Abstract
Purpose
To analyze the expression of somatostatin receptor (SSTR)2a and 5 by immunohistochemistry (IHC) in surgically resected somatotrophic pituitary adenomas and to associate expression rates with tumor size and clinical, biochemical, and histological parameters and response to somatostatin analog (SA) therapy.
Methods
Forty-three microsurgically treated patients with histopathologically proven growth hormone (GH)–producing pituitary adenoma were included (WHO 2017). SSTR subtype expression was analyzed in adenoma tissues using monoclonal antibodies (Abcam, SSTR2a-UMB1, SSTR5-UMB4). Expression rates were classified as low (≤ 20% staining positivity), moderate (21–50%), and high (> 50%). Furthermore, biochemical parameters such as human growth hormone (hGH) and insulin-like growth factor-1 (IGF-1) levels were measured and clinical, biochemical, radiological, and histological data were evaluated.
Results
Of the 43 patients included in this study, 28 were female and 15 were male. The median age was 52 years (range 17–72 years). The median tumor size was 1.2 cm (range: 0.13–3.93 cm). All resected tumors showed positivity for somatotrophic hormone (STH). In all tissue samples, SSTR2a signal expression was detectable in immunohistochemistry, while only 39 samples were positive for SSTR5. Thirty-six samples had a high expression of SSTR2a, while three had a moderate and four a low SSTR2a signal. In comparison, SSTR5 signal was high in 26 out of 43 samples, while seven adenomas showed a moderate and six cases a low expression rate of SSTR5. The median IGF-1 was 714.2 µg/l and the median GH 19.6 mU/l (= 6.53 µg/l). The present study indicates that there is no significant relationship between the expression rates of receptor subtypes and the parameters we analyzed. However, our study revealed that smaller adenomas have a lower baseline GH level (p = 0.015),
Conclusion
IHC with monoclonal antibodies appears to be a suitable method to determine the expression rates of SSTR2a and 5 at protein levels, as it is not possible to draw conclusions regarding receptor subtypes solely on the basis of the parameters analyzed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acromegaly is a rare endocrinological disease caused by an excess of growth hormone (GH), also known as somatotropin. Predominantly, it is related to a benign GH-secreting tumor of the anterior pituitary gland [1]. Very rarely, malignant pituitary tumors lead to acromegaly. Disease prevalence is approximately 40–130 per million, and its annual incidence is estimated at three to four new cases per million individuals [2, 3]. The gender distribution is equal [4].
The therapy of pituitary adenomas is currently based on three different strategies, including transsphenoidal surgery (TSS), pharmacotherapy, and radiotherapy. TSS is the first-line treatment for patients with pituitary adenoma and acromegaly [5]. Full-remission rates up to 70% are reported in the current literature, using endoscopic and microscopic TSS, respectively [6, 7]. In all patients not suitable for surgery or in whom the tumor tissue could not be completely removed, first-generation long-acting somatostatin analogs (LA-SSAs octreotide and lanreotide) are regarded as the primary choice of pharmacotherapy [5]. In patients with resistance to first-generation somatostatin receptor ligands (SRL), pegvisomant, a GH receptor antagonist, and pasireotide, a second generation SRL, may be used [8, 9]. Current meta-analyses demonstrate that the response rate to SSAs is about 56% for GH and 55% for insulin-like growth factor-1 (IGF-1) normalization. In treatment-naïve patients, GH normalization can be achieved in about 40% [10, 11].
Besides their application in incomplete tumor resection, SSAs can be used for preoperative size reduction of pituitary adenomas, as these drugs induce a volume reduction of more than 20% in up to 75% of cases [10, 11].
Somatostatin (SST), in its biologically active forms SST14 and SST28, inhibits the secretion of GH [11]. The effect of SST is mediated by five distinct G-protein-coupled somatostatin receptors (SSTR), namely, SSTR1–5. SSTR2 und SSTR5 are the most prominent in somatotropic adenomas according to the current literature [10, 12,13,14]. Octreotide has the highest affinity to SSTR2 and a low affinity to SSTR3 and SSTR5. Lanreotide also shows the strongest affinity to SSTR2, followed by SSTR1, and shows low affinity to SSTR3 and SSTR5 [13, 15]. Pasireotide, however, has a high affinity to SSTR2 and 3 as well as to 5, and a moderate affinity to SSTR1 [14,15,16,17]. Due to these diverging affinities to SSTR subtypes, histopathological analysis of SSTRs may prove diagnostically significant.
In this study, we examined the expression rates of SSTR2a and 5 in surgically resected somatotropic pituitary adenomas by assessing signal intensity of monoclonal antibody-binding to cognate receptors. Immunohistochemistry (IHC) data were set in relation to tumor size, clinical chemistry, and response to SA therapy.
