Abstract
The authors review the association between diabetes mellitus (DM) and aberrations of lipid metabolism related to DM, diabetic dyslipidemia (DD). DM is considered as a major health burden worldwide and one of the most important modifiable cardiovascular disease (CVD) risk factors. This applies to both the developed and the developing countries, especially the latter. While patients with type 1 DM, 10% of all DM cases, usually do not have dyslipidemia, DD is frequent among patients with type 2 DM (T2DM) (prevalence > 75%) and is mainly a mixed dyslipidemia [increase in triglycerides (TGs), low high-density lipoprotein cholesterol (HDL-C), and small-dense (atherogenic), low-density lipoprotein cholesterol (LDL-C) particles]. The components of DD, which is characterized by quantitative (mentioned above), qualitative, and kinetic abnormalities all contributing to CVD risk, are mostly related to insulin resistance. Statins, ezetimibe, and PCSK9 inhibitors can be used in monotherapy or consecutively in combinations if needed. Statins compose the main drug. For the residual CVD risk after statin treatment, the use of statin-fibrate combinations is indicated only in patients with mixed dyslipidemia. In conclusion, DD is a major health problem worldwide. It is a significant CVD risk factor and should be treated according to current guidelines. The means today exist to normalize all quantitative, qualitative, and kinetic aberrations of DD, thereby reducing CVD risk.
Similar content being viewed by others
Introduction
Over the last few decades, the number of patients with diabetes, especially type 2 (T2DM), has risen to 350 million worldwide [1] and it is estimated that this figure will further increase to 592 million (1 in 10 adults) worldwide by the year 2035 [2, 3]. During the next 20 years, the number of adults with DM is anticipated to increase by 20% in developed countries and by 70% in developing countries [4, 5].
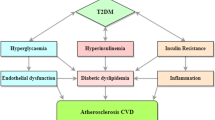
DM is a major risk factor for coronary artery disease (CAD), stroke, peripheral arterial disease (PAD), cardiomyopathy, diabetic nephropathy, diabetic retinopathy, and central as well as peripheral neuropathy [6, 7]. In fact, it has been established that DM is a CVD equivalent. The corollary is that in numerous countries, the escalating rates of DM and its close association with CVD are resulting in an ever heavier disease burden for populations and their health care systems.
One of the major features of DM that is closely and causatively related to its macrovascular complications is diabetic dyslipidemia (DD) [8,9,10].
Type 1 DM (T1DM), if well controlled with insulin, is associated with only a few if any aberrations of lipid metabolism [11]. Indicatively, high low-density lipoprotein cholesterol (LDL-C) was found in 15.8% and high triglyceride-rich lipoproteins (TGLs) in 12.9% in a cohort of young T1DM subjects [11]. Only in patients with poorly controlled T1DM and in those liable to develop obesity or metabolic syndrome (MetS) does DD manifest in a form similar to that linked to T2DM [11]. In T2DM patients, hyperinsulinemia, frequently insulin resistance, and β cell failure are related to DD; there are elevated plasma levels of fasting TRLs (the underlying disorders are hepatic overproduction and delayed clearance of TRLs), small-dense LDL-C particles, and low levels of high-density lipoprotein (HDL) cholesterol [1, 12,13,14].
Epidemiology of DD
Five years ago, a global estimate indicated that 65% of patients with DM had LDL-C levels > 100 mg/dl at baseline, this strongly pointing to the necessity for statin treatment in DM patients [15, 16]. In general, the incidence and prevalence of DD are equivalent to those of DM [15].
In the USA, data from 1980 to 2012 reveal a doubling of the prevalence of DM during the periods 1990–2008 and a plateauing between 2008 and 2012. Nonetheless, the prevalence and incidence of DM has continued to increase among subgroups, including non-Hispanic black and Hispanic subpopulations and those with low education [17]. In Europe, the prevalence of DD is four to sixfold higher in South Asians and African-Caribbeans in the UK compared with European white populations [18]. There is moreover a difference between Eastern vs Western Europe as well as a small gender difference [18], while the current trend is for a steady increase in both DM and DD [18].
Worldwide, close to 80% of people with T2DM live in middle- and low-income countries [19, 20], with a significant proportion of them (41.1 million) living in Latin America [19, 20]. In the latter region, great concern has been expressed regarding the rapidly rising prevalence of DM and DD during the past few decades [19, 20], this epidemic causing a substantial increase not only in CVD deaths but also in diabetic retinopathy, lower limb amputations, and chronic kidney disease (diabetic nephropathy) [19, 20]. Contributing to the increased prevalence of T2D in Latin American countries have been population growth, aging, and major changes in lifestyle, while medical care for T2DM and its complications is inevitably incurring ever greater costs for the national health system as well as substantial expenses for patients and their families [19, 20].
