yeast weekly.
At the end of the test, a floatation technique (using 100 mL water stained with 100 µL of black ink per test vessel) was performed (McKee et al., 2017). Floating F. candida individuals were then photographed using a Samsung Galaxy J4 Core 4 smartphone with a built-in rear HDR camera (1080p/30 frames per second). Adult and juvenile organisms on the images were counted using the “CountThings” application by Dynamic Ventures, Inc. (available on Google Play). The total number of adults and juveniles for each treatment and each river site was recorded.
Metal analyses
Total metal analyses of the sediment samples were done using the EPA (Environmental Protection Agency) 3052 protocol (USEPA, 1996) and metal analysis of sediment–water extracts was performed according to Kingston and Walter (1998). The water extraction procedure provided the concentrations of metals that were supposedly bioavailable to the organisms that might interact with the river sediments. This was useful to help flag those metals and sediment samples that might have deleterious effects on sediment-dwelling organisms in the rivers. The analyses were conducted by Waterlab (Pty) Ltd in Pretoria, Gauteng Province, South Africa. All metal concentrations were measured and expressed in sediment dry weight. Due to the lack of river sediment quality guidelines in South Africa, the national action list guidelines for marine environments were used to evaluate the possible environmental impact of the metals present in the sediment samples. The National Action List can be assessed online at https://www.dffe.gov.za/sites/default/files/docs/marinedisposal_actionlist_technicalreport.pdf. The National Action List is approved and standardized by the South African Department of Environmental Affairs and Tourism. Therein, the sediment, based on the concentration of key metals of interest (As, Cd, Cr, Cu, Hg, Ni, Pb and Zn), is classified as belonging to the Action Level or Prohibition Level categories (Table 2). These guidelines consider the sediment moderately contaminated if the metal concentrations fall between the Action Level and Prohibition Level. The sediment is considered highly contaminated if the concentrations of the metals exceed the Prohibition Level (Table 2). It is stated in the document that the presence of these metals in the sediment does not imply that they could cause adverse biological effects within the ecosystem. Results were further evaluated using six SQGs for freshwater sediments (Long & Morgan, 1991; EC & MENVIQ, 1992; Persaud et al., 1993; Smith et al., 1996; MacDonald et al., 2000; CCME, 2001). From these SQGs, the adverse effects of metals found in sediments are dependent on their concentrations and defined as threshold effect level (TEL), lowest effect level (LEL), minimal effect threshold (MET), effect range low (ERL), probable effect level (PEL), severe effect level (SEL), toxic effect threshold (TET) and effect range median (ERM) (Table 3). Moreover, to provide an additional ecological dimension to the recorded metal concentrations, two indexes from MacDonald et al. (2000) were used, namely the threshold effect concentrations (TEC); the concentrations below which a toxic effect on freshwater organisms will rarely occur, and the probable effect concentrations (PEC); the concentration levels above which adverse effects are likely to occur.
Statistical analysis
Statistical analyses of the survival and reproduction data were performed in GraphPad Prism version 5.00 (GraphPad Software, San Diego, CA, USA, www.graphpad.com). Parametric data were analysed using One-way ANOVA, with Bonferroni post-test. Non-parametric data were analysed using the Kruskal–Wallis ANOVA followed by Dunns’ test. The level of significance was p < 0.05. ToxRat® version 2.10.05 (Toxicity Response Analysis and Testing; ToxRat solutions GmbH, Alsdorf, Germany) was used to calculate median lethal concentrations (LC50) and half maximal effective concentrations (EC50) whenever possible.
Results and discussion
Results
Metal concentrations
The water extraction method only revealed the presence of Zn, at the Metsi-Matsho upper site, at the minute concentration of 0.05 mg.kg−1. In the other rivers and sites, none of the eight metals of interest were present in the water extracts. The results of the total metal analysis for the eight elements of interest are provided in Table 4.