Material and methods
Patients/tumor samples
Forty-three patients, diagnosed with acromegaly, were included in this study. All patients underwent microscopic TSS for biochemical-proven acromegaly in our Department of Neurosurgery, University Medical Center Hamburg-Eppendorf, Germany, between July 2018 and June 2019 (Table 1). The diagnosis of acromegaly was made according to the current guidelines on acromegaly of the Endocrine Society. The guidelines recommend performing IGF-1 measurement in the presence of typical clinical characteristics and/or a pituitary tumor. Patients with an increased or suspect IGF-1 level should be subjected to an oral glucose tolerance test, which, in the case of inadequate suppression of GH plasma concentration levels, will confirm the diagnosis [5]. Finally, diagnosis was made by histopathological analysis of the resected tumor tissue samples (WHO classification 2017). Fourteen tumor samples revealed prolactin co-secretion determined by IHC and increased prolactin levels.
The tumor size was measured on the basis of an MRI, while invasion behavior was determined intraoperatively, as well as by imaging, and finally by histopathologic examination of separately submitted dura specimens and/or sphenoid mucosa. Further patient data, such as age and gender, were assessed.
Immunohistochemistry
The preparation of the tumor specimens was carried out according to standardized laboratory procedures established by the Institute for Neuropathology, University Medical Center Hamburg-Eppendorf. In brief, the intraoperatively obtained samples were fixed in a 4% buffered formalin solution at room temperature, followed by embedding in paraffin and cutting these blocks into 2-4 µm thick sections.
The immunohistochemical procedure to determine the expression rates of SSTR2a and SSTR5 was performed by using two monoclonal antibodies (Abcam, SSTR2a–UMB1 — dilution 1:1000 and SSTR5–UMB4 — dilution 1:200) with the automated Ventana BenchMark XT as specified by the manufacturer.
To assess the staining results of the IHC and thus the expression rate of SSTR2a and SSTR5, we classified the pituitary tumors into four groups in terms of their percentage of immunoreactive cells. Only cell membrane staining was considered positive. No proof of positive-stained cells corresponds to score 0. Up to 20% of immunoreactive cells correlates to score 1 (+ /low), 21–50% positive-stained cells (+ + /moderate) to score 2, and more than 50% positive cells (+ + + /high) to score 3 (Table 2, Fig. 1). In our study, we did not consider the intensity of the staining.
The IHC evaluation was performed by a single pathologist (W.S.) blinded to the clinical data.
Histological classification
Classification of somatotrophic pituitary adenomas into densely granulated (DG) and sparsely granulated (SG) tumors was performed using IHC and anti-cytokeratin antibodies (CAM5.2). Immunohistochemically detected fibrous bodies, which are keratin-positive, small, spherical cytoplasmic inclusions, are classified as SG tumors (Fig. 2). Accordingly, the absence of fibrous bodies characterizes DG adenomas.
IGF-1 and hGH measurement
IGF-1 and human growth hormone (hGH) in patient serum were determined using the Siemens Healthineers IMMULITE 2000XPi solid phase-chemiluminescence immunoassay according to the manufacturer’s instructions at the Institute of Clinical Chemistry and Laboratory Medicine. The Siemens hGH and IGF-1 Sandwich-ELISA tests are based on monoclonal murine-anti-hGH and anti-IGH-1 antibodies, respectively, coupled to beads. Reference range for hGH < 15 mU/l (conversion factor mU/l-µg/l 0.333) and for IGF-1 gender- and age-adjusted reference ranges were used. Measurements were taken prior to surgery, as well as on the first and third postoperative days. Postsurgical GH serum concentrations of < 0.1 µg/l (reported GH nadir without oral glucose tolerance test) indicate disease remission.
Ki-67%
The proliferation marker Ki-67, which is expressed exclusively on the surface of dividing cells, was determined immunohistochemically to evaluate the proliferation rate of the formalin fixed, paraffin embedded tumor samples. The antibody used in our study for detection was MIB-1 (Neo Markers, RM-9106-S, dilution 1:1000). The IHC was conducted with the automated Ventana Benchmark XT staining system, based on the manufacturers’ protocols. The evaluation of the proliferation rate was based on the percentage of positively stained cells. Less than 3% of positively stained cells correspond to a regular not significantly increased proliferation rate of somatotropic tumors, whereas more than 3% of positive cells indicate a significantly increased occurrence of dividing cells (Fig. 3).
Immunohistochemical detection of proliferation marker Ki-67 in formalin fixed, paraffin embedded pituitary adenoma samples. Assessment was based on the percentage of positively stained cells. Less than 3% indicates no increased proliferation rate (A, magnification 440 ×). More than 3% suggests an increased proliferation rate (B, magnification 440 ×)
Statistical analysis
All statistical analyses were performed using the statistical software “IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp.”
