Abstract
Purpose
Treatment guidelines for gender-affirming hormone therapy with estrogen (GAHT-E) recommend specific dosing regimens based on limited data. Well-controlled efficacy trials are essential to tailoring treatment to patient goals as the guidelines recommend. The goal of this study was to take a foundational step toward designing community-centered effectiveness trials for gender-diverse individuals seeking GAHT-E.
Methods
Our team developed a cross-sectional survey based on broad clinical experience and consultation with our community advisory board. The survey included 60 items covering demographics, transition history, goals and priorities for treatment, indicators of treatment success, sexual function goals, and future research priorities. The survey was distributed during the summer of 2021, primarily through social networks designed for gender-expansive individuals seeking treatment with estrogen.
Results
A total of 1270 individuals completed the survey. Overall treatment goals most frequently rated “extremely important” or “very important” were the following: (1) improved satisfaction with life (81%), (2) appearing more feminine (80%), (3) appearing less masculine (77%), (4) improved mental health (76%), and (5) being seen as your true gender by others (75%). The three body characteristics most frequently rated “highest priority” or “high priority” among changes were the following: (1) facial hair (85%), (2) breast shape or size (84%), and (3) body shape (80%). The highest-rated research priority was comparing feminization with different routes of estrogen administration.
Conclusion
The goals and experiences of individuals seeking GAHT-E are diverse. Future clinical trials of GAHT-E should be grounded in the needs and priorities of community stakeholders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatment guidelines for gender-affirming hormone therapy (GAHT) recommend dosing regimens with the caveat that treatment should be tailored to patients’ goals; however, there are few data enabling us to ascertain the efficacy of any specific regimen for achievement of patient-centered endpoints [1,2,3]. Published prospective cohort studies evaluating physical changes achieved via GAHT with estrogen (GAHT-E) offer preliminary evidence of efficacy but are limited by uncontrolled variables [4,5,6,7].
Despite the increase in the types of evidence acceptable for regulatory decisions in the USA, the existing evidence remains inadequate for approval of drugs indicated for GAHT. Well-controlled clinical trials are the gold standard for assessing safety, absolute efficacy, and comparative efficacy of treatment but as of March 28th, 2023, a PubMed search revealed only one randomized controlled trial of GAHT [8]. Burinkul and colleagues demonstrated the feasibility of comparative efficacy trials for GAHT but did not measure patient-centered endpoints such as anatomical change or patient-reported outcome measures (PROMs) [8]. This low quality and volume of evidence is widely cited in treatment guidelines [2, 3], systematic reviews [1], and reports from stakeholder engagement events [9].
A foundational problem for building the nascent literature was succinctly summarized in a report from an FDA community listening session as follows: “… participation would be dependent on the goal/endpoint of the [study]. [Participants] noted that there isn’t a common goal shared by people who identify as trans” [10]. Community members are unlikely to participate in research irrelevant to their treatment goals, and trans and gender-expansive communities have widely varying goals. Thus, investigators must navigate the following two essential decisions: (1) defining and describing appropriately homogenous subgroups for assessing physiological changes and (2) defining “efficacy” to reliably and validly represent their treatment goals [11, 12]. This report describes an initial step in answering these questions in collaboration with community stakeholders.
Methods
Having chosen to focus first on GAHT-E, our research group designed a cross-sectional survey of experiences, expectations, and priorities of individuals seeking GAHT-E in order to prioritize research questions and treatment outcomes. Because few data and no validated survey measures exist on this topic, our research group developed a novel questionnaire based on clinical experience and in consultation with our institution’s community advisory board (CAB). A non-provider team member attended regularly scheduled CAB meetings for advice and feedback. The team subsequently met with two CAB members possessing personal GAHT-E experience to collaboratively review and edit the survey draft, focusing on refining language and broadening the experiences represented; CAB members were paid an honorarium for their time and expertise.