Cr was found to have the highest concentration. It measured 569 mg.kg−1 in the Namahadi River middle site, 526 mg.kg−1 in the Elands (the lower site) and 472 mg.kg−1 in the Metsi-Matsho River middle site. In the Metsi-Matsho River, the concentrations of this element exceeded the action level threshold at both the upper and middle sites but remained below the prohibition level threshold (Table 4). In the Namahadi River, Cr exceeded the action level threshold in the upper reach of the river and the prohibition level threshold in the middle site. In the Elands River, the lower site, the Cr concentration exceeded the prohibition level threshold (Table 4).
The second metal with the highest concentrations was Zn which measured 178 mg.kg−1 in the joint lower site on the Elands River (Table 4). Zn concentrations in the Metsi-Matsho River (93 and 133 mg.kg−1, upper and middle sites, respectively) remained below the action level of 150 mg.kg−1 and never exceeded the prohibition level (i.e., > 750 mg.kg−1). The same was observed in the Namahadi River where Zn was measured at 85 mg.kg−1 and 95 mg.kg−1 in the upper and middle sites, respectively.
Ni was the third highest concentrated metal, measuring 68 mg.kg−1 in the upper site of the Metsi-Matsho River, thus exceeding the action level threshold (Table 2). In the other rivers, it remained below the action level threshold of 50 mg.kg−1. The last metal to exceed the action level threshold was Cu, which measured 66 mg.kg−1 in the Metsi-Matsho upper site but remain below this threshold in all the other rivers. Pb and As never reached levels high enough to exceed the action level threshold, let alone the prohibition level. Cd and Hg were detected in none of the sediment samples assessed in this study (Table 4).
Based on the National Action List Guidelines (Table 2), the sediment samples collected in the present study can be categorized as pollutant class I because their total concentrations of Cd did not exceed 10 mg.kg−1, Hg did not exceed 5 mg.kg−1, As did not exceed 150 mg.kg−1, Cu, Cr, Pb and Ni do not exceed 500 mg.kg−1, and Zn did not 750 mg.kg−1.
Based on the SQGs for freshwater sediments i.e., using Table 3 (Long & Morgan, 1991; EC & MENVIQ, 1992; Persaud et al., 1993; Smith et al., 1996; CCME, 2001), the following was found: Cr concentrations far exceeded all types of SQGs adopted (ERL, MET, LEL, TEL, ERM, TET, SEL and PEL) everywhere. Cu exceeded LEL and MET at the lower site, LEL, MET and TEL at the upper and middle sites of the Namahadi River and far exceeded LEL, MET and TEL at the upper site of the Metsi-Matsho River. Ni was higher than LEL, TEL, ERL, MET and PEL at the lower, middle and upper sites of Namahadi River, while it exceeded LEL, TEL, ERL, MET, PEL, ERM and TET at the upper site of Metsi-Matsho River. Zn exceeded ERL, LEL and TEL at the middle site of the Metsi-Matsho River, whereas it was far above ERL, LEL, TEL and MET values at the joint lower site of the two rivers.
The consensus-based sediment quality guidelines developed by MacDonald et al. (2000), provide a unifying synthesis for assessing sediment quality conditions in freshwater ecosystems. The comparison of metal concentrations to the consensus-based SQGs revealed that metal levels exceeded TEC and PEC in 100% of samples for Cr. Eighty per cent (80%) of samples exceeded TEC (but not PEC) for Cu including the upper site of Metsi-Matsho, the upper and middle sites of Namahadi River and the joint lower site of Elands River. Eighty per cent (80%) of the samples were above TEC (but 20% of samples were higher than PEC) for Ni including the upper site of Metsi-Matsho, the upper and middle sites of Namahadi River and the joint lower site of the two rivers. Forty per cent (40%) of samples exceeded TEC for Zn at the middle site of Metsi-Matsho and the joint lower site of the two rivers. Only the concentrations of Cr and Ni (upper site of Metsi-Matsho River) were higher than PEC, denoting that harmful effects of those metals are likely to occur. Moreover, the mean PEC-Qs for studied sediments were 0.99 and 0.85 for upper and middle sites of Metsi-Matsho River, while the values were 0.86 and 1.12 for upper and middle sites of Namahadi River. The mean PEC-Qs value of the Elands River was 1.08.