However, the biggest problem is DD in South Asian populations (this comprising more than a billion people who live in or come from India, Pakistan, Bangladesh, Sri Lanka, Nepal, Bhutan, Indonesia, Singapore, and Malaysia) [21, 22] who in particular are at very high risk of developing T2DM and CVD [21, 22]. Meanwhile, the overall prevalence of T2DM in South Asia is high and rapidly increasing [21, 22]. As regards DD, the majority of the data come from India, the South Asian country with the largest DM burden, where the prevalence has increased steadily and rapidly over the past four decades [22].
Pathophysiology of DD
T1DM accounts for about 10% of all cases of DM and is characterized by autoimmune destruction of pancreatic β cells, which produce insulin, by CD4+ and CD8+ T cells and macrophages that infiltrate the pancreatic islets [23], the disease occurring most commonly in individuals of European descent [23]. While 10 years ago there were two million people in Europe and North America with T1DM, the incidence is increasing, most likely because of environmental and/or lifestyle changes, which lead to autoimmune response to islet antigens [24].
DD among patients with T2DM is very common (prevalence of 72–85%) [9, 25]. This phenomenon is associated with a substantially increased risk of CVD in comparison to subjects without DM, since DD plays a central role in the genesis and the progression of atherosclerosis [9]. The lipid aberrations of DD are not only quantitative but also qualitative and kinetic [26,27,28]. The main quantitative lipoprotein abnormalities of DD are increased triglycerides (TGs) [it has been clearly shown that TRLs and their remnants are atherogenic [26]] and reduced HDL-C [29, 30]. Increased TRLs are attributed to overproduction and reduced clearance [26]. The main qualitative lipoprotein abnormalities comprise an increase in large very-low-density lipoprotein subfraction (VLDL1) and small-dense LDL-C particles, susceptible to oxidation, as well as increased TG content both in LDL-C and HDL particles, and glycation of apolipoproteins [25, 26]. T2DM DD also includes kinetic abnormalities of lipoproteins such as increased VLDL1 production, decreased VLDL catabolism, and increased HDL catabolism [25, 31]. All the above, which have been proven to be closely linked to each other, constitute major risk factors for the development and evolution of atherosclerosis [32, 33]. In most cases of DM, though LDL-C levels are usually normal, their particles show a reduced turnover which is potentially atherogenic [25]. Such lipoprotein aberrations are frequently associated with insulin resistance (IR), which may affect the activity of lipoprotein lipase (LPL), cholesterol ester transfer protein (CETP), phospholipid transfer protein (PTP), endothelial lipase (EL), and hepatic lipase (HL) [13]. DD is strongly related to insulin resistance, visceral obesity, and non-alcoholic fatty liver disease (NAFLD) [1, 34]. Insulin resistance is associated with excessive fatty acid flux to the liver that leads to VLDL overproduction [1]. Insulin fails to suppress lipolysis and FoxO1 (a transcription factor that plays an important role in the regulation of gluconeogenesis and glycogenolysis by insulin signaling and also negatively regulates adipogenesis [1]), but it is still able to activate rapamycin complex 1 (mTORC1) [1]. The effect on FoxO1 results in increased expression of microsomal triglyceride transfer protein (MTTP) and apoCIII, which promote VLDL overproduction and reduce their clearance [1]. Insulin also inhibits the production rate of apoB48 and secretion of chylomicrons [35], while insulin resistance leads to chronic intestinal overproduction of apoB48, which contributes significantly to both NAFLD and postprandial lipemia, emerging and crucial CVD risk factors [36,37,38,39,40,41].
Genetic studies, though as yet limited, all robustly support the theory that high TRLs or their remnants are causal factors for CVD and total mortality and that low HDL-C is most likely an innocent bystander [1, 42, 43].
Treatment of DD
As in any form of dyslipidemia, the primary target is the achievement of LDL-C levels below a certain value, this in accordance with the CV risk of the patient.
In 2004, the updated National Cholesterol Education Program Adult Treatment Panel III guidelines [44] characterized DM as a CVD equivalent and proposed an LDL-C target goal of < 70 mg/dl (optional value).
