Abstract
Purpose
The aim was to assess the image quality (IQ) in computed tomography angiography (CTA) of the thoracoabdominal aorta utilizing automated tube voltage selection (ATVS) with a subsequently adapted contrast media (CM) injection protocol.
Materials and methods
A total of 104 consecutive patients referred for CTA of the thoracoabdominal aorta were included. Scans were acquired on a 3rd-generation DSCT using ATVS with a quality reference tube voltage and current of 100 kV and 150 mAs. CM protocols were adapted to kV settings by modifying iodine delivery rate (IDR) whilst maintaining an identical injection time (13.3 s): 0.9 gI/s (70 kV), 1.0 gI/s (80 kV), 1.1 gI/s (90 kV) 1.2 gI/s (100 kV). Both objective (attenuation, contrast-to-noise and signal-to-noise) and subjective (4-point Likert scale: 1 = poor/2 = sufficient/3 = good/4 = excellent) IQ were assessed.
Results
ATVS assigned a 70 kV (n = 88) and 90 kV (n = 16) protocol in most patients. Fewer patients were assigned to an 80 kV (n = 4) and 100 kV (n = 1) protocol, these protocols were, therefore, excluded from further analysis. Attenuation on all designated levels of the thoracoabdominal aorta was diagnostic in 99.0% of the scans; 87/88 scans (98.9%) in the 70 kV group and in 16/16 (100%) in the 90 kV group. Overall mean attenuation was 349 ± 72HU for 70 kV and 310 ± 43HU for 90 kV. Subjective IQ was diagnostic in all scans. Overall effective radiation dose for 70 kV and 90 kV was 1.8 ± 0.2 mSv and 3.4 ± 0.7 mSv, respectively.
Conclusions
Adaptation of CM injection protocols (IDR) to ATVS in CTA of the aorta—scanned with 70 kV and 90 kV—is feasible and results in diagnostic image quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Computed Tomography Angiography (CTA) of the aorta is an important imaging method with high diagnostic accuracy for the detection of various aortic diseases [1,2,3,4]. High diagnostic accuracy relies on sufficient intravascular iodine attenuation and contrast-to-noise ratio (CNR) [5]. These factors depend on various features regarding contrast media (CM) administration (e.g. iodine concentration and iodine delivery rate [IDR]), scan protocol (e.g. tube voltage and tube current) and patient related factors (e.g. body weight, heart rate and cardiac output) [6]. Body weight has a substantial impact on vascular attenuation. A fixed injection protocol causes a large variation between different weight groups indicating suboptimal use of CM in different patient weight categories. Patients with a low body weight require less CM volume to be injected in comparison to patients with a higher body weight for diagnostic attenuation. Therefore, optimisation of scan and injection protocols is a must, and various beforementioned features can be used. With regard to the CM injection protocol, the IDR is considered the most important factor [7].
One potential disadvantage of CTA of the aorta is the possible development of post-contrast acute kidney injury (PC-AKI) after CM administration. A higher CM volume might increase the risk of developing PC-AKI, especially in patients with multiple pre-existent risk factors for PC-AKI undergoing CTA of the aorta [8]. Although the development of PC-AKI has been a topic of discussion [9, 10], guidelines still advise to use as low CM volumes as possible. With the optimisation of CM injection protocols, patients only receive the amount of CM volume needed for a diagnostic scan. Another concern is about the long-term, stochastic risks of CT radiation dose, especially in younger patients undergoing frequent follow-up examinations in aneurysm surveillance or endovascular treatment follow-up [3, 11, 12]. Therefore, in CT imaging the “as low as reasonably achievable” (ALARA) principle always applies. Current scanner technology provides sufficient tube current output on lower kV levels, which aids in reducing overall radiation dose. Furthermore, automated tube voltage selection (ATVS) allows for maintained image quality (IQ) with lower overall radiation doses for patients [13,14,15].