Survival and reproduction of Folsomia candida
After the 28-day exposure of F. candida, there was no individual mortality in the control and all the sediment treatments from the Metsi-Matsho, Namahadi and Elands Rivers. Figure 2 presents the reproduction of F. candida after exposure to the sediment samples from all three rivers.
The reproduction of F. candida was statistically similar in all the sediment treatments from Metsi-Matsho upper and Metsi-Matsho middle (p > 0.05, Fig. 2). When compared with F. candida reproduction in the treatments from the Elands (lower site), it was found that in the pure sediment from the Elands (100% sediment treatment), statistically more reproduction occurred than in the 75 and 100% sediment treatment from Metsi-Matsho middle (p < 0.05, Fig. 2).
The half-maximal effective concentration (EC50) was calculated for the sediment collected from all river sites. In the Metsi-Matsho River, the sediment EC50 values were 24% in the upper site, and 54% in the middle site. In the joint lower site on the Elands River, the EC50 had further increased to > 100% (Table 5), indicating a decrease in sediment toxicity when going from the upper to the lower reaches of these river systems.
In the sediments from the Namahadi River, the reproduction of F. candida was similar in all treatments from the upper and middle sites (p > 0.05, Fig. 2). There was no statistical difference between the reproduction rates in the sites on the Namahadi River and the lower site on the Elands (p > 0.05). The EC50 values for the sediments from the Namahadi River decreased from 60% in the upper site to 54% in the middle site, and then it increased to > 100% in the lower site on the Elands River (Table 5). This indicated that, in the Namahadi, sediment toxicity to F. candida increased from the upper to the middle site, and then it decreased dramatically in the lower site on the Elands River.
Discussion
Metal concentrations in the sediment varied between the upper, middle, and lower sites of the three rivers of interest except for Cd and Hg which were not detected in any of the samples investigated. This means that all three rivers were not contaminated with Cd and Hg and that there are no natural sources of these metals along the river courses. However, studies from South Africa, have reported Cd concentrations that fall below the action level (1.5–10.0 mg.kg−1, Awofolu et al., 2005; Gerber et al., 2015) and within the range of the action level (Edokpayi et al., 2016).
Total metal concentrations of Zn and Pb from Elands River (lower site) were much higher than concentrations in sediments from the Metsi-Matsho and Namahadi Rivers. This may be due to the fact that many of the wastewater treatment plants found in Phuthaditjhaba discharge their treated effluents near the Elands River. Moloi et al. (2020) found that effluents from local wastewater treatment plants in Phuthaditjhaba were not exempted from metals and concluded that the concentrations of metals in water from the Elands River originated from multiple sources.
The very high concentrations of Cr (within the action level ranges and even worse, above the prohibition level [> 500 mg.kg−1] at the middle site of the Namahadi River and lower site on the Elands River) were not surprising, since with the establishment of a free economic zone and a simultaneous increase in the population of the QwaQwa region, aquatic systems of the area were expected to undergo an increase in metal contamination. According to Liedtke (2018), the region houses a mosaic of industries ranging from the furniture, textile, leather, and dairy to the agricultural, and aluminium industries (Liedtke, 2018). Such bustling industrial activities, in a region whose population has been increasing since the 1970s (Krige, 1995; Statistics SA, 2011) with reported failed wastewater treatment plants (Moloi et al., 2020; Mosolloane et al., 2019) create ideal conditions for the sort of metal pollution reported in this contribution. The comparisons between the levels of metals in sediments recorded in the QwaQwa region and those reported in other rivers of South Africa (Table 6) show that the rivers of the region could be considered some of the most critically polluted in the country.