In 2011, the Joint Committee of the European Society of Cardiology (ESC)/ European Atherosclerosis Society (EAS) issued guidelines similar to the updated US 2004 guidelines (i.e., diabetes is a high-risk state with an LDL-C target < 70 mg/dl) [45]. However, it included non-HDL-C and apolipoprotein B as alternative targets to LDL-C, mainly in patients with T2DM [46, 47].
The 2013 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults were very different from the previous guidelines [48]. These guidelines did not set specific targets for any CV risk category (instead merely recommending high-moderate-low intensity statins, according to the calculated 10-year CV risk) and did not suggest follow-up of treated patients [48]. These were two of the reasons that these guidelines were not adopted by any other society worldwide, with the exception of the American Diabetes Association (ADA) 2 years (2015) after their publication. DM was considered to be a high-risk category and it was recommended that DM patients be treated with high doses of potent statins if the patient already has overt CVD, and moderate- to high-dose statins for those who had not had a CV event, depending on overall CV risk [48]. The 2015 ACC/AHA report [49] proposes that patients with DM be treated with moderate-intensity statin therapy for adults 40–75 years old, and high-intensity statin therapy for those who have a ≥ 7.5% 10-year CV risk or a prior CV event [48]. In adults with DM who are < 40 or > 75 years of age or with a LDL-C < 70 mg/dl, it was also recommended that physicians should take several factors into account, among which patient preferences, i.e., when they choose to commence, continue, or enhance statin therapy [48].
The 2016 European (ESC/EAS/Others) Guidelines on cardiovascular disease prevention in clinical practice (the Sixth Joint Task Force) suggested that adequate glycemic control decreases the risk of microvascular complications and, to a lesser degree, CVD risk [50]. However, targets should be moderate in the elderly and lower in patients with long-duration DM and those with pre-existing CVD [50].
The 2017 American Diabetes Association guidelines for the management of diabetes advise the use of statins in all patients aged over 40 years. In patients aged between 45 and 70 years with CV risk factors or disease, a high-intensity statin is recommended. Of particular importance, patients in this age group with prior acute coronary syndrome and LDL-C of 50 mg/dl or greater and patients with a history of a CVD event who cannot tolerate high-dose statins should receive double hypolipidemic therapy with a moderate-intensity statin and ezetimibe. Of note, the same recommendations are suggested for patients aged over 75 years who meet the abovementioned criteria [51].
Last but not least, the 2017 American Association of Clinical Endocrinologists and the American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease were recently released. According to these guidelines, patients with T2DM are characterized as high, very high, or extreme risk for CVD (LDL-C target < 100, < 70, and < 55 mg/dl, respectively) [52].
These guidelines took into consideration the benefits of very low levels of LDL-C (53 mg/dl), based on the results of the IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) with ezetimibe (a non-statin drug) added to simvastatin [53, 54] and those of the FOURIER trial with the PCSK9 inhibitor (human antibody) evolocumab (a non-statin drug that reduced CV events by reducing LDL-C at 30 mg/dl) [55, 56]. The 2017 focused update of the 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk [57] included the results of the two above studies and SPIRE I and II that used a humanized anti-PCSK9 antibody, bococizumab, also in the analysis [58], with conclusions similar to those of the endocrinology guidelines [51].
With the use of moderate or potent statins or combinations of statins with non-statin drugs, such as ezetimibe or PCSK9 inhibitors, there is certainty of attaining the LDL-C target set by the CVD risk level for each patient; besides, the baseline LDL-C values for patients with DM are not very high. However, even then a degree of residual CVD risk remains, given that the residual CVD risk with intensive statin therapy, though less, is still unacceptably high. Thus, CVD risk cannot be entirely eliminated, since some of it is attributable to non-modifiable CVD risk factors, such as age, gender, ethnicity or genetic factors. Nevertheless, a good part of it is attributed to atherogenic (mixed) dyslipidemia (AD) [59]. AD consists of a triad of increased TGs, low HDL-C, and increased levels of small-dense LDL-C particles [60]. It is related to insulin resistance conditions such as obesity, metabolic syndrome (MetS), and T2DM [59,60,61] and is considered a potent CVD risk factor [58, 59]. Data from Southeast Asia, mainly India, indicate that the prevalence of AD after statin treatment is higher in this region than in the West (i.e., the USA or Europe), the latter possibly related to a greater degree of insulin resistance in these countries [62, 63]. If lifestyle modification has failed to normalize lipid profile in AD, intervention with pharmacotherapy is needed on top of a statin or other hypocholesterolemic drugs. This should thus be the focus in order to attain all lipid targets (TGs and HDL, besides LDL-C which has already been achieved).