ATVS is a software program which automatically adapt scan settings to the individual patient. ATVS uses scout scans (with information on patient size and tissue density) to automatically adjust the kV setting and mAs to reach a certain CNR level. For slimmer patients, a lower kV setting is selected, and for bigger patients, a higher kV setting. The use of lower kV settings is accompanied by higher iodine attenuation, explained by the fact that mean photon energy (of the lower kV) in the X-ray beam moves closer to the K-edge of Iodine (33.2 keV) [16]. Vice versa, a higher kV setting is accompanied by a lower iodine attenuation. Therefore, the CM injection protocol should be adjusted to the kV setting. With a lower tube voltage, a smaller amount of CM can be applied to achieve the same intravascular attenuation level [17,18,19,20]. Canstein et al. found that with every 10 kV subtraction, the iodine dose can be reduced by 10% (10-to-10 rule) and vice versa [21]. This was tested and proven feasible in a previous study at 80 kV and 100 kV by adjusting the IDR [22].The aim of this study was to assess feasibility and image quality of CM injection protocols (IDR) adapted to ATVS in a cohort of patients undergoing CTA of the aorta.
Materials and methods
Ethics and patient population
The study design was approved by the local ethical committee and the institutional review board. A waiver of written informed consent was obtained from the local ethical committee (METC 15-4-247). Between January 2016 and April 2017, all patients referred for CTA of the thoracoabdominal aorta were considered eligible for inclusion. A non-randomized study design was deemed appropriate, since ATVS takes the patient size and scan length into account when assigning the optimal kV setting.
The following exclusion criteria for CTA in our department were applicable; allergy to iodinated CM, pregnancy, and renal insufficiency (defined as glomerular filtration rate (GFR) < 30 mL/min/1.73 m2). Furthermore, patients were excluded if a combined examination protocol was applied, e.g. additional portal venous assessment of the upper abdomen, as this required a different CM protocol with larger volumes for parenchymal enhancement.
Patient characteristics (age, sex, weight, height and BMI) were collected and analysed. Patients were analysed in groups according to the kV setting designated by automated tube voltage selection (ATVS; CAREkV™, Siemens Healthineers, Forchheim, Germany): the possible kV settings in this study ranged from 70 to 120 kV, in increments of 10 kV.
CTA scan protocol
Scans were performed using a 3rd generation dual-source CT scanner (DSCT, Somatom Force, Siemens) with a slice collimation of 2 × 192 × 0.6 mm; gantry rotation time of 0.5 s. A pitch value of 0.6 was chosen to increase scan time and prevent the scanner from outrunning the CM bolus in the aorta. The scan acquisition time varied depending on scan range. All patients received a scan protocol with a quality reference tube voltage of 100 kVref and quality reference tube current of 150 mAsref (CareDose 4D™, Siemens). The slider position in the current study was set at 11; the vascular setting. Scan delay was determined via bolus tracking by placing a circular region of interest (ROI) in the descending aorta at the level of the pulmonary trunk. A threshold of 100 HU was used with a post bolus tracking delay of 5 s for table movement followed by a breath hold command. Image reconstruction was performed on 1 mm slices with an increment of 0.8 mm using an iterative reconstruction vascular kernel (Bv36; Advanced Modelled Iterative Reconstruction [ADMIRE], strength 3).
CAREkV™ is ATVS software developed to reduce radiation dose while keeping CNR constant [15]. Scout-scans and the resulting patient attenuation are used to calculate patient-specific mAs curves for all possible kV settings. These mAs curves are composed with help of different parameters, e.g. scan length, patient anatomy, scan type and tissue of interest. The scanner calculates estimated radiation dose and the optimal dose efficiency in terms of kV and mAs combination is determined for each individual patient [23].
Radiation dose monitoring software (Radimetrics™, Bayer) was used to record the CTDIvol (mGy), dose-length product (DLP; mGy*cm) and the effective dose (mSv) for each CTA. The software estimates the effective dose using patient age and sex to determine accurate tissue weighing factors as described by the ICRP publication 103 [24].
CM injection protocol
CM consisted of 300 mg 1/mL Iopromide (Ultravist, Bayer Healthcare, Berlin, Germany), prewarmed to body temperature (37 °C; 99 °F), injected using a dual-head CT power injector (Stellant, Bayer).