Qualitative data are presented as absolute and relative (%) frequencies, while quantitative data are presented as median (min, max). Spearman’s rho test was performed to determine whether the expression of SSTR2a and SSTR5 has an influence on the response to pharmacotherapy, measured by the IGF-1 (ULN), in patients pretreated with SSA. Using linear regression analysis with preoperative IGF-1 level and GH level as dependent variables, respectively, adjusted for age and gender, their relationship with parameters such as tumor size, histology, invasion behavior, and expression rates of SSTR2a and SSTR5 was assessed. For the analysis of correlations between the SSTR expression rates and nominal variables, the Fisher exact test was performed.
A P-value of < 0.05 was considered a statistically significant result.
Results
Patients
The tumor samples that were histologically examined were taken from 43 patients of whom 28 were female and 15 were male. The median age at time of surgery was 52 years (range 17–72 years). Macroadenomas, i.e., tumors larger than or equal to 1 cm, were detected in 25 cases, while microadenomas (< 1 cm) were found in 18 patients. The median tumor size was 1.2 cm (range 0.13–3.93 cm). The median of presurgically determined growth-hormone level was 19.6 mU/l (range 4.8–150 mU/l), while the median baseline IGF-1 level was 714.2 µg/l (range 139.4–1122 µg/l) and the median presurgical IGF-1 level (/ULN) was 3.12 µg/l (range 0.63–5.01 µg/l).
Pretreatment
Eight out of the 43 patients underwent a surgical intervention beforehand, of whom four received pharmacotherapy afterwards, which was maintained up to the next operation. One of them had combined therapy with cabergoline (dopamine D2-receptor agonist) and lanreotide (a SSA). The other three received octreotide (SSA). Two patients without previous surgical intervention received cabergoline, and another octreotide.
There was no significant influence of SSTR2a on the effect of pharmacotherapy with octreotide or lanreotide as assessed by age- and gender-adjusted IGF-1 concentration level after pharmacotherapy (ρ=-0.354, p = 0.559), while a more significant correlation with regard to SSTR5 was observed (ρ=-0.738, p = 0.155). However, there was just one patient who reached an age- and gender-normalized IGF-1 level under medical therapy (Table 3).
IGF-1/hGH
In terms of presurgical IGF-1 levels, no statistically significant relationship was found regarding tumor invasion, tumor size, or expression rates of SSTR2a and SSTR5 (Table 5).
As expected, a smaller tumor size was associated with lower GH levels (P = 0.015, Table 4). Out of 15 patients with microadenomas in whom a presurgical GH measurement was performed, 11 (73.33%) had GH values below 5 µg/l. In contrast, among patients with macroadenomas, only two out of 20 (10%) had a level of less than 5 µg/l.
Remission
After surgery, 20 of 42 patients (47.6%) showed a GH level of < 1 µg/l at the time of the second measurement (day 3). In seven of these cases, the value was below < 0.4 µg/l. In contrast, eight patients (19.1%) had a GH level of > 3.5 µg/l. Seven of them were macroadenomas (87.5%), of which two showed invasive growth. The GH level of the remaining 14 cases was between 1 µg/l and 3.5 µg/l, of which nine were below 2 µg/l. In one case, no results were present for the second measurement.
Forty patients showed a decrease in IGF-1 level, of whom seven (17.5%) had already reached an age-normalized IGF-1 serum concentration at the second postoperative measurement (day 3).
Pituitary function
In 38 cases (88%), there was no insufficiency of the pituitary axes after surgery. In most cases with postsurgical pituitary deficits, there was already a loss of function before intervention (80%; 4/5). Three patients showed insufficiency of the pituitary gland presurgically, which recovered afterwards. Depending on each case, functional disorders such as corticotropic, thyrotropic, gonadotropic insufficiencies, and/or central diabetes insipidus were present, and an appropriate therapy and follow-ups were initiated.
Histology
All 43 removed adenoma tissue samples used in our study stained immunohistochemically positive for somatotrophic hormone (STH), of which 14 were also positive for prolactin. A histological classification into DG and SG adenomas was made (DG 55.8%, SG 44.19%). An increased proliferation rate (Ki67 > 3%) was observed in 15 specimens (34.9%) (Table 1).
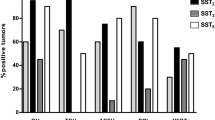
Expression of SSTR2a was detected in all tumor specimens, whereas SSTR5 expression was absent in four samples.