To estimate the ecotoxicity of the sediments from the Metsi-Matsho, Namahadi and Elands Rivers, a comparative study was carried out with sediments from different reaches of these rivers using the springtails F. candida. The endpoints assessed i.e., reproduction and survival, are of paramount relevance as they are the most important functions in the life cycle of an organism (Vezzone et al., 2018; Woin & Brönmark, 1992). The present study revealed that the reproduction of F. candida was much more sensitive than survival and this was more evident when the substrate was made up of 100% of sediments. This finding was consistent with previous studies on the toxicity of sediments to springtails (Cesar et al., 2015a; Vezzone et al., 2018).
When comparing the chronic toxicity of sediments, three factors should be taken into consideration: (i) the effect of the mixture of metals on the toxicity of each single metal, (ii) the effect of sediment properties on the toxicity of the metals and (iii) the (probable) effect of other toxicants present in the sediments, that can jointly influence the toxicity of all the toxicants in the matrix.
The use of SQGs is a quick way to interpret sediment quality as it provides useful information on the metals of concern and the sediment samples that are likely to be toxic to sediment-dwelling organisms (MacDonald et al., 2000; Sahli et al., 2021). According to the consensus-based sediment quality criteria, the mean quotient (m-PEC-Qs) of measured metal concentrations predicted sediment samples to be toxic (m-PEC-Qs ˃ 0.5), indicating a high potential harmful effect to benthic fauna (MacDonald et al., 2000; Niu et al., 2009). Thus, all the studied sediments were potentially toxic to freshwater organisms.
The sediment toxicity tests indicated that F. candida responded differently to the sediment samples. Indeed, the ecotoxicity results provided the same type of information as the SQGs except for the sediments of Elands River where the expected toxic effects on the reproduction of F. candida could not be observed. It was observed that the collembolans exposed to 100% sediment from the Elands River significantly increased their numbers compared to 75–100% sediment from Metsi-Matsho middle. The improvement of reproductive performance may be related to additive (beneficial) effects of certain metal ions from treated effluents discharged in the river. Regarding the treated effluents discharged in the Elands River, study by Moloi et al. (2020) reported significantly high concentrations of certain metal ions in water samples after discharge, in particular Mg, Fe, Pb and Zn. Indeed, in the present study, the micronutrient Zn steadily increased from the upper to the lower reaches of the rivers with its highest concentration found in the joint lower site of the Elands River (Table 4). Cu, the other micronutrient assessed, had one of its highest concentrations, when compared to the main rivers, in the joint lower site as well (Table 4).
The EC50 results were markedly the lowest for the upper Metsi-Matsho (24%) compared to the other sites (Table 5). Interestingly, according to the consensus-based SQGs (MacDonald et al., 2000) the sediments from the upper Metsi-Matsho exhibited a complex mixture of two metals (Cr and Ni) with concentrations above PEC. The SQGs have been designed for aquatic organisms (e.g., Chironomus riparius), however, using other organisms (e.g., terrestrial ones, see Höss et al., 2010) may make this approach a promising one for evaluating sediment toxicity using terrestrial organisms too. Folsomia candida has been found a suitable candidate for the assessment of trace metals in sediments (Cesar et al., 2015a; Vezzone et al., 2018). A study by Vezzone et al. (2018) comparing dredged sediments doses of 0%, 2.5%, 5%, 10%, and 20% from the Rodrigo de Freitas Lagoon (Brazil) supplemented with either artificial soil (70% of quartz sand, 20% of kaolin and 10% of coconut shells dust) or natural soils (chernosol, ferralsol), found a reproduction EC50 values of 2.8 and 4.9%, which are far lower than those found in this study (24, 54, 60, and > 100%, Table 5). In a previous study, Cesar et al. (2015a), amended dredged sediments from the Guanabara Bay basin in Brazil with chernosol and ferralsol and estimated EC50 value of 9.52%.
In the present study, generally, the mixture of sediments with OECD soil tended to decrease the reproduction of collembolans, except for the joint lower site (the Elands River) whose sediment was found to be the optimum breeding matrix for F. candida. Perhaps the combination and levels of metals at this site made the pure sediment (i.e., 100% of the Elands River sample) most conducive to oogenesis in the test organism.