Patients received a CM injection protocol according to the selected kV setting. The various CM injection parameters are listed in Table 1. A clinically validated CM injection protocol for 80 kV was used and adapted for the other kV settings (70 kV and 90 kV, 100 kV, 110 kV and 120 kV) [22]. The IDR of 80 kV was known, and for every 10 kV increment subtraction or addition, the IDR was decreased or increased with 10% [21]. Since the CM concentration was known and a fixed injection time (IT) of 13.3 s was used, the other injection protocol parameters could be calculated. A saline chaser was injected afterwards with a volume of 40 mL at the same flow rate as the CM bolus.
Data analysis
All CT-images were analysed using axial slices and multi-planar reconstruction (MPR) on a dedicated post processing workstation (Syngo-Via™, Siemens Healthineers). To assess the true axial planes perpendicular to the vascular wall on multiple pre-defined levels of the aorta, a centreline was automatically drawn in the aorta and both right and left iliac and femoral arteries. Curved multiplanar reconstructions (MPRs) were used to draw region of interests (ROIs) in the aorta (for examples, see Figs. 1 and 2).
Example of curved MPR of the thoracoabdominal aorta. Patient with a BMI of 28 kg/m2; scan protocol: 90 kV, 164 mAseff, CTDIvol 4.75 mGy, effective radiation dose of 4 mSv, image quality was graded excellent. Three-dimensional reconstruction of the entire aorta (left) shows an abdominal aortic aneurysm (AAA). Vessel definition, with help of a centreline drawn in the aorta (right), and axial views (small boxes) on the same anatomical level (stripe) also show a short dissection. The axial views are used to measure intravascular attenuation, and vessel wall and plaques can be avoided with more precision
Example of curved MPR of the thoracoabdominal aorta. Patient with a BMI of 20 kg/m2; scan protocol: 70 kV, 172 mAseff, CTDIvol 2.02 mGy, effective radiation dose of 1.6 mSv, image quality was graded excellent. Three-dimensional reconstruction of the entire aorta (left) shows an abdominal aortic aneurysm (AAA). The axial views are used to measure intravascular attenuation, and vessel wall and plaques can be avoided with more precision
Image analysis: primary endpoint
The primary endpoint was the proportion of scans with a mean intravascular attenuation above 200 HU at multiple pre-defined vascular segments in the aorta (see below). The minimal diagnostic attenuation value for the evaluation of aortic pathologies is considered to be 200 HU [25, 26]. Objective image quality analysis was performed by one radiology resident (N.E, 5 years of experience) by measuring intravascular attenuation in Hounsfield Units (HU) in predefined vascular segments. ROI’s were manually drawn as large as possible in the ascending aorta (AA), aortic arch (Arch), descending aorta (DA), abdominal aorta (AbA), right and left common iliac artery (RCIA and LCIA) and right and left common femoral arteries (RCFA and LCFA) [20]. Special care was taken to avoid measurements of the vessel wall, stents and atherosclerotic plaques. Image noise was defined as the standard deviation (SD) of the intravascular attenuation and measured at the level of the AbA [27]. Overall mean intravascular attenuation was defined as the mean of the sum of all different anatomic levels in one scan. Overall mean intravascular attenuation (per-patient) and attenuation at the different anatomic levels (per-segment) were reported.
Image analysis: secondary endpoints
CNR was calculated as intravascular attenuation minus intramuscular attenuation, divided by the SD of the intramuscular attenuation [19, 20, 22, 28]. SNR was calculated as intravascular attenuation divided by the SD of the intravascular attenuation [19, 22, 27].
Subjective image quality was determined by two readers in consensus (N.E. and M.D.) using a four-point Likert scale with regard to intravascular attenuation, presence of artefacts and image noise: 1 = non-diagnostic image quality, 2 = sufficient image quality, 3 = good image quality, 4 = excellent image quality.
Statistical analysis
Statistical analysis was performed with Statistical Package for the Social Sciences (IBM SPSS statistics, Chicago IL, USA, version 25.0). Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables as absolute numbers and percentages (%). Categorical variables were expressed as absolute numbers and percentages.
