Key summary points
To study the application of a systematic protocol for specialized CGA in patients with lymphoma over 70 years of age.
AbstractSection FindingsPatients were classified by level of frailty, with different groups showing statistically significant differences in overall survival, response to treatment, and likelihood of increased frailty at the end of treatment.
AbstractSection MessageThis study suggests that standardized, systematic CGA performed by geriatricians permits patient classification by level of frailty, helps in decision-making, and predicts clinical outcomes.
Abstract
Purpose
A study analyzing the application of a protocol of comprehensive geriatric assessment (CGA) in older patients with lymphoma was carried out to allow frailty-based patient classification and individualized treatment.
Methods
Lymphoma patients older than 70 years referred to the Geriatric Clinic at a tertiary hospital between May 2016 and March 2021 were included. The assessment protocol included comorbidity, polypharmacy, nutritional, functional, and mental status, geriatric syndromes, and life expectancy. CGA enabled patient classification into four groups (Type I to Type IV) based on frailty assessment instrument scoring and clinical, functional, and mental status. Variables were compared using parametric and non-parametric statistical tests and Kaplan–Meier survival curves.
Results
Ninety-three patients (55.9% women) were included. Median age was 81.1 years (± 5.7). 23 patients (24.7%) were classified as robust (type I), 30 (32.3%) as pre-frail (type II) with potentially reversable deficits, 38 (40.9%) as frail (type III), and 2 (2.2%) as requiring palliative care (type IV). Patients received oncospecific treatment with modifications carried out in 64.5% of cases based on CGA results. Differences in overall survival (p = 0.002), response to treatment (p < 0.001) and likelihood of increased frailty (p = 0.024) were observed, with type III–IV patients showing significantly worse outcomes.
Conclusion
Performance of standardized, systematic CGA by geriatricians permits older lymphoma patients to be classified according to frailty, with significant differences in terms of clinical outcomes across groups. We propose incorporating CGA performed by geriatricians as part of the multidisciplinary care team to optimize therapeutic strategy for these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In industrialized countries, around 45% of new hematological malignancy (HM) diagnoses are made in patients aged 75 years or older [1]. HMs comprise a spectrum of different diseases, of which lymphomas are the most common; non-Hodgkin’s lymphoma (NHL) encompasses more than 20 subtypes of lymphoid diseases, classified by morphological, cytogenetic and immunophenotypic characteristics. NHL mainly affects the older population, with a median age at diagnosis of 67 years [2]. Its incidence and mortality rank 12th worldwide according to GLOBOCAN 2020 [3], with the highest incidence observed in Australia and New Zealand, Northern America, Northern Europe, and Western Europe (> 10/100,000 inhabitants for both sexes combined). In Spain, the incidence of NHL is 7.5–9.1/100,000 inhabitants for both sexes combined.
Older patients with HMs are characterized by decreased physiological reserve, leading to reduced treatment tolerance, and complicating the diagnostic and therapeutic decision-making process. Although chronological age itself is not an accurate marker of an individual patient’s biological situation and should not be used as a discriminatory variable when deciding on a therapeutic option [4], older patients with HM often present age-related characteristics that influence prognosis and must be considered when choosing the most appropriate treatment. Therapeutic decisions must be based, not only on the tumor’s characteristics, but also on the patient’s physical, mental, and social ability to tolerate treatment [5], underlining the importance of multidimensional, multidisciplinary assessment.
The European Society for Medical Oncology’s consensus document for the management of older patients with lymphoma focuses on several key aspects [6], including identification of frail patients and individualized treatment. While robust older patients may be eligible for curative treatment at full doses, frail patients with comorbidities may require treatment modifications. Developing robust strategies to detect vulnerable patients at risk of complications is the key to personalizing treatment for older HM patients. Comprehensive geriatric assessment (CGA) has proven its efficacy in aiding treatment decision-making and guiding outcomes in different studies [7,8,9,10], and uses specific instruments to identify patient frailty, which confers increased vulnerability to adverse events (disability, hospitalization, and death). The International Society of Geriatric Oncology (SIOG) and National Comprehensive Cancer Network (NCCN) consensus guidelines recommend the use of CGA in all older cancer patients [11, 12].