In the present study, no information on the geological properties of the sediments was recorded. Apart from sediment properties, the contribution of other contaminants in the sediments might have influenced the observed results. However, it is unlikely to successfully measure all the toxicants present in such complex river systems, especially given the interest in metals shown in this study. Our approach nevertheless revealed the intricate ecotoxic dynamics between sediments collected at different reaches of the rivers of interest, with surprisingly low toxic effects in the upper site assessed.
Conclusion
In this study, the quality of sediments across all the river sites was assessed with the aid of the combination of F. candida bioassay and sediment quality guidelines (SQGs) for freshwater ecosystems. The hindering effects of the sediment samples on the reproduction of F. candida indicate existing toxicological threats to aquatic species in these rivers, especially sediment-dwelling ones. Cr contamination particularly far exceeded the prohibition level in the Namahadi and Elands Rivers, as well as the concentration levels previously reported in South African rivers. The diverse sources of river pollution in the study area could be wastewater effluents from the Phuthaditjhaba wastewater treatment plant, domestic and industrial waste disposal in waterways, runoff from agricultural lands, leaching from landfill sites near the rivers, and atmospheric depositions amongst others. The findings of this study highlight the need for uniquely South African sediment quality guidelines for freshwater systems and the adoption of macroinvertebrate bioassays from various trophic levels for the assessment of sediment quality.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Akhtar, N., Syakir, M. I., Bhawani, S. A., & Umar, K. (2021). Various natural and anthropogenic factors responsible for water quality degradation: A review. Water, 13, 2660. https://doi.org/10.3390/w13192660
Awofolu, O. R., Mbolekwa, Z., Mtshemla, V., & Fatoki, O. S. (2005). Levels of trace metals in water and sediment from Tyume River and its effects on an irrigated farmland. Water SA, 31, 87–94. https://doi.org/10.4314/wsa.v31i1.5124
Bellinger, P. F., Christiansen, K. A., Janssens, F. (2023). 1996–2023. Checklist of the Collembola of the world. http://www.collembola.org. Accessed 25 Januray 2024.
Benettayeb, A., & Haddou, B. (2023). New biosorbents based on the seeds, leaves and husks powder of Moringa oleifera for the effective removal of various toxic pollutants. International Journal of Environmental Analytical Chemistry, 18(103), 6859–6884. https://doi.org/10.1080/03067319.2021.1963714
Benettayeb, A., Ghosh, S., Usman, M., Seihoub, F. Z., Sohoo, I., Chia, C. H., & Sillanpää, M. (2022a). Some well-known Alginate and Chitosan modifications used in adsorption: A review. Water, 14, 1353. https://doi.org/10.3390/w14091353
Benettayeb, A., Usman, M., Tinashe, C. C., Adam, T., & Haddou, B. (2022b). A critical review with emphasis on recent pieces of evidence of Moringa oleifera biosorption in water and wastewater treatment. Environmental Science and Pollution Research, 29, 48185–48209. https://doi.org/10.1007/s11356-022-19938-w
Benettayeb, A., Seihoub, F. Z., Pal, P., Ghosh, S., Usman, M., Chia, C. H., Usman, M., & Sillanpää, M. (2023). Chitosan nanoparticles as potential nano-sorbent for removal of toxic environmental pollutants. Nanomaterials, 13, 447. https://doi.org/10.3390/nano13030447
Binning, K., & Baird, D. (2001). Survey of heavy metals in the sediments of the Swartkops River Estuary, Port Elizabeth South Africa. Water SA, 27, 461–466. https://doi.org/10.4314/wsa.v27i4.4958
Canadian Council of Ministers of the Environment (2001). Canadian sediment quality guidelines for the protection of aquatic life: Summary tables; Canadian environmental guidelines; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada.