Results
Patient characteristics
A total of 153 consecutive patients referred for CTA of the thoracoabdominal aorta were considered eligible for inclusion. A total of 44 patients were excluded because aberrant scan parameters or a different CM injection protocol was used (n = 42; e.g. a multiphase scan protocol was used instead of the requested study protocol), or because the aortic tract was not depicted in its entirety (n = 2). The use of ATVS resulted in the following group distribution: 88 patients (80.7%) for 70 kV, four patients (3.7%) for 80 kV, 16 patients (14.7%) for 90 kV and one patient for 100 kV (0.9%). ATVS did not select a 110 kV or 120 kV setting for any patient. Since the 80 and 100 kV groups consisted of less than five patients each, these were excluded for detailed statistical analysis. Thus, a total of 104 patients were included for further analysis.
Mean age in the 70 kV and 90 kV group was 68.2 ± 12.5 years and 66.5 ± 11.6 years, respectively. Mean weight was 78.5 ± 16.8 kg in the 70 kV group and 83.6 ± 19.7 in the 90 kV group. A total of 52 patients (50%) had a BMI ≥ 25 kg/m2 (40 patients in the 70 kV group and 12 patients in the 90 kV group), the rest of the patients had a BMI < 25 kg/m2. All patient characteristics are depicted in Table 2.
Radiation dose and injection parameters
All radiation dose parameters are listed in Table 3. Mean effective radiation dose was 1.8 ± 0.2 mSv in the 70 kV group and 3.4 ± 0.7 mSv in the 90 kV group.
Mean CM volumes and flow rates for the different kV settings were 39.9 ± 0.9 mL and 2.9 ± 0.1 mL/s for 70 kV and 48.9 ± 0.1 mL and 3.6 ± 0.0 mL/s for 90 kV (Table 4).
Image quality-per-patient level
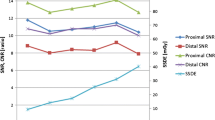
Mean intravascular attenuation on a per-patient level was diagnostic (≥ 200 HU) in 99.0% of all scans; mean intravascular attenuation was diagnostic in 87/88 scans (98.9%) in the 70 kV group and in all scans (16/16; 100%) in the 90 kV group. In one patient of the 70 kV group, the scanner outrunned the bolus. Hence, the attenuation level was below 200 HU in four distal segments (i.e. RCIA, LCIA, RCFA, LCFA). Overall mean attenuation for different kV groups was 349 ± 72 HU for 70 kV and 310 ± 43 HU for 90 kV. Overall mean CNR was 15 ± 6 for the 70 kV and 18 ± 7 for the 90 kV group. Overall mean SNR was 11 ± 4 for the 70 kV and 14 ± 4 for the 90 kV group.
All image quality parameters are depicted in Table 5.
Image quality per-segment level
Attenuation on a per-segment level was diagnostic in 94.8% of all scans (≥ 200 HU). Figures 3 and 4 show the attenuation and corresponding CNR values at the different anatomic levels of the aorta. Attenuation fell below 200 HU in the abdominal and lower levels of the aorta in 13 scans of the 70 kV (14.8%) and two scans of the 90 kV group (12.5%) (AbA, RCIA, LCIA, RCFA and LCFA) due to the scanner outrunning the bolus (n = 10; 8 in 80 kV and 2 in 90 kV), aortic stenosis (n = 2), aortic dissection (n = 2) and high BMI (29.7 kg/m3) with a 70 kV protocol (n = 1).
Discussion
Adaptation of IDR to ATVS resulted in a diagnostic mean overall intravascular attenuation of the thoracoabdominal aorta, a sufficiently high CNR and a sufficient SNR in nearly all (99.0%) scans. Furthermore, the use of ATVS predominantly resulted in an image acquisition with low kV settings (e.g. 70 kV and 90 kV). By using the attenuation effect of low kV settings drawing closer to the K-edge of iodine, CM volumes of 40 mL (70 kV) and 46 mL (90 kV) were used without loss of diagnostic overall image quality.