Population definition
Our research group sought to prioritize affirmation, concision, clarity, and precision in defining the target population. Our goal was to specify a group likely to have relatively similar endogenous hormone environments and treatment responses while affirmatively including individuals of varied identities and experiences. Our study thus focused on the chosen treatment modality over the inherent characteristics of participants by defining the primary inclusion criterion as “seeking GAHT-E.” Gender identity and GAHT-E were not referred to as “feminizing” to avoid assumptions or implications of binary gender. Instead, GAHT-E was defined as “medications taken for the purpose of altering your body toward body characteristics such as fat distribution across the body, breast growth, and finer body hair. This can include medications like estrogen, spironolactone, or others.”
CAB input and clinical experience indicated that defining this population by natal sex inherently invalidated their lived identities; thus, our research group sought to develop a population definition without reference to natal sex. Our team was unable to develop another approximation of endogenous hormone environment that was as clear and concise—though lacking precision—as the widely used “sex assigned at birth.” CAB members suggested “sex listed on your original birth certificate,” which was abbreviated to “listed ‘male’ at birth” or “LMAB,” hoping its similarity to the established term “assigned male at birth” or “AMAB” would enhance clarity while reducing invalidating and oppressive connotations.
Our final population definition included individuals listed as “male” on their original birth certificates who were 18 years of age or older and seeking GAHT-E. For initial communication (e.g., flyers), the target population was described as “treatment-seeking trans and gender-expansive people listed as ‘male’ at birth.” GAHT-E was not specified for the sake of concision, reasoning that GAHT-E experience or interest would, in any case, be common among respondents. Additionally, the research group did not want to exclude individuals interested only in other treatments, including androgen blockade monotherapy or surgical therapies.
Survey design
This study was reviewed and approved by the UCLA South General Institutional Review Board. Our survey of 60 items covering demographics, transition history, treatment goals and experiences, and research priorities was distributed using Qualtrics, an online survey tool. As an incentive and token of gratitude, individuals could enter a raffle for one of eight gift cards regardless of participation. To prevent linking survey responses to identifiable raffle entries, a separate entry form accessible only through a restricted link provided upon completion or declination of the survey was created.
Prior to any questions, the electronic survey presented study information, including a statement that participation was voluntary and an item requesting consent to continue. To ensure respondents met inclusion criteria, three initial screening items excluded respondents who (1) did not consent, (2) were younger than 18 years of age, or (3) were listed as “female” or “intersex” on their original birth certificates. Built-in tools of the Qualtrics platform were utilized to discourage multiple participation and bot responses as well as to remove identifiers, such as IP address, from the final dataset.
Item text and response options were based on personal experiences, patient reports, and CAB consultation. Given the paucity of existing data to guide scale development, face-valid single items were created to be analyzed individually. Several items included an additional free-text option to solicit additional information where available response items were insufficient. For affirmation and brevity, logic structures were used to avoid displaying questions not applicable to the respondent. For example, participants were asked if they had a penis prior to questions about erectile function, which were then displayed only following an affirmative response. Broadly inclusive definitions of terms were utilized (e.g., defining “sex” as “any activity you use for erotic stimulation whether you are alone or with another person. This includes masturbation, oral sex with a partner, penetrative sex, and a wide range of other activities”).
In contrast, some items were designed restrictively to simplify future analyses. For instance, the complex reality of gender identity was balanced with the statistical simplicity of finite, mutually exclusive choices with the item “With which of these gender identities do you most identify?” Insight on item acceptability and utility was sought through the “a gender not listed here” option, which included a free-text field.
Recruitment
The survey was distributed as follows: by (1) emailing patients who previously agreed to receive research opportunities, (2) sharing survey flyers with community allies, and (3) social media and community forum posts. By utilizing our community connections and prioritizing online outreach, the study team hoped to reach a more diverse convenience sample than our institution’s primarily white, non-Hispanic, and privately insured population [13]. A team member engaged with relevant online communities led an outreach effort focused on groups created by and for treatment-seeking individuals using relevant terms (e.g., “MtF,” “transfeminine,” and “GAHT”).