Cesar, R., Natal-da-Luz, T., Sousa, J. P., Colonese, J., Bidone, E., Castilhos, Z., Egler, S., & Polivanov, H. (2014). Disposal of dredged sediments in tropical soils: Ecotoxicological effects on earthworms. Environmental Monitoring Assessment, 186, 1487–1497. https://doi.org/10.1007/s10661-013-3468-9
Cesar, R., Natal-da-Luz, T., Bidone, E., Castilhos, Z., Polivanov, H., & Sousa, J. P. (2015a). Disposal of dredged sediments in tropical soils: Ecotoxicological evaluation based on bioassays with springtails and enchytraeids. Environmental Science and Pollution Research, 22, 2916–2924. https://doi.org/10.1007/s11356-014-3559-3
Cesar, R., Natal-da-Luz, T., Silva, F., Bidone, E., Castilhos, Z., Polivanov, H., & Sousa, J. P. (2015b). Ecotoxicological assessment of a dredged sediment using bioassays with three species of soil invertebrates. Ecotoxicology, 24, 414–423. https://doi.org/10.1007/s10646-014-1390-8
Crouau, Y., Chenon, P., & Gisclard, C. (1999). The use of Folsomia candida (Collembola, Isotomidae) for the bioassay of xenobiotic substances and soil pollutants. Applied Soil Ecology, 12(2), 103–122. https://doi.org/10.1016/S0929-1393(99)00002-5
Culik, M. P., & Zeppelini, D. (2003). Diversity and distribution of Collembola (Arthropoda: Hexapoda) of Brazil. Biodiversity Conservation, 12(6), 1119–1143. https://doi.org/10.1023/A:1023069912619
Daskalakis, K. D., & O’connor, T. P. (1995). Normalization and elemental sediment contamination in the coastal United States. Environmental Science and Technology, 29(2), 470–477. https://doi.org/10.1021/es00002a024
Degroot, A. J., Zschuppe, K. H., & Salomons, W. (1976). Standardization of methods of analysis for heavy metals in sediments. Hydrobiologia, 92, 689–695.
EC, MENVIQ (Environment Canada and Ministere de l’Envionnement du Quebec) (1992). Interim criteria for quality assessment of St. Lawrence River sediment. Environment Canada.
Edokpayi, J. N., Odiyo, J. O., Popoola, O. E., & Msagati, T. A. M. (2016). Assessment of trace metals contamination of surface water and sediment: A case study of Mvudi River. South Africa. Sustainability, 8, 135. https://doi.org/10.3390/su8020135
Gerber, R., Smit, N. J., van Vuren, J. H. J., Nakayama, S. M. M., Yohannes, Y. B., Ikenaka, Y., Ishizuka, M., & Wepener, V. (2015). Application of a sediment quality index for the assessment and monitoring of metals and organochlorines in a premier conservation area. Environmental Science and Pollution Research, 22, 19971–19989. https://doi.org/10.1007/s11356-015-5206-z
Handschin, E. (1955). Considérations sur la position systématique des Collemboles., Mémoires de la Société Royale d'Entomologie de Belgique, Tome Vingt-Septième.
Heininger, P., Höss, S., Claus, E., Pelzer, J., & Traunspurger, W. (2007). Nematode communities in contaminated river sediments. Environmental Pollution, 146(1), 64–76. https://doi.org/10.1016/j.envpol.2006.06.023
Höss, S., Haitzer, M., Traunspurger, W., Gratzer, H., Ahlf, W., & Steinberg, C. (1997). Influence of particle size distribution and content of organic matter on the toxicity of copper in sediment bioassays using Caenorhabditis elegans (Nematoda). Water, Air, and Soil Pollution, 99, 689–695.
Höss, S., Ahlf, W., Fahnenstich, C., Gilberg, D., Hollert, H., Melbye, K., et al. (2010). Variability of sediment-contact tests in freshwater sediments with low-level anthropogenic contamination—Determination—of toxicity thresholds. Environmental Pollution. https://doi.org/10.1016/j.envpol.2010.05.013
International Organization For Standardisation (ISO) (1999). Soil quality – inhibition of reproduction of Collembola (Folsomia candida) by soil pollutants. ISO 11267, Geneva, Switzerland.