The current study shows that with IDR adapted to ATVS, a diagnostic objective and subjective image quality can be achieved in nearly all scans. In some scans the attenuation fell < 200 HU due to the scanner outrunning the CM bolus, a large abdominal aortic aneurysm (AAA), an aortic stenosis resulting in a decreased attenuation, an extensive thoracoabdominal dissection were the contrast filled the false instead of the true lumen, or a patient with a high BMI (29.7 kg/m2) scanned with 70 kV, where a low CM volume of 40 mL was administered. Figure 3 shows a lower mean intravascular attenuation in the peripheral arteries and a relatively large SD in the 70 kV group. The first can be explained by the scanner outrunning the CM bolus peripherally in the patients with a large aneurysm or significant stenosis. In this situation, it is possible that the table speed was too fast, and a longer CM bolus (e.g. 46 mL in the 90 kV group) or a decreased flow rate is needed to widen the peak of the enhancement curve to prevent this from happening. Another solution could be the use of a double-level test bolus to calculate the individual injection duration [29]. The wide SD could be due to the large variations in body size between the patients. Patient with a high body weight (or BMI) have an increased blood volume compared to slimmer patients. Since CM is diluted by the circulating blood, an increased blood volume causes more diluted CM [30]. Thus, an increased CM volume, for instance via additional optimisation to body weight, is needed in bigger patients to reach the same intravascular attenuation. Furthermore, the use of low kV settings increases the image noise in bigger patients, caused by the increased volume of surrounding tissue. A higher IDR and larger CM volume in patients with a higher BMI is needed. Indeed, although the study group was small, the 90 kV group had no cases of non-diagnostic scans.
Previously used one-size-fits-all protocols, where every patient receives the same amount of CM volume (ca. 120 mL) with the same kV setting (120 kV), resulted in suboptimal image quality [22, 31]. Technical development in scan technique allow for usage of lower kV settings (70–90 kV). These lower kV settings, coming closer to the K-edge of iodine (33.2 keV), result in a higher intravascular attenuation [16]. Thus, lower CM volumes can be used with a 70 kV scan acquisition compared to 120 kV. In the current study, the CM injection protocol was adapted to the tube voltage by adjusting the IDR based on a previously used CM injection protocol [22]. With every 10 kV subtraction, the iodine dose can be reduced by 10% (10-to-10 rule) [21]. When using ATVS, the 10-to-10 rule can be used to adapt the CM injection protocol to tube voltage in CTA of the aorta.
The diagnostic image quality required for CTA of the aorta varies per indication; for instance, assessment of aorta diameters can be accomplished even at relatively low image quality, whereas assessment of aortic dissection, rupture or endoleakage post-EVAR requires the full picture. In the current study, the objective and subjective image quality was good to excellent in 99% of patients. Therefore, the proposed protocols may be used for all indications.
Disadvantages of CT imaging and administering CM are the use of radiation dose and the possible development of PC-AKI. Even though there is an ongoing discussion regarding the potential development of PC-AKI, guidelines still advocate preventive hydration in high-risk individuals to minimise the risk of PC-AKI [32, 33]. In addition, overall CM volume reduction is a straight forward method to minimise the risk as well. The current study showed that with adaptation of CM injection protocols to ATVS, CM volumes of 40 mL and 49 mL for respectively 70 kV and 90 kV are feasible. Furthermore, a low radiation dose is important in patients who require lifelong follow-up, to keep the cumulative radiation dose as low as possible in these patients. In the current study, ATVS assigned a tube voltage of 70 kV and 90 kV to almost all patients and this resulted in a radiation dose of 1.8 mSv for 70 kV and 3.4 mSv for 90 kV. Unfortunately, the patient groups of 80 kV and 100 kV where too small for statistical analysis and had to be excluded. Higher kV settings (110 kV and 120 kV) were not assigned. An important factor contributing to lower tube voltage in this study is the pitch setting used; 0.6. CT scanners have a limitation with regard to the maximum photon output in a certain amount of time, which usually corresponds to a tradeoff between pitch and maximum tube output [34]. A low pitch corresponds to slower table movement and thus longer scan time. The scanner has enough time to provide a sufficiently high tube current to reach the desired diagnostic image quality. Consequently, a lower kV setting can be chosen by the software. With a high pitch the scan time decreases, the tube may not be able to provide a high enough tube current per second (mAs) required at low kV settings.