High expression rates for SSTR2a with more than 50% immunoreactive cells were found in 36 cases; in three samples, the number of positive cells was between 21 and 50%, which represents a moderate expression rate, and four had a low SSTR2a signal with only up to 20% positivity. Lower expression rates of SSTR2a were often associated with previous operations and occurred more frequently with SG tumors (Figs. 4 and 5). Out of seven tissue samples which showed a moderate or low expression rate with respect to SSTR2a, five were SG (71.4%). However, these characteristics were also present in samples with strong expression. Out of seven tumors that grew invasively, six (85.7%) had a high expression rate of SSTR2a.
In comparison, 26 out of 43 adenomas showed more than 50% positive-stained cells (high expression) regarding SSTR5, while seven samples showed moderate and six a low expression rate. With regard to SSTR5, lower or no expression was more frequent with DG tumors (76.5%, 13/17, Fig. 5), and DG were also positive for prolactin in 9/14 cases (64.3%). SG tumors with no or lower expression of SSTR5 were all, except for one, associated with prior surgery (75%, 3/4). Furthermore, a low level or no expression of SSTR5 was mostly associated with adenoma size smaller than 1 cm (70%, 7/10, Fig. 6).
With regard to prolactin co-secreting adenomas, 13 showed high expression of SSTR2a, and only one had a low SSTR2a expression rate. High expression of SSTR5, however, was seen in only six GH/PRL samples, while three showed moderate, three low, and two no expression of SSTR5.
Discussion
In this study, we investigated expression of SSTR2a and SSTR5 in surgically resected somatotropic adenomas of the pituitary gland.
The expression of somatostatin receptors in different tissues has been ascertained in other studies, mostly by RT-PCR, in situ hybridization, scintigraphy, receptor autoradiography, or IHC with polyclonal antibodies [18,19,20,21,22]. In our case, we used IHC with two monoclonal antibodies in an automated staining procedure. These specific antibodies have been shown to be sensitive and reliable in previous studies, and they show no cross-reaction with proteins other than the targeted ones, as can occur with polyclonal antibodies [23, 24]. As far as the automatic procedure is concerned, according to recent data, it seems to be an advantageous method for determining SSTR expression rates in tumor tissues [25].
Whereas several studies describe receptor expression rates at mRNA level, we performed receptor-protein analysis by using IHC [19, 26,27,28,29,30,31]. Some studies show a correlation between these two methods [29, 31, 32]. However, in this respect, the various studies are still ambiguous, which is probably due to the process that takes place to turn mRNA into a protein [32,33,34,35].
An advantage of IHC compared to other methods is its ability to provide information on the sub-cellular localization of SSTR [36, 37]. In our case, as expected for G-protein-coupled receptors, signals were mainly located on the cell membrane. Additionally, an immunoreactive score can be generated, which provides information about the SSTR expression rate (Table 2). In comparison to other studies, we did not consider the intensity of the staining, but concentrated exclusively on the percentage of immunoreactive cells to avoid a subjective assessment of the staining intensity [23, 28, 35, 36, 38, 39]).
The occurrence of SSTR2a and SSTR5 in all or at least the majority of somatotropic adenomas was reported in earlier studies [25,26,27, 35, 40, 41]. According to the literature, pharmacotherapy with somatostatin analogs to inhibit hormone secretion and reduce cell proliferation seems to be associated with the presence of mainly subtypes SSTR2 and SSTR5 [10, 12,13,14].
Our data showed that the majority of GH-secreting tumors studied had a high expression of both SSTR2a and SSTR5 (83.72% and 60.47% respectively), which is also consistent with previous publications [18, 25].
In contrast to the surveys of Thodou et al. and Jaquet et al., SSTR2a (100%) occurred more frequently compared to SSTR5 (90.7%) [18, 27]. Other authors, however, confirmed our result [25, 30, 35]. One reason for these differing findings may be the different methods used in the individual studies to determine the receptors’ expression.
With regard to the expression rates of SSTR2a and SSTR5, there is no significant difference between GH/PRL tumors and pure GH tumors. This is consistent with previous analyses, but differs from the research of Casarini et al., who found a higher incidence of SSTR5 in co-secreting adenomas [18, 19, 25, 27]. Unlike this result, our study showed that five out of ten tumors (50%) with no or low expression of SSTR5 are GH/PRL tumors. A further investigation of this aspect with a larger cohort is required to draw firmer conclusions regarding the therapy with pasireotide in GH/PRL tumors, a pharmaceutical substance with a high affinity to SSTR 5 [15, 17].
Contrary to our assumption, SSTR2a expression had no influence on the response to pharmacotherapy with octreotide or lanreotide measured by IGF-1 levels after long-term medication. There was a negative correlation between the gender- and age-adjusted IGF-1 levels and SSTR5 expression.
We also cannot corroborate on the basis of our study the results of Fougner et al. and Plöckinger et al. showing a possible effect of prior medical treatment on SSTR expression (mainly SSTR2a) [38, 40]. While these two research studies suggest SSTR2a reduction by presurgical SSA therapy, four out of five of our patients pretreated with SSAs exhibited high and one moderate SSTR2a expression levels.