The administration of a statin-fibrate combination seems the best solution [64]. In the major study “Action to Control Cardiovascular Risk in Diabetes” (ACCORD), the combination of fenofibrate (160 mg/d) and simvastatin (20–40 mg/d) in 5518 patients with T2DM did not reduce the rate of fatal or non-fatal CVD events, in comparison with simvastatin monotherapy [65]. However, an analysis of the ACCORD group of patients with AD showed a 31% reduction in CVD events [65]. The patients in ACCORD with T2DM and AD at baseline were unexpectedly few (only 17%) [65]. Given that this is not the proportion seen in everyday practice (usually > 70%), there is a possibility that there was a patient selection bias. A substantial number of ACCORD patients came from Veteran Affairs Administration Institutions and these might not be representative of the general population. This could have been the reason that the fenofibrate-simvastatin combination did not meet the primary endpoint in the entire study, but only marginally in a small part of it. The results of the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study also showed clinical CVD benefit in patients with both elevated TG levels and low HDL-C levels [66].
The 10-year (n = 8982) Acute Coronary Syndrome Israeli Survey (ACSIS) reported a significantly lower risk of 30-day major adverse CVD events rate (up to 66% in DM patients) and a 46% reduction during mid-term follow-up (1 year) in all patients with acute coronary syndrome (ACS) receiving combined statin/fibrate compared with those on statin monotherapy [67]. Analyses of fibrate trials [66, 68] and a meta-analysis of all fibrate survival studies demonstrated that there was a reduction of 35% in major CVD events in patients with AD but of only 6% in those with plain hypercholesterolemia [69]. Thus, as Prof. Tenenbaum (the primary investigator of the Israeli Survey) said, “If it ain’t broke, don’t fix it” [70].
All the above suggest that the statin/fibrate combination is useful in AD and reduces residual CVD risk but should not be administered in patients with plain hypercholesterolemia, as it is not efficacious [71,72,73,74].
However, fibrates seem to have a beneficial effect on microvascular complications of DM, which contribute to the residual CVD risk increase [75]. In a large meta-analysis of 290 clinical studies, it was reported that fenofibrate substantially slowed in T2DM the progression of new diabetic retinopathy and pre-existing retinopathy at baseline, reduced the progression of urinary albumin excretion, and halved DM-related amputations, the first cause of non-traumatic amputations worldwide [76].
During the last 3 years, fixed combinations of pravastatin with fenofibrate and simvastatin with fenofibrate (the rosuvastatin with fenofibrate fixed combination has been announced) have been approved to increase compliance among patients who are already on a large number of drugs.
Alternatively, a statin/omega-3 combination can be prescribed. However, recent data imply that there is moderate strength of evidence of no effect at all on blood pressure (BP) or lipids and low strength of evidence of no effect on CVD risk [77]. Of course, given that fibrates are metabolized in the kidneys and cannot be administered in patients with chronic kidney disease (CKD with a glomerular filtration rate < 45 ml/min), except for the 60 mg formulation which is unfortunately not available in all countries, the statin (atorvastatin or pitavastatin)/omega-3 combination is mandatory for the treatment of AD in these patients, especially in CKD patients with a nephritic syndrome.
After publication of the Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE, n = 25,000 adults) results, on January 11, 2013, the European Medicines Agency (EMA) ordered the withdrawal of the niacin-laropiprant combination [78]; the drug was withdrawn by its company (https://www.reuters.com/ article/us-merck-cholesteroldrug-withdrawal/merck-begins-overseas-recall-of-hdl-cholesterol-drug) because among patients with CVD, the extended-release niacin-laropiprant (Tredaptive®) used for almost 4 years in Europe and China on top of statin treatment did not significantly reduce the risk of major CVD events but substantially increased the risk of serious adverse events [79]. Moreover, niacin had an adverse effect on glucose metabolism and was not the first choice of hypolipidemic drug [80].
As described above, several options are available that help in adjustment of LDL-C and HDL-C levels. However, little is known about the impact of hypolipidemic drugs on the third component of AD lipid alterations, increased small-dense LDL-C. The impact of statins on small-dense LDL-C has not been completely clarified, with studies offering contradictory findings [81,82,83]. By contrast, ezetimibe resulted in a moderate reduction of small-dense LDL-C and a greater reduction in large and medium LDLs [84]. Fibrates and niacin were found to be efficacious in reducing small-dense LDL-C [85].