Despite the relevance of CGA for older patients diagnosed with cancer, to the best of our knowledge, there are no studies reporting large series of older patients with lymphoma who have undergone standardized CGA for personalized oncospecific treatment. Our study, carried out in a tertiary care hospital (Madrid, Spain), presents the application of a systematic protocol for specialized CGA in patients with lymphoma over 70 years of age, allowing frailty-based patient classification, individualized care recommendations, and treatment personalization. We also report patient outcomes after the start of oncospecific treatment.
Methods
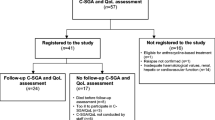
Lymphoma patients over 70 years of age referred to the Geriatric Hematology clinic at the Fundación Jiménez Díaz University Hospital (Madrid, Spain) for specialized CGA between May 1st, 2016, and March 31st, 2021, were included in the study. All patients presented a recent diagnosis of lymphoma and had been approved for oncospecific treatment by the hospital’s tumor committee. Patients undergoing second or subsequent lines of treatment were excluded. No other exclusion criteria were defined.
Patients approved for oncospecific therapy were referred to the geriatric hematology clinic as a part of our clinical pathway for assessment using a systematic CGA protocol. Evaluation was carried out by a qualified geriatrician with broad experience in geriatric oncology and hematology. This assessment was usually performed 1–2 weeks before starting oncospecific treatment. Neither patient classification nor subsequent interventions delayed the start of treatment.
The CGA protocol included the domains recommended by Mohile et al. [13]: assessment of comorbidity; presence of polypharmacy; nutritional, functional, and mental status; physical performance tests; life expectancy; and the presence of geriatric syndromes such as urinary or fecal incontinence, falls, or history of depression or dementia. Information was stored and retrieved for analysis from the hospital’s electronic health record, Casiopea® (Inetum).
Socio-demographic variables (age, sex, and living arrangements (alone or accompanied)) and Eastern Cooperative Oncology Group (ECOG) [14] performance status were collected. The Barthel Index [15] (Basic activities of daily living), the Lawton and Brody Index [16] (Instrumental activities of daily living) and the FAC [17] (Functional Ambulation Categories) scale for mobility were used to assess functional status. Mental status was assessed using the Pfeiffer Short Portable Mental Status Questionnaire (SPMSQ) [18]. The Global Deterioration Scale [19] (GDS) was used to describe patients’ cognitive status, and the Yesavage scale [20] to assess the presence of depression. Previous diagnoses of depression were also recorded. Nutritional screening was carried out using the Mini Nutritional Assessment Short Form [21] (MNA-SF) and body mass index (BMI). Comorbidity was assessed using the Cumulative Illness Rating Scale-Geriatric [22] (CIRS-G). Polypharmacy was defined as the simultaneous prescription of 5 or more drugs. Life expectancy was calculated based on the patient’s medical history and baseline situation, using the ePrognosis application (www.eprognosis.com). A score equal to or greater than 12 predicted 5-year mortality. Analytical parameters such as albumin, lactate dehydrogenase (LDH) and hemoglobin values were also recorded. The Short Physical Performance Battery [23] (SPPB) and the FRAIL [24] questionnaire were used to assess frailty. Assessment tools were selected based on usual geriatric practice and on past research on geriatric oncology carried out by our team.
Data were manually extracted from the electronic health record, including: type and characteristics of the different lymphomas, including cell of origin, grade, lymphoma subtype, revised international prognostic index (R-IPI) [25], presence or absence of B symptoms, and extranodal involvement; oncospecific treatment data, including start and end date, and treatment modifications; and treatment results, including tolerance, need for treatment modifications, reason for modification, toxicity, and severity (grade > 2)). Severity of adverse events was evaluated using the Common Terminology Criteria for Adverse Events (CTCAE) [26]. Response to treatment, disease progression, relapse, and the date of relapse when applicable were also recorded.
Interventions implemented by the geriatrician after CGA, including nutritional interventions, physical activity recommendations, antidepressant prescriptions, polypharmacy reduction strategies, and social interventions were recorded. Information on patient mortality and use of health resources (number of emergency room visits and number of hospital admissions) during the year after starting treatment was also collected.
Intervention model: comprehensive assessment, patient classification, and multidisciplinary approach
Comprehensive geriatric assessment enabled patient classification using a modification of the criteria proposed by Balducci and Extermann [27, 28] based on frailty assessment instrument scoring and clinical, functional, and mental status. Four groups were identified: Type I (“fit” patients with no detected deficits); Type II (“pre-frail” patients with potentially correctable deficits); Type III (“frail” patients, with irreversible deficits); and Type IV (disabled patients and those with a poor overall prognosis). A similar classification has been used in a previous study [29].