Jackson, V. A., Paulse, A. N., Odendaal, J. P., & Khan, W. (2009). Investigation into the metal contamination of the Plankenburg and Diep Rivers, Western Cape. South Africa. Water SA, 35(3), 289–299. https://doi.org/10.4314/wsa.v35i3.76766
Ju, Y.-R., Chen, C.-F., Lim, Y. C., Tsai, C.-Y., Chen, C.-W., & Dong, C.-D. (2022). Developing ecological risk assessment of metals released from sediment based on sediment quality guidelines linking with the properties: A case study for Kaohsiung Harbor. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2022.158407
Kingston, H. M., & Walter, P. J. (1998). The art and science of microwave sample preparations for trace and ultratrace elemental analysis. In A. Montaser (Ed.), Inductively coupled mass spectrometry (pp. 33–81). Wiley-VCH.
Krige, S. (1995). Demographic profile of the Free State. University of the Free State.
Liedtke, S. (2018). DTI completes R50m phase 1 upgrade of Phuthaditjhaba Industrial Park. http://www.engineeringnews.co.za.
Long, E. R., Morgan, L. G. (1991). The potential for biological effects of sediment-sorbed contaminants tested in the National Status and Trends Program. NOAA Technical Memorandum NOS OMA 52, National Oceanic and Atmospheric Administration, Seattle, WA
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39, 20–31. https://doi.org/10.1007/s002440010075
McKee, M. S., Engelke, M., Zhang, X., Lesnikov, E., Koser, J., Eickhorst, T., & Filser, J. (2017). Collembola reproduction decreases with aging of silver nanoparticles in a sewage sludge-treated soil. Frontiers in Environmental Science, 5, 19. https://doi.org/10.3389/fenvs.2017.00019
Moeletsi, M. E., & Walker, S. (2012). Rainy season characteristics of the Free State province of South Africa with reference to rain-fed maize production. Water SA, 38, 775–782. https://doi.org/10.4314/wsa.v38i5.17
Moloi, M., Ogbeide, O., & Voua Otomo, P. (2020). Probabilistic health risk assessment of heavy metals at wastewater discharge points within the Vaal River Basin, South Africa. International Journal of Hygiene and Environmental Health. https://doi.org/10.1016/j.ijheh.2019.113421
Mosolloane, P. M., Bredenhand, E., & Voua Otomo, P. (2019). Laboratory assessment of the ecotoxic effects of sewage sludge from the Maluti-Drakensberg region on a terrestrial oligochaete species. Ecotoxicology, 28(1), 86–91. https://doi.org/10.1007/s10646-018-2002-9
Motholo, L. F. (2014). Characterization of macro-and micro-invertebrates and assessment of water quality in dams and rivers of QwaQwa. Master Dissertation, University of the Free State.
Niu, H., Deng, W., Wu, Q., & Chen, X. (2009). Potential toxic risk of heavy metals from sediment of the Pearl River in South China. Journal of Environmental Sciences, 21(8), 1053–1058. https://doi.org/10.1016/s1001-0742(08)62381-5
OECD (Organization for Economic Co-operation and Development) (2004). Guideline for testing of chemicals No. 222, Earthworm ReproductionTest (Eisenia fetida/andrei). Paris, France.
Persaud, D., Jaagumagi, R., Hayton, A. (1993). Guidelines for the protection and management of aquatic sediment quality in Ontario. Water Resources Branch, Ontario Ministry of the Environment, Toronto.
Sahli, L., Belhiouani, H., Pérez, K. F., Okki, M. E. H. E., Afri-Mehennaoui, F.-Z., Férard, J.-F., & Mehennaoui, S. (2021). Assessment of freshwater sediment quality: Potential ecological risk and ecotoxicity tests as complementary approaches. Chemistry and Ecology, 37(3), 219–233. https://doi.org/10.1080/02757540.2020.1853106
Smith, S. L., MacDonald, D. D., Keenleyside, K. A., Ingersoll, C. G., & Field, J. (1996). A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. Journal of Great Lakes Research, 22(3), 624–638. https://doi.org/10.1016/S0380-1330(96)70985-1
Stats SA (2011). Phuthaditjhaba: http://www.statssa.gov.za/?page_id=4286&id=7197. (Accessed 14 May 2023).