In the current study, the scanner assigned 70 kV and 90 kV to most patients. Previous studies with ATVS also found that a majority of CTA scans can be performed on the lower spectrum of possible kV settings (80–90 kV) [15, 35,36,37]. These studies did not scan any patient with 70 kV, whereas in our study 70 kV was assigned in the majority of patients. Several explanations can be found: the different quality reference kV and mAs, the slider position and pitch, the latter explained above. The quality reference kV and mAs can be set pre-acquisition to determine a user preferred image quality. Based on these references, the scanner calculates the optimal tube settings (kV and mAs) for each patient to reach a comparable image quality (CNR) as with the reference settings. With a higher reference kV and/or mAs, higher tube settings are needed to ensure the same image quality and the software will increase kV and/or mAs.
This study has some limitations. First, it is a single-centre study with a small population. A larger cohort might result in a larger number of patients chosen in higher kV settings (e.g. 100 kV and 110 kV). Furthermore, a larger cohort would improve the power of the results. Secondly, this study was executed as a feasibility study and therefore did not focus on a direct comparison with a control group.
To conclude, adaptation of IDR to ATVS (70 kV and 90 kV) in CTA of the aorta is feasible and results in diagnostic image quality.
Abbreviations
- PC-AKI:
-
Post-contrast acute kidney injury
- ATVS:
-
Automated tube voltage selection
- BMI:
-
Body mass index
- CM:
-
Contrast media
- CNR:
-
Contrast-to-noise ratio
- CTA:
-
Computed tomography angiography
- DSCT:
-
Dual source computed tomography
- IDR:
-
Iodine delivery rate
- IQ:
-
Image quality
- SNR:
-
Signal-to-noise ratio
References
Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(41):2873–926. https://doi.org/10.1093/eurheartj/ehu281.
Agarwal PP, Chughtai A, Matzinger FR, Kazerooni EA. Multidetector CT of thoracic aortic aneurysms. Radiographics. 2009;29(2):537–52. https://doi.org/10.1148/rg.292075080.
Soares Ferreira R, Bastos GF. Next generation post EVAR follow-up regime. J Cardiovasc Surg (Torino). 2017;58(6):875–82. https://doi.org/10.23736/S0021-9509.17.10100-X.
Hallett RL, Ullery BW, Fleischmann D. Abdominal aortic aneurysms: pre- and post-procedural imaging. Abdom Radiol (New York). 2018;43(5):1044–66. https://doi.org/10.1007/s00261-018-1520-5.
Behrendt FF, Bruners P, Keil S, Plumhans C, Mahnken AH, Stanzel S, et al. Impact of different vein catheter sizes for mechanical power injection in CT: in vitro evaluation with use of a circulation phantom. Cardiovasc Intervent Radiol. 2009;32(1):25–31. https://doi.org/10.1007/s00270-008-9359-8.
Bae KT, Tran HQ, Heiken JP. Uniform vascular contrast enhancement and reduced contrast medium volume achieved by using exponentially decelerated contrast material injection method. Radiology. 2004;231(3):732–6. https://doi.org/10.1148/radiol.2313030497.
Mihl C, Wildberger JE, Jurencak T, Yanniello MJ, Nijssen EC, Kalafut JF, et al. Intravascular enhancement with identical iodine delivery rate using different iodine contrast media in a circulation phantom. Invest Radiol. 2013;48(11):813–8. https://doi.org/10.1097/RLI.0b013e31829979e8.
Lameire NH. Contrast-induced nephropathy—prevention and risk reduction. Nephrol Dial Transplant. 2006;21(6):i11-23. https://doi.org/10.1093/ndt/gfl215.
Aycock RD, Westafer LM, Boxen JL, Majlesi N, Schoenfeld EM, Bannuru RR. Acute kidney injury after computed tomography: a meta-analysis. Ann Emerg Med. 2018;71(1):44–53. https://doi.org/10.1016/j.annemergmed.2017.06.041.