With the aim of optimizing patients’ health status before, during, and after treatment, and providing personalized care throughout the therapeutic process [30,31,32], the geriatrician conducting CGA made specific recommendations for each of the deficits or problems detected during assessment. Patient-tailored recommendations included nutritional advice, prescription of specific physical exercise using the VIVIFRAIL program (web.vivifrail.com) [33], adjustments in polypharmacy (according to the STOPP/START criteria [34]), and control of cardiovascular risk factors. Patients were scheduled for follow-up appointments every 3 months during the first year of treatment, or more frequently if needed. We collected data from the follow-up appointments to detect increased frailty (measured using functional scales or the appearance of new geriatric syndromes). At follow-up appointments, only functional tests (the Barthel Index and Lawton Index) were performed.
After CGA and patient classification had been carried out, the hospital’s multidisciplinary lymphoma committee, which includes hematologists, pathologists, nuclear medicine specialists, radiologists, hospital pharmacists, and geriatricians, selected oncospecific treatment for each patient. Type I (“fit”) patients were prescribed standard oncospecific treatment; patients classified as types II, III and IV were prescribed adapted regimens featuring lower doses, longer intervals between cycles, and drugs with lower risk of cardiotoxicity.
Statistical analysis
Qualitative variables were presented as frequencies and percentages, and quantitative variables as mean and standard deviation or median and quartiles, depending on distribution. Comparisons of qualitative variables were performed using Pearson’s chi-squared test or Fisher’s exact test. Comparisons of quantitative variables were performed using one-way ANOVA or Student’s t test for those variables summarized as mean and standard deviation, and Kruskal–Wallis or Wilcoxon’s rank test for those variables summarized as median and quartiles. Overall survival (OS) time was computed from the date of diagnosis to either the date of the last visit that the patient was known to be alive or the date of death from any cause. Survival curves for each group of patients were estimated using the Kaplan–Meier method and compared using a log-rank test. Multivariate analysis of survival was performed using the Cox proportional hazard ratio (HR) with a 95% confidence interval (CI), considering all variables that had been shown to be significantly associated with survival in the univariate analysis. Statistical analyses were performed using R version 4.1.2 (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
The study was approved by the Hospital Clinical Research Ethics Committee of the Fundación Jiménez Díaz University Hospital (EO121-21_FJD).
Results
Between May 1st, 2016, and March 31st, 2021, 93 patients aged 70 years and above with a recent diagnosis of lymphoma underwent CGA at our hospital. Median age at assessment was 81.1 years (± 5.7 years), and 55.9% of patients were women. The majority of lymphomas diagnosed were NHL (94.5%), high-grade (66.7%), and B-cell (91.4%). The most frequent lymphoma subtype was diffuse large B-cell lymphoma (48.4%), followed by marginal (10.8%) and follicular (9.7%) lymphoma. Most patients presented a high R-IPI (51.6%) and extranodal involvement (75.3%) and did not present B symptoms (55.9%). Most patients were assessed before starting oncospecific treatment. Only 20% (mainly patients with diffuse large B-cell lymphoma) were assessed after having received prephase treatment or the first cycle of chemotherapy.
Regarding classification, 23 patients (24.7%) were classified as robust (type I), 30 patients (32.3%) as pre-frail (type II) with potentially reversable deficits, 38 patients (40.9%) as frail (type III), and only 2 patients (2.2%) as presenting with a poor overall prognosis or requiring palliative care (type IV). Type III and IV patients were analyzed as a group. This decision was made after data collection, because only two type IV patients were identified, which made comparison with other groups impossible.
Patients’ clinical characteristics, and the differences between groups, are presented in Table 1. We observed significant differences regarding comorbidity across groups, with a higher CIRS-G score in type III–IV patients (p 0.006). Likewise, functional status was worst in the type III–IV group, with lower overall Barthel Index (p 0.005), FAC (p < 0.001) and Lawton Index (p < 0.001) scores. Higher GDS (p 0.008) and lower Pfeiffer questionnaire scores (p 0.034) in the type III–IV group indicated significantly worse cognitive status compared to other groups. Significant differences in frailty scores (SPPB (p < 0.001) and FRAIL questionnaire (p 0.002)) and analytical parameters (hemoglobin (p 0.021), albumin (0.006)) were also observed, with patients in the type III–IV groups showing increased frailty and lower hemoglobin and serum albumin levels.