Lastly, physicians are commonly concerned about the potential effects of hypolipidemic drugs on glucose metabolism, which might alter the management of hyperglycemia in the diabetes setting. Nevertheless, it should be borne in mind that most hypolipidemic drugs seem to ameliorate glucose homeostasis. Fibrates were found to improve glucose homeostasis through activation of PPAR-alpha and increases in adiponectin levels [86, 87]. Similarly, ezetimibe seems to increase insulin sensitivity in patients with increased insulin resistance [88]. Omega-3 fatty acids were shown to provide benefits in insulin sensitivity and glucose homeostasis in obese animals and in others with high insulin resistance states. With regard to the impact of fatty acids in humans, cross-sectional studies have pointed to a beneficial impact and interventional studies also demonstrate a neutral or favorable effect on glucose metabolism [89]. In contrast, statins have shown conflicting findings. On the one hand, the anti-inflammatory properties of statins, the increase in pancreatic isle blood flow, and the alteration of adipokine levels are thought to result in a favorable impact on glucose homeostasis. On the other hand, lipophilic statins were found to decrease insulin secretion. Furthermore, statins have been implicated in decreased availability of isoprenoids and thus reduction of insulin sensitivity. Of note, pravastatin has appeared to be the only exception, with consistent findings supporting a protective role of pravastatin on insulin sensitivity [90, 91].
Conclusions
Diabetic dyslipidemia is highly prevalent in patients with T2DM (> 75%). It is usually a mixed (atherogenic) hyperlipidemia and comprises a major CV risk factor. It is commonly related to insulin resistance and is characterized by moderate increases of LDL-C, elevations of TGs, low HDL-C, and small-dense (atherogenic) LDL-C particles. The cornerstone of treatment, as in all dyslipidemias, is based on statins and the primary goal is that of LDL-C, as per the comorbidities described in the newer (above analyzed) guidelines. In a limited number of cases, i.e., patients with very high LDL-C who are mainly exposed to very high to extreme CVD risk, ezetimibe or even a PCSK9 inhibitor can be added (to the statin). After statin treatment, a part of the modifiable residual CVD risk is due to high TGs and low HDL-C. This is the case for a statin-fibrate combination which has been proven to further reduce CVD events and ameliorate microvascular complications of DM (retinopathy, nephropathy, and amputations). Fixed combinations of statin-fibrate increase patient compliance to therapy.
References
Taskinen MR, Borén J (2015) New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis 239:483–495
Guariguata L, Whiting DR, Hambleton I et al (2014) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103:137–149
Zimmet PZ, Magliano DJ, Herman WH et al (2014) Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol 2:56–64
Whiting DR, Guariguata L, Weil C et al (2011) 2011 IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 94:311–321
Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87:4–14
Grundy SM, Benjamin IJ, Burke GL et al (1999) Diabetes and cardiovascular disease. A statement for healthcare professionals from the American Heart Association. AHA SCIENTIFIC STATEMENT. Circulation 100:1134–1146
No authors listed 1999 Diabetes mellitus: a major risk factor for cardiovascular disease. A joint editorial statement by the American Diabetes Association; the National Heart, Lung, and Blood Institute; the Juvenile Diabetes Foundation International; the National Institute of Diabetes and Digestive and Kidney Diseases; and the American Heart Association. Circulation 100:1132–1133
Vijayaraghavan K (2010) Treatment of dyslipidemia in patients with type 2 diabetes. Lipids Health Dis 9:144
Turner RC, Millns H, Neil HA et al (1988) Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS 23). BMJ 316:823–828
Farmer JA (2008) Diabetic dyslipidemia and atherosclerosis: evidence from clinical trials. Curr Diab Rep 8:71–77
Bulut T, Demirel F, Metin A (2017) The prevalence of dyslipidemia and associated factors in children and adolescents with type 1 diabetes. J Pediatr Endocrinol Metab 30(2):181–187
Taskinen MR (2005) Type 2 diabetes as a lipid disorder. Curr Mol Med 5:297–308
Chapman MJ, Ginsberg HN, Amarenco P et al (2011) Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J 32:1345–1361