Luk L, Steinman J, Newhouse JH. Intravenous contrast-induced nephropathy-the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169–75. https://doi.org/10.1053/j.ackd.2017.03.001.
Motta R, Rubaltelli L, Vezzaro R, Vida V, Marchesi P, Stramare R, et al. Role of multidetector CT angiography and contrast-enhanced ultrasound in redefining follow-up protocols after endovascular abdominal aortic aneurysm repair. Radiol Med. 2012;117(6):1079–92. https://doi.org/10.1007/s11547-012-0809-x.
Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–84. https://doi.org/10.1056/NEJMra072149.
Bischoff B, Hein F, Meyer T, Hadamitzky M, Martinoff S, Schomig A, et al. Impact of a reduced tube voltage on CT angiography and radiation dose: results of the PROTECTION I study. JACC Cardiovasc Imaging. 2009;2(8):940–6. https://doi.org/10.1016/j.jcmg.2009.02.015.
Meinel FG, Canstein C, Schoepf UJ, Sedlmaier M, Schmidt B, Harris BS, et al. Image quality and radiation dose of low tube voltage 3rd generation dual-source coronary CT angiography in obese patients: a phantom study. Eur Radiol. 2014;24(7):1643–50. https://doi.org/10.1007/s00330-014-3194-x.
Winklehner A, Goetti R, Baumueller S, Karlo C, Schmidt B, Raupach R, et al. Automated attenuation-based tube potential selection for thoracoabdominal computed tomography angiography: improved dose effectiveness. Invest Radiol. 2011;46(12):767–73. https://doi.org/10.1097/RLI.0b013e3182266448.
Mahesh M. MDCT physics: the basics: technology, image quality and radiation dose. 1st ed. Philadelphia: Lippincott Williams and Wilkins; 2009.
Funama Y, Awai K, Nakayama Y, Kakei K, Nagasue N, Shimamura M, et al. Radiation dose reduction without degradation of low-contrast detectability at abdominal multisection CT with a low-tube voltage technique: phantom study. Radiology. 2005;237(3):905–10. https://doi.org/10.1148/radiol.2373041643.
Nakayama Y, Awai K, Funama Y, Hatemura M, Imuta M, Nakaura T, et al. Abdominal CT with low tube voltage: preliminary observations about radiation dose, contrast enhancement, image quality, and noise. Radiology. 2005;237(3):945–51. https://doi.org/10.1148/radiol.2373041655.
Kok M, Mihl C, Hendriks BM, Altintas S, Kietselaer BL, Wildberger JE, et al. Optimizing contrast media application in coronary CT angiography at lower tube voltage: evaluation in a circulation phantom and sixty patients. Eur J Radiol. 2016;85(6):1068–74. https://doi.org/10.1016/j.ejrad.2016.03.022.
Kok M, Mihl C, Seehofnerova A, Turek J, Jost G, Pietsch H, et al. Automated tube voltage selection for radiation dose reduction in CT angiography using different contrast media concentrations and a constant iodine delivery rate. Am J Roentgenol. 2015;205(6):1332–8. https://doi.org/10.2214/AJR.14.13957.
Korporaal LG. Reduction of contrast agent dose at low kV settings. 2017.
Kok M, de Haan MW, Mihl C, Eijsvoogel NG, Hendriks BM, Sailer AM, et al. Individualized CT angiography protocols for the evaluation of the aorta: a feasibility study. J Vasc Interv Radiol. 2016;27(4):531–8. https://doi.org/10.1016/j.jvir.2016.01.009.
SHwG K. CARE kV: automated dose-optimized selection of X-ray tube voltage. 2011. https://www.usahealthcaresiemenscom/siemens_hwemhwem_ssxa_websites-contextroot/wcm/idc/groups/public/@us/@imaging/documents/download/mdaw/ndq2/~edisp/low_dose_carekv-00308417pdf. Accessed 2017.
The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 2007;37(2–4):1–332. https://doi.org/10.1016/j.icrp.2007.10.003.