No significant differences regarding lymphoma subtypes and clinical characteristics were found between groups. Treatment was chosen on a tailored basis, considering patients’ values and preferences, by the hospital’s multidisciplinary lymphoma tumor board. Oncospecific treatment differed significantly across groups (p < 0.001), with adapted regimens (64.5%) being more frequent in type II and III–IV patients (p 0.001) (Table 2). The rate of subsequent treatment modifications did not vary between groups. 14% of patients discontinued treatment due to side effects, with similar rates of severe (> grade 2) toxicity and discontinuation due to toxicity observed across groups. There were no differences regarding the need for subsequent treatment adjustments (modification or discontinuation of the initial treatment). With regards to geriatric intervention (Table 3), nutritional recommendations were given to 82.2%, oral nutritional supplements were prescribed to 39.8%, and an individualized physical exercise program was prescribed to 59.1% of patients. General exercises were recommended to all, but 60% of patients were prescribed specific, individualized exercise using the VIVIFRAIL program.
Regarding response to treatment, overall survival, and frailty at the end of treatment (Table 4), significant differences were observed. 53.8% of patients achieved complete response at the end of treatment, 34.4% achieved partial response, 9.7% showed no response to treatment, and 2.2% died during treatment (2 patients, one from the type I group and another from the type III–IV group). At follow-up (median follow-up 27.3 months, range 18–74 months), 25.8% patients presented a relapse, with no differences between groups. Survival analysis was performed using Kaplan–Meier curves (Fig. 1); we found that fit (type I) patients presented higher survival rates compared to those in the type III–IV group (42.5 ± 19.6 months versus 23.7 ± 20.5 months, p 0.002). Increased frailty at the end of treatment during the follow-up period was much less frequent in type I patients compared with type III–IV patients (9.1% vs 39.5%, p 0.024). No differences were observed regarding the use of hospital resources, including hospital admissions and emergency department visits, during the year after starting treatment.
We performed univariate and multivariate analyses using a COX regression model to identify predictors of mortality (Table 5). As expected, age and moderate-to-severe dependence measured using the Barthel Index were found to correlate to mortality, while higher LDH levels (< 250 mg/dL) and the presence of geriatric syndromes almost reached statistical significance (p = 0.076 and p = 0.062, respectively). The regression model showed acceptable goodness of fit, with a C-statistic of 0.70.
Discussion
This study presents a cohort of older patients with NHL assessed with a systemic CGA protocol to enable individualized oncospecific treatment. The goal of our research was to determine if the application of a systemic CGA protocol in older patients with a recent diagnosis of lymphoma could enable patient classification according to frailty profiles, prescription of geriatric care recommendations, and tailored oncospecific treatment. We also aimed to describe the impact of CGA on clinical outcomes.
CGA yielded exhaustive information on patients’ functional capacity, comorbidity, level of frailty, nutritional status, cognitive status, geriatric syndromes, and estimated survival. These data allowed us to classify patients into three groups (type I, II, and III–IV). Although no differences were observed regarding the types of lymphoma diagnosed across groups, patient classification permitted individualized care, including personalized geriatric recommendations to improve nutritional status, physical condition, and cardiovascular risk factors, as well as frailty-based adaptation of oncospecific treatments. During follow-up, toxicity rates were similar for the different groups, and no differences in the use of hospital resources were observed, leading us to consider that initial treatment had been chosen appropriately. Despite the use of more intensive regimens in groups I–II, no increase in hospitalization rates and emergency room visits was observed. However, mortality rates and frailty among surviving patients were significantly higher in the type III–IV group.
Previous attempts have been made to classify older patients with lymphoma according to non-hematologic characteristics. However, there is no definitive consensus on the most appropriate instruments, scores, or scales for classification. Some studies (most of which have been performed in an older population with diffuse large B-cell lymphoma) have identified a series of prognostic factors associated with worse clinical outcomes and lower survival, using domains such as functional impairment, dependence for basic or instrumental activities, presence of malnutrition and comorbidity [36, 37]. Tools focusing on these domains are capable of identifying frailty more accurately than clinician judgment or performance status (PS) alone [38].