Analysis
Using SAS version 9.4 (SAS Institute, Cary, NC, USA), data was examined for anomalous response patterns such as duplicate responses and outliers. Once data was determined to be free of such artifacts, built-in analysis and reporting tools on the Qualtrics platform were used to produce descriptive statistics. Given our descriptive goal, the study did not include a priori hypotheses or conduct significance tests. Instead, we sought to describe response variance and free-text additions. Categorical responses are reported as proportions of responders to respective individual items. For items with many free-text responses, answers were categorized by simple topic (e.g., body part described) and reported by number of respondents addressing each topic. The recommendations in the Checklist for Reporting of Survey Studies (CROSS) were followed in developing this report [14].
Results
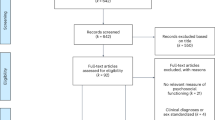
Of 1729 individuals who accessed and responded to at least one screening or consent item, 1270 eligible individuals (73%) completed the survey between June 8 and July 31, 2021. Details for participant eligibility are displayed in Fig. 1.
Demographics
Respondent demographics are presented in Table 1. Though 90% of respondents reached the survey via social media, our recruitment strategy generally did not meet our goal of a sample more diverse than that of our local program. Predominantly, respondents were white (77%), under age 35 (84%), and residing in the USA (81%).
Regarding gender identity, one respondent commented that “transgender female” did not make sense because “female” is a sex category, while “woman” is a gender category. Another respondent also added “transgender woman” as free text. Overall, 14 individuals (1%) selected “a gender not listed here.” Of associated free-text responses, only “demigirl,” listed twice, was listed more than once.
Treatment goals
Figure 2 displays the overall goals of gender-affirming treatment. Treatments are listed in order of the frequency with which they were rated “extremely important.” Similarly, physical features prioritized for change are listed in Fig. 3. Free-text responses are described in Table 2.
Subjective efficacy indicators
Table 3 lists potential experiences during GAHT-E in the order of frequency endorsed as indicators as to whether GAHT-E was working or not. Free-text positive indicators added by multiple respondents included qualitative changes to sexuality, changes in body odor, changes to face shape, and reduced head hair loss. Additions to negative indicators were similar to (e.g., changes in body odor) or opposite of (e.g., head hair loss) positive indicators.
Research priorities
From a list of potential research questions, respondents selected up to three as the most important. These are listed in Table 4 by proportion of respondents endorsing them. Research questions added in free-text responses were similar in their focus on assessing efficacy and safety, additionally suggesting comparing different types of estrogen, testing estrogen monotherapy, and measuring head hair loss as an outcome.
Discussion
This study emphasizes the immense variance in identities, experiences, and treatment goals among individuals seeking GAHT-E. For example, although respondents rated head hair as a “low priority” or “not a priority” more frequently than any other feature, nearly 60% still rated it as “highest priority” or “high priority.” Furthermore, reducing head hair loss was the only specific endpoint listed as a research priority in free-text responses. Similarly, while 16% of individuals felt that increased sex drive was a sign of treatment efficacy, 14% reported that decreased sex drive was a sign of efficacy. Speaking to the complexity of gender identity, 24% of our respondents identified as male or transgender male, and 87% of that group reported current or former use of GAHT-E compared to 82% of the entire sample. Their GAHT-E use indicates that this group was appropriately included and that centering inclusion criteria on treatment choice allowed us to recruit a group who may not have responded to identity-based recruitment or may have been excluded by identity-based screening items.
Just as informative are the commonalities in our data. Current guidelines for GAHT-E recommend matching treatment regimens with individualized goals, but research evaluating how to personalize treatment is lacking [2, 3]. Respondents’ highest-rated research priority was comparing routes of estrogen administration in facilitating feminization. Among known outcomes of GAHT-E, respondents most prioritized breast growth, facial and body hair reduction, and body shape changes. The highest prioritized overall treatment goal was “satisfaction with life.” These data provide a foundational step toward incorporating the needs of the community into future research and patient care by roughly outlining which treatment goals should be prioritized when designing efficacy studies. While a few prospective cohort studies have attempted to assess some of these differences in physical changes with varying levels or routes of estrogen [4,5,6], the results have been limited by uncontrolled variables. Active-control trials can provide vital information by varying a single aspect of treatment (e.g., route of administration).