These differing results are probably due to our limited number of five patients who received pharmacological pre-treatment with somatostatin analogs.
With regard to gender and age, there was no difference in SSTR2a and SSTR5 expression rate in our population.
In terms of SSTR2a expression, tumor size did not seem to play a significant role either. These results are in agreement with the findings of Plöckinger et al., as well as of Corbetta et al., who also failed to reveal a correlation between SSTR2 and tumor size [26, 40]. Even though our results did not show a statistically significant correlation between SSTR5 and tumor size, macroadenomas appear to have a slightly higher SSTR5 expression rate than microadenomas.
Additionally, while Corbetta et al. did not find any correlation between SSTR2 mRNA level and tumor invasiveness, our findings indicated that invasive tumors have a high expression of SSTR2a (85.71%, 6/7) [26]. This result is presumably due to our low number of invasively growing tumors.
The assumption that smaller adenomas have lower baseline GH level was confirmed in our present study (p = 0.015) and is in accordance with the previous report of Kaltsas et al. ([42]). Similarly to Casarini et al., however, we found no significant correlation between SSTR expression and pre-surgical GH and IGF-1 levels [19] (Tables 4 and 5).
Looking at the GH levels at time of the second postoperative measurement, GH suppression below 1 µg/l indicates a high likelihood of complete remission [43,44,45]. Values below 0.4 µg/l may have an even higher predictive value for remission [43].
In contrast, GH levels > 3.5 µg/l lead to relapse, with a very high chance of up to 100%, according to the aforementioned studies [43,44,45]. Our study showed that cases with GH values > 3.5 µg/l after surgery were mainly macroadenomas.
Regarding the histologic classification, the ratio in our study was 55.81% (DG) to 44.19% (SG). This result is in accordance with other studies in the literature, wherein a wide range of variation can be found, the latter probably due to the different ways of categorizing such tumors [41, 46,47,48].
Looking at the somatostatin receptor distribution among DG and SG tumors, our analysis revealed that the majority of DG (91.67%, 22/24) had high expression of SSTR2a, while it was slightly less in SG (73.68%, 14/19), which has been confirmed in several studies [25, 28, 29, 39, 49]. In comparison, 11 DG (45.83%, 11/24) and 15 SG (78.95%, 15/19) showed a high expression rate of SSTR5. This leads to the hypothesis that DG show a higher response to medical treatment with somatostatin analogs such as octreotide, which have a high affinity to SSTR2, as supported by the analysis of Ezzat et al. In their study, DG adenomas showed a higher decrease in GH levels by inhibition of GH secretion during pharmacotherapy with octreotide than did SG adenomas [50].
Similarly to the majority of previous reports, our findings demonstrated that SG compared to DG more often occurred at younger age and had a larger volume at the time of diagnosis, which may be partly explained by the increased Ki67 in SG and young patients, as this was seen in about half of the cases in our cohort [25, 29, 41, 46, 48, 51, 52].
In terms of invasiveness, however, there was no remarkable difference between SG (4/7 cases 57.14%) and DG (3/7cases 42.86%). In this respect, there is controversy among the various authors of previous studies. While in the study of Chinezu et al. there is evidence that SG have a more invasive character, the study of Brzana et al. shows no correlation between invasiveness and the different subtypes [25, 41]. One reason for these diverse research results could be the subjective assessment of tumor size and invasiveness based on an MRI.
Regarding gender, the majority of studies, including ours, could not find any correlation [28, 46, 47]. However, the study of Mazal et al. revealed that SG are more common in women [51].
Conclusion
Our study demonstrating that it is apparently not possible to determine receptor subtypes solely on the basis of the analysis of parameters, we determined that IHC is a useful method to ascertain the best possible treatment for each individual.
Our study demonstrates the feasibility of IHC examination of SSTR2a and SSTR5 in GH adenomas.
Today, TSS still is the first-line therapy for acromegaly. However, there are cases in which complete surgical removal of the tumor is not possible. In these cases, pharmacotherapy with somatostatin analogs is recommended. Since the response to medical treatment is to a certain extent related to the expression of SSTR in the target tissue, a reproducible method is necessary as a routine procedure for determination of SSTR expression. This is relevant in order to be able to determine which is the most effective SSA individually for each patient, since the various analogs have diverging affinities to SSTR2a and SSTR5. The technique of IHC with monoclonal antibodies, which we used in our study, may prove to be the most convenient method. Following further investigation into its potential, it could be established as the main method in histological procedures to facilitate decision-making as to the most efficacious medication.