Existing studies seeking to predict unfavorable outcomes in older patients with lymphoma include research published by the Italian Lymphoma Foundation [39] (FIL), featuring a simplified geriatric assessment including basic and instrumental activities of daily living, comorbidity, and age, and Miura et al.’s study [40] describing the development of the ACA Index to predict outcomes using age, comorbidity, and albumin blood levels. Liu et al. [41] combined the ACA index with an assessment of functional status (IADL) to create the IADL-ACA (IACA) index for patients ≥ 65 years of age with diffuse large B-cell lymphoma, allowing patient classification into three risk groups—low, intermediate, and poor—observing significant differences in overall response rate, cumulative incidence of treatment-related mortality, relapse rate, and 2-year overall survival. Although our study did not aim to create a predictive score, we were able to detect different areas that can help to classify older patients with lymphoma and that could potentially serve as a basis for the creation of predictive models using a larger series.
Two aspects of our study should be highlighted. On one hand, the detection of frailty through CGA revealed unmet needs for geriatric intervention to improve patients’ overall health status. These interventions (physical exercise programs, nutritional support, and psychological interventions) have also been carried out in other cancer settings and have been described as positively influencing patient outcomes [42, 43]. On the other hand, the information gathered through CGA and patient classification allowed the lymphoma committee to tailor treatment for each patient. To the best of our knowledge, this is the first study reporting systematic individualization of oncospecific treatment according to frailty status. For example, in Tucci’s study [39], patients were treated according to the attending physician’s clinical judgement regardless of category, while Garrick et al. [44] report that frailty had a slight influence on the choice of treatment, leading to a change of treatment in only 21.7% of cases.
Adjusting treatment according to patients’ characteristics allowed us to achieve a higher percentage of complete responses in type I and II patients, similar to that observed in younger populations (60–80% 5-year complete response rates, depending on the subtype of lymphoma), without increasing toxicity, use of health resources, or need for treatment. These results indicate appropriate choice of initial treatment by the lymphoma committee. Other studies, such as Corre et al. [45], have demonstrated similar results in advanced non-small-cell lung cancer, with CGA-based individualized treatment failing to improve treatment outcomes but slightly reducing treatment toxicity. Mohile et al. [46] report that older patients with advanced cancer undergoing CGA (incurable solid tumors or lymphoma) experience less grade 3–5 toxicity than their non-CGA counterparts. These studies highlight the importance of CGA-guided interventions to improve outcomes, although more specific studies are needed to determine how CGA-tailored treatment can reduce toxicity for older individuals with lymphoma.
Strengths of this study include the thoroughness with which geriatric assessment was performed, and the close clinical follow-up patients received during treatment. We believe that CGA carried out by an expert physician, instead of using standalone frailty scales, is one of the greatest strengths of the study. In our opinion, CGA-mediated patient selection enabled the lymphoma committee to carry out comprehensive evaluation and therapeutic decision-making, while geriatric intervention during oncospecific treatment played an important role in the study’s results. In routine care, re-performing frailty assessments after the start of an oncological treatment together with non-oncological frailty interventions would allow us to assess whether and to what degree a patient is responsive to such management. This would enable a multidisciplinary team to decide whether frailty interventions should be continued, escalated, de-escalated, or stopped. Furthermore, frailty monitoring over time may guide hematologists to increase or decrease the intensity of oncospecific treatment. To date, however, no studies have explored the utility of repeated frailty assessments to guide continuous adaptation of ongoing cancer treatment, and how to perform re-assessment [35].
One of our study’s limitations is the lack of a control group, which could have helped us to understand the implications of this care strategy better. Moreover, we cannot draw robust conclusions regarding different lymphoma subtypes due to the small sample size. To overcome these limitations, prospective randomized trials using CGA as a stratum criterion should be planned. We expect future studies to validate the efficacy of CGA-based therapy across different lymphoma subgroups.
In conclusion, the oncohematogeriatric approach to care using CGA enables geriatric intervention in older patients with lymphoma, classifies patients according to their frailty status, and aids the decision-making process by allowing individualized treatment tailored to patients’ overall condition and personal preferences. Our results reinforce the value of multidisciplinary teams that include geriatricians to personalize oncospecific therapy according to the clinical, functional and frailty status of each patient. This study is one of the first to demonstrate oncohematogeriatric assessment and intervention and its influence on treatment outcomes.