An expansive review of methods to quantitatively assess prioritized outcomes of GAHT-E is outside the scope of this article; however, it should be noted that one substantial barrier to efficacy trials is that most available measures that were developed with cisgender samples have not been validated in gender-diverse populations, and when used have often been found to be of mixed utility in assessing GAHT-E effects [15,16,17,18,19,20]. To cite an example, most methods that evaluate breast growth assess volume, which translates easily as a continuous variable for analysis but may not represent patient preferences for overall breast shape [21]. Measurement tools should be grounded in the needs of the relevant population and thoroughly capture the patient experience [22]. The GENDER-Q, for instance, is a modular set of PROMs currently in field testing after development in collaboration with gender-diverse stakeholders which promises to be ideal for GAHT trials [23]. The GENDER-Q may be an ideal model to help update existing anatomical measurement tools to become more patient-centric.
Acknowledging diverse treatment goals is also a requisite for providing an informed, individualized approach to patient care. To illustrate, individuals who view increased libido as a sign of treatment failure may benefit from aggressive testosterone suppression. Conversely, individuals who want to maintain erectile function and libido may benefit from a liberal testosterone goal and early conversations about phosphodiesterase inhibitors. While it is not clear that adjusting testosterone goals directly affects libido, approaching treatment with an open mind, querying specific goals, and allowing patients room to explore such possibilities may improve the doctor-patient relationship, empower patients to be active participants in their care, and ultimately help providers gain understanding of ways to personalize treatment.
Eliciting patient goals at an initial intake visit can also elucidate which adjunctive therapies to utilize. For example, those who rate body hair reduction as a top priority should be referred for laser or electrolysis early in transition, while those who prioritize voice change warrant an early referral for voice therapy. Similarly, if androgenic alopecia is a large source of dysphoria, it may be worthwhile discussing the addition of minoxidil to initial hormone therapy. It is also important for providers to be mindful that not all individuals with gender incongruence desire hormone therapy. Discussing specific treatment goals may reveal that utilizing exclusively non-hormonal treatments such as scalp hair restoration surgery, laser hair removal, and voice therapy is more suitable for some.
Limitations
This report describes methods and findings of initial steps toward designing efficacy trials for GAHT and as such is limited in its generalizability. Because the survey was developed based on patient-reported experiences, personal experiences, and the advice of our CAB, our decisions were substantially shaped by our locality and the patients seen at our center. The study team had hoped to reach a broader population via global recruitment within online communities to assess broader validity, but ultimately our sample was substantially similar to those individuals seen in our clinics.
Although it speaks to the efficacy of our recruitment efforts, having 90% of participants recruited from social media may bias our results. Social media offer important spaces for building transgender, nonbinary, and other gender-expansive communities [24,25,26,27], but as with physical spaces, access is not always equitable [28, 29]. Further, the online groups from which survey respondents were recruited often focused on starting gender-affirming treatments, likely contributing to our sample’s relatively short duration of hormone therapy. The groups frequently included such terms as “MtF” or “transfeminine” in titles and descriptions and may have appealed less to nonbinary users; only 3.5% of our respondents reported identifying most with a nonbinary identity, while the Williams Institute estimated that 32.1% of transgender adults in the USA identify as non-binary [30].
Future studies should more directly target communities that our study left out, preferably via qualitative methods that allow participants greater freedom to express experiences different from those encoded in a structured questionnaire. Additionally, the majority of our study participants were aged 18–35. Future studies should target more specific age ranges to assess whether age affects goals and expectations. Our findings represent only a starting point for characterizing community research priorities; different methods are essential to broadening and deepening our understanding.
Conclusions
Well-controlled efficacy trials will be essential to gaining regulatory approval for the specific use of GAHT medications, refining clinical care guidelines, tailoring individual treatment plans, and ultimately understanding how to safely and effectively support gender-diverse patients. To best do so, trial designs must be grounded in the needs, priorities, and experiences of actual GAHT users. Based on our collaboratively developed community survey, the medical community should prioritize trials comparing estrogen administration routes in their effects on breast growth, body hair, body shape, and overall well-being. Our findings can serve as a starting point for GAHT-E trials and our methods as one approach to meeting the challenge of their design.
Data availability
Data supporting the findings in this report are fully anonymous and available upon reasonable request to the corresponding author.