Isomaa B, Almgren P, Tuomi T et al (2001) Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24:683–689
Dake AW, Sora ND (2016) Diabetic dyslipidemia review: an update on current concepts and management guidelines of diabetic dyslipidemia. Am J Med Sci 351:361–365
Center for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States. http://www.cdc.gov/ diabetes/pubs/ statsreport14/national-diabetes-report-web.pdf; 2014 Accessed 20.9.17
Geiss LS, Wang J, Cheng YJ et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA 312: 1218–1226
Forouhi NG, Merrick D, Goyder E (2006) Diabetes prevalence in England, 2001—estimates from an epidemiological model. Diabetic Med 23:189–197
Wild S, Roglic G, Green A et al (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053
Arredondo A, De Icaza E (2011) The cost of diabetes in Latin America: evidence from Mexico. Value Health 14(Suppl 1):S85–S88
Gujral UP, Pradeepa R, Weber MB, Narayan KM, Mohan V (2013) Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann N Y Acad Sci 1281:51–63
Mohan V (2004) Why are Indians more prone to diabetes. J Assoc Physicians India 52:468–474
Williams GM, Long AE, Wilson IV et al (2016) Beta cell function and ongoing autoimmunity in long-standing, childhood onset type 1 diabetes. Diabetologia 59:2722–2726
Long AE, Gillespie KM, Rokni S, Bingley PJ, Williams AJ (2012) Rising incidence of type 1 diabetes is associated with altered immunophenotype at diagnosis. Diabetes 61:683–686
Vergès B (2015) Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia 58:886–899
Taskinen MR (2003) Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia 46:733–749
Chahil TJ, Ginsberg HN, 2006 Diabetic dyslipidemia. Endocrinol Metab Clin North Am 35: 491–510, vii–viii
Vergès B (2005) New insight into the pathophysiology of lipid abnormalities in type 2 diabetes. Diabetes Metab 31:429–439
Doucet J, Le Floch JP, Bauduceau B, Verny C (2012) GERODIAB: glycaemic control and 5-year morbidity/mortality of type 2 diabetic patients aged 70 years and older: 1. Description of the population at inclusion. Diabetes Metab 38:523–530
Tziomalos K, Athyros VG, Karagiannis A, Kolovou GD, Mikhailidis DP (2009) Triglycerides and vascular risk: insights from epidemiological data and interventional studies. Curr Drug Targets 10:320–327
Wang J, Stancakova A, Soininen P et al (2012) Lipoprotein subclass profiles in individuals with varying degrees of glucose tolerance: a population-based study of 9399 Finnish men. J Intern Med 272:562–572
Arca M, Pigna G, Favoccia C (2012) Mechanisms of diabetic dyslipidemia: relevance for atherogenesis. Curr Vasc Pharmacol 10:684–686
Mikhailidis DP, Elisaf M, Rizzo M et al (2011) European panel on low density lipoprotein (LDL) subclasses: a statement on the pathophysiology, atherogenicity and clinical significance of LDL subclasses: executive summary. Curr Vasc Pharmacol 9:531–532
Athyros VG, Alexandrides TK, Bilianou H et al (2017) The use of statins alone, or in combination with pioglitazone and other drugs, for the treatment of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and related cardiovascular risk. An expert panel statement. Metabolism 71:17–32
Abumrad NA, Davidson NO (2012) Role of the gut in lipid homeostasis. Physiol Rev 92:1061–1085
Xiao C, Lewis GF (2012) Regulation of chylomicron production in humans. Biochim Biophys Acta 1821:736–746
Veilleux A, Grenier E, Marceau P et al (2014) Intestinal lipid handling: evidence and implication of insulin signaling abnormalities in human obese subjects. Arterioscler Thromb Vasc Biol 34:644–653
Xiao C, Dash S, Morgantini C et al (2014) New and emerging regulators of intestinal lipoprotein secretion. Atherosclerosis 233:608–615
Axelsen M, Smith U, Eriksson JW, Taskinen MR, Jansson PA (1999) Postprandial hypertriglyceridemia and insulin resistance in normoglycemic first-degree relatives of patients with type 2 diabetes. Ann Intern Med 131:27–31
Borén J, Matikainen N, Adiels M, Taskinen MR (2014) Postprandial hypertriglyceridemia as a coronary risk factor. Clin Chim Acta 431:131–142
Fujioka Y, Ishikawa Y (2009) Remnant lipoproteins as strong key particles to atherogenesis. J Atheroscler Thromb 16:145–154
Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP (2015) Genetic, epidemiologic and clinical data strongly suggest that fasting or non-fasting triglycerides are independent cardiovascular risk factors. Curr Med Res Opin 31:435–438