We propose incorporating a CGA protocol and ensuring the presence of geriatricians as part of a multidisciplinary care team as part of the optimal therapeutic strategy for older patients with lymphoma. If multidisciplinary or geriatric inputs are not available, it is important to design a predefined intervention plan [46] for these patients. Moving forward, there is a need for further studies on the role of CGA regarding prognosis and management of older adults with lymphoma. Future randomized studies should focus on providing evidence for optimal therapeutic options guided by geriatric assessment [47].
Availability of data and materials
The datasets generated and/or analyzed in the current study are not publicly available, but available from the corresponding author upon reasonable request.
References
REDECAN. Red Española de Registros de Cáncer, https://redecan.org/es/;2022 [Accessed 10 February 2022]
Surveillance, Epidemiology, and End Results (SEER), https://surveillance.cancer.gov/; 2022 [Accessed 13 March 2022]
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A (2018) Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol 19(6):e305–e316
Koll TT, Rosko AE (2018) Frailty in hematologic malignancy. Curr Hematol Malig Rep 13(3):143–154
Buske C, Hutchings M, Ladetto M, et al (2018) ESMO Lymphoma Consensus Conference Panel Members. ESMO Consensus Conference on malignant lymphoma: general perspectives and recommendations for the clinical management of the elderly patient with malignant lymphoma. Ann Oncol 29(3):544–562
Abel GA, Keplin HD (2018) Frailty and the management of hematologic malignancies. Blood 131(5):515–524
Tucci A, Ferrari S, Bottelli C, Borlenghi E, Drera M, Rossi G (2009) A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy. Cancer 115(19):4547–4553
Klepin HD (2019) Ready for prime time: role for geriatric assessment to improve quality of care in hematology practice. Blood 134(23):2005–2012
Hamaker ME, Prins MC, Stauder R (2014) The relevance of a geriatric assessment for elderly patients with a haematological malignancy–a systematic review. Leuk Res 38(3):275–283
Wildiers H, Heeren P, Puts M et al (2014) International Society of geriatric oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 32(24):2595–2603
Hurria A, Wildes T, Blair SL et al (2014) Senior adult oncology, version 2. 2014: clinical practice guidelines in oncology. J Natl Compr Canc Netw 12(1):82–126
Mohile SG, Dale W, Somerfield MR et al (2018) Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol 36(22):2326–2347
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol 5(6):649–655
Caballero-Martinez MJ, Cabrero-Garcia J, Richart-Martinez M et al (2009) The Spanish version of the Barthel index and the Katz index of activities of daily-living: a structured review. Arch Gerontol Geriatr 49(1):77–84
Alarcón T (2006) Escala de Lawton de actividades de la vida diaria. In: Cruz Jentoft AJ, González Montalvo JI, Alarcón Alarcón T, Rexach Cano L (eds) Curso sobre el uso de escalas de valoración geriátrica. Prous Science, Madrid, pp 39–43
Alarcón T (2006) Clasificación functional de la marcha (escala FAC). In: Cruz Jentoft AJ, González Montalvo JI, Alarcón Alarcón T, Rexach Cano L (eds) Curso sobre el uso de escalas de valoración geriátrica. Prous Science, Madrid, pp 73–76
González-Montalvo JI, Alarcón-Alarcón MT, Salgado-Alba A (1993) Valoración del estado mental en el anciano. In: Salgado A, Alarcón MT (eds) Valoración del paciente anciano. Masson, Barcelona, pp 73–103
Cruz Jentoft AJ (2006) Global deterioration scale (GDS) de Reisberg. In: Cruz Jentoft AJ, González Montalvo JI, Alarcón Alarcón T, Rexach Cano L (eds) Curso sobre el uso de escalas de valoración geriátrica. Prous Science, Madrid, p 115119
Martínez de la Iglesia J, Onís-Vilches MC, Dueñas-Herrero R, Albert-Colomer C, Aguado-Taberné C et al (2002) Versión española del cuestionario de Yesavage abreviado (GDS) para el despistaje de depresión en mayores de 65 años: adaptación y validación. Medifam 12(10):26–40
Alarcón T (2006) Miniexamen nutricional (mininutritional assessment). In: Cruz Jentoft AJ, González Montalvo JI, Alarcón Alarcón T, Rexach Cano L (eds) Curso sobre el uso de escalas de valoración geriátrica. Prous Science, Madrid, pp 175–179