Tietjen, J. H., & Lee, J. J. (1984). The use of free-living nematodes as a bioassay for estuarine sediments. Marine Environmental Research, 11(4), 233–251.
Traunspurger, W., Haitzer, M., Höss, S., Beier, S., Ahlf, W., & Steinberg, C. (1997). Ecotoxicological assessment of aquatic sediments with Caenorhabditis elegans (nematoda): a method for testing liquid medium and whole-sediment samples. Environmental Toxicology and Chemistry, 16(2), 245–250. https://doi.org/10.1002/etc.5620160221
USEPA (United States Environmental Protection Agency) (1997b). An assessment of sediments from the Upper Mississippi River. Final report—June, 1997. EPA 823-R-97–005, Columbia, MO.
USEPA (United States Environmental Protection Agency) (1996). Method 3052: Microwave assisted acid digestion of siliceous and organically based sediments. In: Test methods for evaluating solid waste, physical/chemical methods - SW-846. Washington, DC.
USEPA (United States Environmental Protection Agency) (1997a). The incidence and severity of sediment contamination in surface waters of the United States. Volume 1: National sediment quality survey. EPA 823-R-97–006, Office of Science and Technology, Washington, DC.
USEPA (United States Environmental Protection Agency) (2007). Method 1699, pesticides in water, soil, sediment, biosolids, and tissue by HRGC/HRMS. DC 20460 USA.
Ustaoğlu, F., & Tepe, Y. (2019). Water quality and sediment contamination assessment of Pazarsuyu Stream, Turkey using multivariate statistical methods and pollution indicators. International Soil and Water Conservation Research, 7(1), 47–56. https://doi.org/10.1016/j.iswcr.2018.09.001
Vezzone, M., Cesar, R., Polivanov, H., Serrano, A., Siqueira, D., Abreu, L., Bianchi, M., Correia, M. E., Castilhos, Z., & de Campos, T. (2018). Ecotoxicological evaluation of dredged sediments from Rodrigo de Freitas Lagoon (Rio de Janeiro State, Brazil) using bioassays with earthworms and collembolans. Environmental Earth Sciences, 77, 743. https://doi.org/10.1007/s12665-018-7930-4
Woin, P., & Brönmark, C. (1992). Effect of DDT and MCPA (4-chloro-2-methylphenoxyacetic acid) on reproduction of the pond snail, Lymnaea stagnalis L. Bulletin of Environmental Contamination and Toxicology, 48(1), 7–13. https://doi.org/10.1007/BF00197476
World Health Organization (WHO) (2003). The right to water. http://www2.ohchr.org/english/issues/water/docs/Right_to_Water.pdf
Yabe, J., Ishizuka, M., & Unemura, T. (2010). Current levels of heavy metal pollution in Africa. Journal of Veterinary Medical Science, 72(10), 1257–1263. https://doi.org/10.1292/jvms.10-0058
Acknowledgements
We thank an anonymous reviewer for her/his valuable comments towards the improvement of this manuscript. This study was supported by National Research Foundation (NRF), award number MND190920478274.
Funding
Open access funding provided by University of the Free State. This study was supported by National Research Foundation (NRF), award number MND190920478274.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
This study is part of a larger project entitled “Ecotoxicological and bacteriological assessment of water resources in the Afromontane region of the eastern Free State”. Approved by the Animal Research Ethics of the University of the Free State (South Africa) on 03-May-2016. Project Number: UFS-AED2016/0067.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kamdem, M.M., Kubheka, N., Nyoka, N.WK. et al. Using Folsomia candida (Collembola) for the ecological assessment of sediment samples from three rivers from the QwaQwa region, South Africa. Int J Energ Water Res (2024). https://doi.org/10.1007/s42108-024-00282-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42108-024-00282-3