Abbreviations
- DG:
-
Densely granulated
- hGH:
-
Human growth hormone
- IGF-1:
-
Insulin-like growth factor-1
- IHC:
-
Immunohistochemistry
- IRS:
-
Immunoreactive score
- LA-SSAs:
-
Long-acting somatostatin analogs
- PRL:
-
Prolactin
- SA:
-
Somatostatin analog
- SG:
-
Sparsely granulated
- SRL:
-
Somatostatin receptor ligands
- SST:
-
Somatostatin
- SSTR:
-
Somatostatin receptor
- STH:
-
Somatotrophic hormone
- TSS:
-
Transsphenoidal surgery
- ULN:
-
Upper limit of normal
References
Zahr R, Fleseriu M (2018) ‘Updates in diagnosis and treatment of acromegaly.’ Eur. Endocrinol 14(2):57. https://doi.org/10.17925/EE.2018.14.2.57
Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J (2009) Acromegaly. Best Pract Res Clin Endocrinol Metab 23(5):555–574. https://doi.org/10.1016/j.beem.2009.05.010
Holdaway IM, Rajasoorya C (1999) Epidemiology of acromegaly. Pituitary 2(1):29–41. https://doi.org/10.1023/A:1009965803750
Gurel MH, Han Y, Stevens AL, Furtado A, Cox D (2017) Treatment adherence and persistence with long-acting somatostatin analog therapy for the treatment of acromegaly: a retrospective analysis. BMC Pharmacol Toxicol 18(1):22. https://doi.org/10.1186/s40360-017-0124-y
Katznelson L et al (2014) Acromegaly: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 99(11):3933–3951. https://doi.org/10.1210/jc.2014-2700
Briceno V et al (2017) Efficacy of transsphenoidal surgery in achieving biochemical cure of growth hormone-secreting pituitary adenomas among patients with cavernous sinus invasion: a systematic review and meta-analysis. Neurol Res 39(5):387–398. https://doi.org/10.1080/01616412.2017.1296653
Sun H, Brzana J, Yedinak CG, Gultekin SH, Delashaw JB, Fleseriu M (2014) Factors associated with biochemical remission after microscopic transsphenoidal surgery for acromegaly. J Neurol Surg Part B Skull Base 75(1):47–52. https://doi.org/10.1055/s-0033-1354578
Gadelha MR et al (2014) Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol 2(11):875–884. https://doi.org/10.1016/S2213-8587(14)70169-X
Shimon et al (2017) Somatotropinomas inadequately controlled with octreotide may over-respond to pasireotide: the importance of dose adjustment to achieve long-term biochemical control’. HORMONES 16(1). https://doi.org/10.14310/horm.2002.1722
Franck SE et al (2017) Somatostatin receptor expression in GH-secreting pituitary adenomas treated with long-acting somatostatin analogues in combination with Pegvisomant. Neuroendocrinol 105(1):44–53. https://doi.org/10.1159/000448429
Freda PU (2002) Somatostatin analogs in acromegaly. J Clin Endocrinol Metab 87(7):3013–3018. https://doi.org/10.1210/jcem.87.7.8665
Liu W et al (2017) Expression of somatostatin receptor 2 in somatotropinoma correlated with the short-term efficacy of somatostatin analogues. Int J Endocrinol 2017:1–7. https://doi.org/10.1155/2017/9606985
Melmed S (2009) Acromegaly pathogenesis and treatment. J Clin Invest 119(11):3189–3202. https://doi.org/10.1172/JCI39375
Colao A et al (2014) Pasireotide versus octreotide in acromegaly: a head-to-head superiority study. J Clin Endocrinol Metab 99(3):791–799. https://doi.org/10.1210/jc.2013-2480
Samson SL (2015) Pasireotide in acromegaly: an overview of current mechanistic and clinical data. Neuroendocrinol 102(1–2):8–17. https://doi.org/10.1159/000381460
Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G (2002) SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol 146(5):707–716. https://doi.org/10.1530/eje.0.1460707
Fleseriu M, Cuevas-Ramos D (2016) Pasireotide: a novel treatment for patients with acromegaly. Drug Des Devel Ther. 227. https://doi.org/10.2147/DDDT.S77999.