Stefanutti C, Labbadia G, Athyros VG (2014) Hypertriglyceridaemia, postprandial lipaemia and non-HDL cholesterol. Curr Pharm Des 20:6238–6248
Grundy SM, Cleeman JI, Merz CN et al (2004) Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Circulation 110:227–239
Catapano AL, Reiner Z, De Backer G, European Society of Cardiology (ESC); European Atherosclerosis Society (EAS) et al (2011) ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis 217:3–46
Stock J (2012) News from the literature: focus on joint ESC/EAS dyslipidemia guidelines. Atherosclerosis 220:42–44
Khavandi M, Duarte F, Ginsberg HN, Reyes-Soffer G (2017) Treatment of dyslipidemias to prevent cardiovascular disease in patients with type 2 diabetes. Curr Cardiol Rep 19:7
Stone NJ, Robinson JG, Lichtenstein AH, American College of Cardiology/American Heart Association Task Force on Practice Guidelines et al (2014) 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129(25 Suppl 2):S1–S45
American Diabetes Association (2015) Cardiovascular disease and risk management. Diabetes Care 38(Supplement 1):S49–S57
Piepoli MF, Hoes AW, Agewall S (2016) European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 252:207–274
Jellinger PS, Handelsman Y, Rosenblit PD et al (2017) American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract 23(Suppl 2):1–87
American Diabetes Association (2017) Cardiovascular disease and risk management. Sec. 9. In Standards of Medical Care in Diabetes-2017. Diabetes Care 40:S75–S87
Cannon CP, Blazing MA, Giugliano RP, IMPROVE-IT Investigators et al (2015) Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 372:2387–2389
Katsiki N, Athyros VG, Mikhailidis DP (2016) More news from IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial). Hormones (Athens) 15:5–7
Sabatine MS, Giugliano RP, Keech AC, FOURIER Steering Committee and Investigators et al (2017) Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 376:1713–1722
Katsiki N, Athyros VG, Mikhailidis DP, Mantzoros C (2017) Proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors: shaping the future after the further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk (FOURIER) trial. Metabolism 74:43–46
Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2017 Focused update of the 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 70:1785–1822
Ridker PM, Revkin J, Amarenco P, SPIRE Cardiovascular Outcome Investigators et al (2017) Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med 376:1527–1539
Fruchart JC, Sacks F, Hermans MP et al (2008) Residual Risk Reduction Initiative (R3I): The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in dyslipidaemic patient. Diab Vasc Dis Res 5:319–335
Kiran Musunuru K (2010) Atherogenic dyslipidemia: cardiovascular risk and dietary intervention. Lipids 45:907–914
Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP Dyslipidaemia of obesity, metabolic syndrome and type 2 diabetes mellitus: the case for residual risk reduction after statin treatment. Open Cardiovasc Med J 5:24–34
Athyros VG, Doumas M, Karagiannis A (2016) Differential residual dyslipidemia/cardiovascular risk after statin treatment between Asian-Indians and western whites. Call for action. Indian Heart J 68:596–598
Katsiki N, Koumaras C, Athyros VG, Karagiannis A (2012) Thinking beyond traditional cardiovascular risk factors: the role of arterial stiffness in targeting residual risk. Angiology 63:9–11
Athyros VG, Wierzbicki AS (2014) Statin-fibrate combination therapy is safe and effective in normalizing lipid profile and in keeping cardiovascular event rates low. Curr Med Res Opin 30:57–58
ACCORD Study Group, Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd (2010) Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 362:1563–1574
Keech A, Simes RJ, Barter P, FIELD study investigators et al (2005) Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366:1849–1861
Tenenbaum A, Medvedofsky D, Fisman EZ et al (2012) Cardiovascular events in patients received combined fibrate/statin treatment versus statin monotherapy: Acute Coronary Syndrome Israeli Surveys data. PLoS One 7:e35298
Rubins HB, Robins SJ, Collins D et al (2002) Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs high-density lipoprotein intervention trial (VA-HIT). Arch Intern Med 162:2597–2604
Sacks FM, Carey VJ, Fruchart JC (2010) Combination lipid therapy in type 2 diabetes. N Engl J Med 363:692–694