References
Haupt C, Henke M, Kutschmar A et al (2020) Antiandrogen or estradiol treatment or both during hormone therapy in transitioning transgender women. Cochrane Database Syst Rev 11(11):Cd013138. https://doi.org/10.1002/14651858.CD013138.pub2
Coleman E, Radix AE, Bouman WP et al (2022) Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health 23(Suppl 1):S1–s259. https://doi.org/10.1080/26895269.2022.2100644
Hembree WC, Cohen-Kettenis PT, Gooren L et al (2017) Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 102(11):3869–3903. https://doi.org/10.1210/jc.2017-01658
de Blok CJM, Dijkman BAM, Wiepjes CM et al (2021) Sustained breast development and breast anthropometric changes in 3 years of gender-affirming hormone treatment. J Clin Endocrinol Metab 106(2):e782–e790. https://doi.org/10.1210/clinem/dgaa841
de Blok CJM, Klaver M, Wiepjes CM et al (2018) Breast development in transwomen after 1 year of cross-sex hormone therapy: results of a prospective multicenter study. J Clin Endocrinol Metab 103(2):532–538. https://doi.org/10.1210/jc.2017-01927
Klaver M, de Blok CJM, Wiepjes CM et al (2018) Changes in regional body fat, lean body mass and body shape in trans persons using cross-sex hormonal therapy: results from a multicenter prospective study. Eur J Endocrinol 178(2):163–171. https://doi.org/10.1530/eje-17-0496
Tebbens M, Nota NM, Liberton N et al (2019) Gender-affirming hormone treatment induces facial feminization in transwomen and masculinization in transmen: quantification by 3D scanning and patient-reported outcome measures. J Sex Med 16(5):746–754. https://doi.org/10.1016/j.jsxm.2019.02.011
Burinkul S, Panyakhamlerd K, Suwan A et al (2021) Anti-androgenic effects comparison between cyproterone acetate and spironolactone in transgender women: a randomized controlled trial. J Sex Med 18(7):1299–1307. https://doi.org/10.1016/j.jsxm.2021.05.003
National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) (2020) Second Annual SGM Health Research Listening Session. https://dpcpsi.nih.gov/sites/default/files/2020%20SGM%20Health%20Research%20Listening%20Session%20Summary%20Document.pdf. Accessed
U.S. Food and Drug Administration (2021) Healthcare challenges and unmet medical needs of transgender adults - FDA-requested listening session (Session #1). Patient listening sessions summaries. https://www.fda.gov/media/153932/download. Accessed
Evans SR (2010) Fundamentals of clinical trial design. J Exp Stroke Transl Med 3(1):19–27. https://doi.org/10.6030/1939-067x-3.1.19
Yin G (2012) Clinical trial design: Bayesian and frequentist adaptive methods. Clinical Trial Design: Bayesian and Frequentist Adaptive Methods. https://doi.org/10.1002/9781118183335
Gaither TW, Williams K, Mann C et al (2022) Initial clinical needs among transgender and non-binary individuals in a large, urban gender health program. J Gen Intern Med 37(1):110–116. https://doi.org/10.1007/s11606-021-06791-9
Sharma A, Minh Duc NT, Luu Lam Thang T et al (2021) A consensus-based checklist for reporting of survey studies (CROSS). J Gen Intern Med 36(10):3179–3187. https://doi.org/10.1007/s11606-021-06737-1
Bennett JP, Liu YE, Quon BK et al (2022) Assessment of clinical measures of total and regional body composition from a commercial 3-dimensional optical body scanner. Clin Nutr 41(1):211–218. https://doi.org/10.1016/j.clnu.2021.11.031
Bulstrode N, Bellamy E, Shrotria S (2001) Breast volume assessment: comparing five different techniques. Breast 10(2):117–123. https://doi.org/10.1054/brst.2000.0196
Choppin SB, Wheat JS, Gee M et al (2016) The accuracy of breast volume measurement methods: a systematic review. Breast 28:121–129. https://doi.org/10.1016/j.breast.2016.05.010
Clennon EK, Martin LH, Fadich SK et al (2022) Community engagement and patient-centered implementation of patient-reported outcome measures (PROMs) in gender affirming surgery: a systematic review. Curr Sex Health Rep 14(1):17–29. https://doi.org/10.1007/s11930-021-00323-6