Thodou E (2006) Mapping of somatostatin receptor types in GH or/and PRL producing pituitary adenomas. J Clin Pathol 59(3):274–279. https://doi.org/10.1136/jcp.2005.026914
Casarini APM et al (2009) Acromegaly: correlation between expression of somatostatin receptor subtypes and response to octreotide-lar treatment. Pituitary 12(4):297–303. https://doi.org/10.1007/s11102-009-0175-1
Reubi JC, Schaer JC, Waser B, Mengod G (1994) Expression and localization of somatostatin receptor SSTR1, SSTR2, and SSTR3 messenger RNAs in primary human tumors using in situ hybridization. Cancer Res 54(13):3455–3459
Volante M et al (2007) Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol 20(11):1172–1182. https://doi.org/10.1038/modpathol.3800954
Körner M, Waser B, Schonbrunn A, Perren A, Reubi JC (2012) ‘Somatostatin receptor subtype 2A immunohistochemistry using a new monoclonal antibody selects tumors suitable for in vivo somatostatin receptor targeting’: Am. J Surg Pathol 36(2):242–252. https://doi.org/10.1097/PAS.0b013e31823d07f3
Lupp A, Hunder A, Petrich A, Nagel F, Doll C, Schulz S (2011) Reassessment of sst5 somatostatin receptor expression in normal and neoplastic human tissues using the novel rabbit monoclonal antibody UMB-4. Neuroendocrinology 94(3):255–264. https://doi.org/10.1159/000329876
Fischer T, Doll C, Jacobs S, Kolodziej A, Stumm R, Schulz S (2008) Reassessment of sst 2 somatostatin receptor expression in human normal and neoplastic tissues using the novel rabbit monoclonal antibody UMB-1. J Clin Endocrinol Metab 93(11):4519–4524. https://doi.org/10.1210/jc.2008-1063
Chinezu L et al (2014) Expression of somatostatin receptors, SSTR2A and SSTR5, in 108 endocrine pituitary tumors using immunohistochemical detection with new specific monoclonal antibodies. Hum Pathol 45(1):71–77. https://doi.org/10.1016/j.humpath.2013.08.007
Corbetta S et al (2001) Somatostatin receptor subtype 2 and 5 in human GH-secreting pituitary adenomas: analysis of gene sequence and mRNA expression. Eur J Clin Invest 31(3):208–214. https://doi.org/10.1046/j.1365-2362.2001.00786.x
Jaquet P et al (2000) Human somatostatin receptor subtypes in acromegaly: distinct patterns of messenger ribonucleic acid expression and hormone suppression identify different tumoral phenotypes 1. J Clin Endocrinol Metab 85(2):781–792. https://doi.org/10.1210/jcem.85.2.6338
Kiseljak-Vassiliades K et al (2015) Differential somatostatin receptor (SSTR) 1–5 expression and downstream effectors in histologic subtypes of growth hormone pituitary tumors. Mol Cell Endocrinol 417:73–83. https://doi.org/10.1016/j.mce.2015.09.016
Kato M et al (2012) Differential expression of genes related to drug responsiveness between sparsely and densely granulated somatotroph adenomas. Endocr J 59(3):221–228. https://doi.org/10.1507/endocrj.ej11-0177
Zatelli MC et al (2005) Dopamine receptor subtype 2 and somatostatin receptor subtype 5 expression influences somatostatin analogs effects on human somatotroph pituitary adenomas in vitro. J Mol Endocrinol 35(2):333–341. https://doi.org/10.1677/jme.1.01876
Kumar U et al (2005) Somatostatin receptors in primary human breast cancer: quantitative analysis of mRNA for subtypes 1–5 and correlation with receptor protein expression and tumor pathology. Breast Cancer Res Treat 92(2):175–186. https://doi.org/10.1007/s10549-005-2414-0
Pisarek H, Krupiński R, Kubiak R, Borkowska E, Pawlikowski M, Winczyk K (2011) Differential expression of somatostatin receptor subtype-related genes and proteins in non-functioning and functioning adrenal cortex adenomas. Mol Med Rep 4(5):963–969. https://doi.org/10.3892/mmr.2011.519
Pisarek H, Pawlikowski M, Kunert-Radek J, Radek M (2009) Expression of somatostatin receptor subtypes in human pituitary adenomas – immunohistochemical studies. Endokrynol Pol 60(4):240–251
de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C, (2009) Global signatures of protein and mRNA expression levels. Mol Biosyst 10.1039.b908315d. https://doi.org/10.1039/b908315d.