Tenenbaum A, Fisman EZ et al (2010) “If it ain’t broke, don’t fix it”: a commentary on the positive-negative results of the ACCORD lipid study. Cardiovasc Diabetol 9:24
Wierzbicki AS, Mikhailidis DP, Wray R, Schacter M, Cramb R, Simpson WG, Byrne CB (2003) Statin-fibrate combination: therapy for hyperlipidemia: a review. Curr Med Res Opin 19(3):155–168
Athyros VG, Papageorgiou AA, Kontopoulos AG (2001) Statin-fibrate combinations in patients with combined hyperlipidemia. Atherosclerosis 155:263–264
Athyros VG, Papageorgiou AA, Athyrou VV et al (2002) Atorvastatin versus four statin-fibrate combinations in patients with familial combined hyperlipidaemia. J Cardiovasc Risk 9:33–39
Athyros VG, Papageorgiou AA, Hatzikonstandinou HA et al (1997) Safety and efficacy of long-term statin-fibrate combinations in patients with refractory familial combined hyperlipidemia. Am J Cardiol 80:608–613
Mitsiou EK, Athyros VG, Karagiannis A, Mikhailidis DI (2012) Is there a role for hypolipidaemic drug therapy in the prevention or treatment of microvascular complications of diabetes? Open Cardiovasc Med J 6:28–32
Czupryniak L, Joshi SR, Gogtay JA, Lopez M (2016) Effect of micronized fenofibrate on microvascular complications of type 2 diabetes: a systematic review. Expert Opin Pharmacother 17:1463–1473
Balk EM, Lichtenstein AH 2017 Omega-3 fatty acids and cardiovascular disease: summary of the 2016 Agency of Healthcare Research and Quality Evidence Review. Nutrients 9. https://doi.org/10.3390/nu9080865
European Medicines Agency 2013 European medicines agency confirms recommendation to suspend Tredaptive, Pelzont and Trevaclyn. January 18, 201
The HPS2-THRIVE Collaborative Group (2014) Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 371:203–212
Katsiki N, Athyros VG, Karagiannis A, Mikhailidis DP (2015) Nicotinic acid and new-onset diabetes. Horm Metab Res 47:544–545
Packard C, Caslake M, Shepherd J (2000) The role of small, dense low density lipoprotein (LDL): a new look. Int J Cardiol 74:S17–S22
Rizzo M, Berneis K (2006) The clinical relevance of low-density-lipoproteins size modulation by statins. Cardiovasc Drugs Ther 20:205–217
Ip S, Lichtenstein AH, Chung M, Lau J, Balk EM (2009) Systematic review: association of low-density lipoprotein subfractions with cardiovascular outcomes. Ann Intern Med 150:474–484
Gouni-Berthold I, Mikhailidis DP, Rizzo M (2012) Clinical benefits of ezetimibe use: is absence of proof, proof of absence? Expert Opin Pharmacother 13:1985–1988
Superko HR, Berneis KK, Williams PT, Rizzo M, Wood PD (2005) Gemfibrozil reduces small low-density lipoprotein more in normolipemic subjects classified as low-density lipoprotein pattern B compared with pattern A. Am J Cardiol 96:1266–1272
Simental-Mendía LE, Simental-Mendía M, Sánchez-García A et al (2017) Effect of fibrates on glycemic parameters: a systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacol Res
Sahebkar A, Watts GF (2013) Fibrate therapy and circulating adiponectin concentrations: a systematic review and meta-analysis of randomized placebo-controlled trials. Atherosclerosis 230:110–120
Tsunoda T, Nozue T, Yamada M, Mizuguchi I, Sasaki M, Michishita I (2013) Effects of ezetimibe on atherogenic lipoproteins and glucose metabolism in patients with diabetes and glucose intolerance. Diabetes Res Clin Pract 100:46–52
Flachs P, Rossmeisl M, Kopecky J (2014) The effect of n-3 fatty acids on glucose homeostasis and insulin sensitivity. Physiol Res 63:S93–S118
Kostapanos MS, Liamis GL, Milionis HJ, Elisaf MS (2010) Do statins beneficially or adversely affect glucose homeostasis? Curr Vasc Pharmacol 8:612–631
Kostapanos MS, Agouridis AP, Elisaf MS (2015) Variable effects of statins on glucose homeostasis parameters and their diabetogenic role. Diabetologia 58:1960–1961
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
VGA has given talks, attended conferences, and participated in trials sponsored by MSD, Sanofi, and Amgen. MD has given talks and attended conferences sponsored by Menarini, WinMedica, Bayer, Boehringer-Ingelheim, Merck, and Unipharma. AK has given talks, attended conferences, and participated in trials sponsored by WinMedica. The rest have no conflict of interest whatsoever.
Rights and permissions
About this article
Cite this article
Athyros, V.G., Doumas, M., Imprialos, K.P. et al. Diabetes and lipid metabolism. Hormones 17, 61–67 (2018). https://doi.org/10.1007/s42000-018-0014-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-018-0014-8