Bae KT. Optimization of contrast enhancement in thoracic MDCT. Radiol Clin N Am. 2010;48(1):9–29. https://doi.org/10.1016/j.rcl.2009.08.012.
Weininger M, Barraza JM, Kemper CA, Kalafut JF, Costello P, Schoepf UJ. Cardiothoracic CT angiography: current contrast medium delivery strategies. Am J Roentgenol. 2011;196(3):W260–72. https://doi.org/10.2214/AJR.10.5814.
Leipsic J, Labounty TM, Heilbron B, Min JK, Mancini GB, Lin FY, et al. Adaptive statistical iterative reconstruction: assessment of image noise and image quality in coronary CT angiography. Am J Roentgenol. 2010;195(3):649–54. https://doi.org/10.2214/AJR.10.4285.
Kok M, Turek J, Mihl C, Reinartz SD, Gohmann RF, Nijssen EC, et al. Low contrast media volume in pre-TAVI CT examinations. Eur Radiol. 2016;26(8):2426–35. https://doi.org/10.1007/s00330-015-4080-x.
Nishii T, Watanabe Y, Shimoyama S, Kono AK, Sofue K, Mori S, et al. Tailored duration of contrast material injection in high-pitch computed tomographic aortography with a double-level test bolus method. Invest Radiol. 2017;52(5):274–80. https://doi.org/10.1097/RLI.0000000000000340.
Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology. 2010;256(1):32–61. https://doi.org/10.1148/radiol.10090908.
Hendriks BM, Kok M, Mihl C, Bekkers SC, Wildberger JE, Das M. Individually tailored contrast enhancement in CT pulmonary angiography. Br J Radiol. 2016;89(1061):20150850. https://doi.org/10.1259/bjr.20150850.
Nijssen EC, Rennenberg RJ, Nelemans PJ, Essers BA, Janssen MM, Vermeeren MA, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non-inferiority trial. Lancet. 2017;389(10076):1312–22. https://doi.org/10.1016/S0140-6736(17)30057-0.
Nijssen EC, Nelemans PJ, Rennenberg RJ, van Ommen V, Wildberger JE. To hydrate or not to hydrate? Lessons learned from the AMACING trial. Chin J Acad Radiol. 2019;1(1):2–5. https://doi.org/10.1007/s42058-019-00008-z.
Tack D, Kalra MK, Gevenois PA. Radiation dose from multidetector CT. Berlin: Springer; 2012.
Goetti R, Winklehner A, Gordic S, Baumueller S, Karlo CA, Frauenfelder T, et al. Automated attenuation-based kilovoltage selection: preliminary observations in patients after endovascular aneurysm repair of the abdominal aorta. Am J Roentgenol. 2012;199(3):W380–5. https://doi.org/10.2214/AJR.11.8029.
Lurz M, Lell MM, Wuest W, Eller A, Scharf M, Uder M, et al. Automated tube voltage selection in thoracoabdominal computed tomography at high pitch using a third-generation dual-source scanner: image quality and radiation dose performance. Invest Radiol. 2015;50(5):352–60. https://doi.org/10.1097/RLI.0000000000000133.
Nijhof WH, Baltussen EJ, Kant IM, Jager GJ, Slump CH, Rutten MJ. Low-dose CT angiography of the abdominal aorta and reduced contrast medium volume: assessment of image quality and radiation dose. Clin Radiol. 2016;71(1):64–73. https://doi.org/10.1016/j.crad.2015.10.007.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JEW receives institutional grants from AGFA, Bard, Bayer, GE, Optimed, Philips and Siemens and personal fees (speakers bureau) from Bayer and Siemens. MD receives institutional grants from Philips, Siemens and Bayer and personal fees (speakers bureau) from Cook, Siemens and Bayer. CM receives personal fees (speakers bureau) from Bayer and Siemens.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eijsvoogel, N.G., Hendriks, B.M.F., Kok, M. et al. Automated tube voltage selection and adapted contrast media injection protocols in CT angiography of the thoracoabdominal aorta. Chin J Acad Radiol 4, 116–124 (2021). https://doi.org/10.1007/s42058-021-00065-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42058-021-00065-3