Casar-Borota O et al (2013) Expression of SSTR2a, but not of SSTRs 1, 3, or 5 in somatotroph adenomas assessed by monoclonal antibodies was reduced by octreotide and correlated with the acute and long-term effects of octreotide. J Clin Endocrinol Metab 98(11):E1730–E1739. https://doi.org/10.1210/jc.2013-2145
Schmid HA et al (2012) Monoclonal antibodies against the human somatostatin receptor subtypes 1–5: development and immunohistochemical application in neuroendocrine tumors. Neuroendocrinology 95(3):232–247. https://doi.org/10.1159/000330616
Hofland LJ et al (1999) Immunohistochemical detection of somatostatin receptor subtypes sst1 and sst2A in human somatostatin receptor positive tumors. J Clin Endocrinol Metab 84(2):775–780. https://doi.org/10.1210/jcem.84.2.5497
Fougner SL, Borota OC, Berg JP, Hald JK, Ramm-Pettersen J, Bollerslev J (2008) The clinical response to somatostatin analogues in acromegaly correlates to the somatostatin receptor subtype 2a protein expression of the adenoma. Clin Endocrinol (Oxf) 68(3):458–465. https://doi.org/10.1111/j.1365-2265.2007.03065.x
Mayr B, Buslei R, Theodoropoulou M, Stalla GK, Buchfelder M, Schöfl C (2013) Molecular and functional properties of densely and sparsely granulated GH-producing pituitary adenomas. Eur J Endocrinol 169(4):391–400. https://doi.org/10.1530/EJE-13-0134
Plöckinger U et al (2008) Selective loss of somatostatin receptor 2 in octreotide-resistant growth hormone-secreting adenomas. J Clin Endocrinol Metab 93(4):1203–1210. https://doi.org/10.1210/jc.2007-1986
Brzana J, Yedinak CG, Gultekin SH, Delashaw JB, Fleseriu M (2013) Growth hormone granulation pattern and somatostatin receptor subtype 2A correlate with postoperative somatostatin receptor ligand response in acromegaly: a large single center experience. Pituitary 16(4):490–498. https://doi.org/10.1007/s11102-012-0445-1
Kaltsas GA et al (2001) Predictors of the outcome of surgical treatment in acromegaly and the value of the mean growth hormone day curve in assessing postoperative disease activity. J Clin Endocrinol Metab 86(4):1645–1652. https://doi.org/10.1210/jcem.86.4.7398
Rotermund R, Burkhardt T, Rohani Z, Jung R, Aberle J, Flitsch J (2018) Value of early postoperative random growth hormone levels and nadir growth hormone levels after oral glucose tolerance testing in acromegaly. Growth Horm IGF Res 41:64–70. https://doi.org/10.1016/j.ghir.2018.03.002
Kim EH, Oh MC, Lee EJ, Kim SH (2012)Predicting long-term remission by measuring immediate postoperative growth hormone levels and oral glucose tolerance test in acromegaly. Neurosurgery 70(5):1106–1113. https://doi.org/10.1227/NEU.0b013e31823f5c16.
Takahashi JA, Shimatsu A, Nakao K, Hashimoto N (2004) Early postoperative indicators of late outcome in acromegalic patients. Clin Endocrinol (Oxf) 60(3):366–374. https://doi.org/10.1046/j.1365-2265.2003.01900.x
Kiseljak-Vassiliades K et al (2015) Growth hormone tumor histological subtypes predict response to surgical and medical therapy. Endocrine 49(1):231–241. https://doi.org/10.1007/s12020-014-0383-y
Obari A et al (2008) Clinicopathological features of growth hormone-producing pituitary adenomas: difference among various types defined by cytokeratin distribution pattern including a transitional form. Endocr Pathol 19(2):82–91. https://doi.org/10.1007/s12022-008-9029-z
Bakhtiar Y et al (2010) Relationship between cytokeratin staining patterns and clinico-pathological features in somatotropinomae. Eur J Endocrinol 163(4):531–539. https://doi.org/10.1530/EJE-10-0586
Fougner SL, Casar-Borota O, Heck A, Berg JP, Bollerslev J (2012) Adenoma granulation pattern correlates with clinical variables and effect of somatostatin analogue treatment in a large series of patients with acromegaly. Clin Endocrinol (Oxf) 76(1):96–102. https://doi.org/10.1111/j.1365-2265.2011.04163.x
Ezzat S, Kontogeorgos G, Redelmeier DA, Horvath E, Harris AG, Kovacs K (1995) In vivo responsiveness of morphological variants of growth hormone-producing pituitary adenomas to octreotide. Eur J Endocrinol 133(6):686–690. https://doi.org/10.1530/eje.0.1330686
Mazal et al Prognostic relevance of intracytoplasmic cytokeratin pattern, hormone expression profile, and cell proliferation in pituitary adenomas of akromegalic patients - PubMed. https://pubmed.ncbi.nlm.nih.gov/11495005/ (Accessed 16 Sep 2020).
Bando H, et al (1992) Differences in pathological findings and growth hormone responses in patients with growth hormone-producing pituitary adenoma. Endocrinol Jpn 39(4):355–363. https://doi.org/10.1507/endocrj1954.39.355
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by Lena Rass, Amir-Hossein Rahvar, and Roman Rotermund. The first draft of the manuscript was written by Lena Rass and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics committee of the medical board Hamburg and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lena Rass and Amir-Hossein Rahvar contributed equally to this work
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rass, L., Rahvar, AH., Matschke, J. et al. Differences in somatostatin receptor subtype expression in patients with acromegaly: new directions for targeted therapy?. Hormones 21, 79–89 (2022). https://doi.org/10.1007/s42000-021-00327-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-021-00327-w