Oles N, Darrach H, Landford W et al (2022) Gender affirming surgery: a comprehensive, systematic review of all peer-reviewed literature and methods of assessing patient-centered outcomes (Part 1: Breast/Chest, Face, and Voice). Ann Surg 275(1):e52–e66. https://doi.org/10.1097/SLA.0000000000004728
Wierckx K, Gooren L, T'Sjoen G (2014) Clinical review: breast development in trans women receiving cross-sex hormones. J Sex Med 11(5):1240–1247. https://doi.org/10.1111/jsm.12487
O'Connell RL, Stevens RJ, Harris PA et al (2015) Review of three-dimensional (3D) surface imaging for oncoplastic, reconstructive and aesthetic breast surgery. Breast. 24(4):331–342. https://doi.org/10.1016/j.breast.2015.03.011
Crossnohere NL, Brundage M, Calvert MJ et al (2021) International guidance on the selection of patient-reported outcome measures in clinical trials: a review. Qual Life Res 30(1):21–40. https://doi.org/10.1007/s11136-020-02625-z
Klassen AF, Kaur M, Johnson N et al (2018) International phase I study protocol to develop a patient-reported outcome measure for adolescents and adults receiving gender-affirming treatments (the GENDER-Q). BMJ Open 8(10):e025435. https://doi.org/10.1136/bmjopen-2018-025435
Heinz M (2012) Transmen on the web: inscribing multiple discourses. In: Ross K (ed) The handbook of gender, sex, and media. p 326–343. https://doi.org/10.1002/9781118114254.ch20
Selkie E, Adkins V, Masters E et al (2020) Transgender adolescents’ uses of social media for social support. J Adolesc Health 66(3):275–280. https://doi.org/10.1016/j.jadohealth.2019.08.011
Cannon Y, Speedlin S, Avera J et al (2017) Transition, connection, disconnection, and social media: examining the digital lived experiences of transgender individuals. J LGBT Issues Couns 11(2):68–87. https://doi.org/10.1080/15538605.2017.1310006
Rothbaum B, Etengoff C, Uribe E (2022) Transgender community resilience on YouTube: constructing an informational, emotional, and sociorelational support exchange. J Community Psychol 50(5):2366–2384. https://doi.org/10.1002/jcop.22781
Haimson OL, Delmonaco D, Nie P et al (2021) Disproportionate removals and differing content moderation experiences for conservative, transgender, and black social media users: marginalization and moderation gray areas. Proc ACM Hum-Comput Interact 5(CSCW2):466. https://doi.org/10.1145/3479610
Hernandez K, Faith B (2023) Online but still falling behind: measuring barriers to internet use ‘after access’. Internet. Pol Rev 12(2). https://doi.org/10.14763/2023.2.1713
Wilson BD and Meyer IH (2021) Nonbinary LGBTQ adults in the United States. https://williamsinstitute.law.ucla.edu/wp-content/uploads/Nonbinary-LGBTQ-Adults-Jun-2021.pdf. Accessed
Author information
Authors and Affiliations
Contributions
SG: conceptualization, methodology, supervision, writing the original draft, reviewing, and editing. JW: conceptualization, methodology, writing the original draft, reviewing, and editing. KW: conceptualization, methodology, data curation, formal analysis, writing the original draft, reviewing, and editing. AW: conceptualization, methodology, reviewing, and editing. SF: conceptualization, methodology, reviewing, and editing. RP: reviewing and editing. AG: conceptualization. SK: conceptualization, methodology, supervision, reviewing, and editing.
Corresponding author
Ethics declarations
Consent to participate
Informed consent was obtained from all study participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grock, S., Weinreb, J., Williams, K.C. et al. Priorities for efficacy trials of gender-affirming hormone therapy with estrogen: collaborative design and results of a community survey. Hormones 23, 287–295 (2024). https://doi.org/10.1007/s42000-024-00532-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-024-00532-3