Linn BS, Linn MW, Gurel L (1968) Cumulative illness rating scale. J Am Geriatr Soc 16(5):622–626
Guralnik JM, Simonsick EM, Ferrucci L et al (1994) A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49(2):M85-94
Morley JE, Malmstrom TK, Miller DK (2012) A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 16(7):601–608
Sehn LH, Berry B, Chhanabhai M et al (2007) The revised international prognostic index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 109(5):1857–1861
Freites-Martinez A, Santana N, Arias-Santiago S, Viera A (2021) Using the common terminology criteria for adverse events (CTCAE – Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed). 112(1):90–92
Balducci L, Extermann M (2000) Management of cancer in the older person: a practical approach. Oncologist 5(3):224–237
Balducci L, Beghe C (2000) The application of the principles of geriatrics to the management of the older person with cancer. Crit Rev Oncol Hematol 35(3):147–154
Ramírez-Martín R, Pérez-Rodríguez P, Menéndez-Colino R et al (2022) Prehabilitation and perioperative geriatric care in patients aged over 80 years with colorectal cancer: results of a cross-speciality geriatrics program. J Geriatr Oncol 13(6):813–820
Soubeyran P, Terret C, Bellera C et al (2016) Role of geriatric intervention in the treatment of older patients with cancer: rationale and design of a phase III multicenter trial. BMC Cancer 16(1):932
Rodríguez Couso M (2020) Geriatric interventions in the older with cancer based on comprehensive geriatric assessment. Optimization areas: what the geriatrician can contribute to the multidisciplinar team. Nutr Hosp 34(Spec No. 1):38–47.
Pi-Figueras Valls M, Hormigo Sánchez A, Rodríguez Couso M, et al (2019) Maniobras de intervención en el anciano oncológico (I)(II). In: Molina Garrido MJ, Balducci L (ed) Fundamentos en Oncogeriatría. Tratado de Oncología médica en el paciente anciano. Madrid: Eds Mederic S.L p. 239–328
Izquierdo M (2019) Prescripción de ejercicio físico. El programa Vivifrail como modelo. Nutr Hosp 36:50–56
Delgado Silveira E, Montero Errasquín B, Muñoz García M, Vélez-Díaz-Pallarés M, Lozano Montoya I, Sánchez-Castellano C, Cruz-Jentoft AJ (2015) Mejorando la prescripción de medicamentos en las personas mayores: una nueva edición de los criterios STOPP-START [Improving drug prescribing in the elderly: a new edition of STOPP/START criteria]. Rev Esp Geriatr Gerontol 50(2):89–96
Goede V (2023) Frailty and cancer: current perspectives on assessment and monitoring. Clin Interv Aging 28(18):505–521. https://doi.org/10.2147/CIA.S365494
Nabhan C, Smith SM, Helenowski I et al (2012) Analysis of very elderly (≥80 years) non-Hodgkin lymphoma: impact of functional status and co-morbidities on outcome. Br J Haematol 156(2):196–204
Wieringa A, Boslooper K, Hoogendoorn M et al (2014) Comorbidity is an independent prognostic factor in patients with advanced-stage diffuse large B-cell lymphoma treated with R-CHOP: a population-based cohort study. Br J Haematol 165(4):489–496
Akhtar OS, Huang L-W, Tsang M et al (2022) Geriatric assessment in older adults with non-Hodgkin lymphoma: a young international society of geriatric oncology (YSIOG) review paper. J Geriatr Oncol 13(5):572–581
Tucci A, Martelli M, Rigacci L et al (2015) Italian lymphoma foundation (FIL). Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B-cell lymphoma: a prospective multicenter evaluation in 173 patients by the lymphoma italian foundation (FIL). Leuk Lymphoma 56(4):921–926
Miura K, Konishi J, Miyake T et al (2017) A host-dependent prognostic model for elderly patients with diffuse large B-cell lymphoma. Oncologist 22(5):554–560
Liu H, Zhang CL, Feng R, Li JT, Tian Y, Wang T (2018) Validation and refinement of the age, comorbidities, and albumin index in elderly patients with diffuse large B-cell lymphoma: an effective tool for comprehensive geriatric assessment. Oncologist 23(6):722–729
Minnella EM, Carli F (2018) Prehabilitation and functional recovery for colorectal cancer patients. Eur J Surg Oncol 44(7):919–926
Goh N, Tan K-Y (2019) Effect of multidisciplinary prehabilitation-rehabilitation on out- comes after colorectal surgery in elderly patients. Asian J Gerontol Geriatr 14(1):5–9
Garric M, Sourdet S, Cabarrou B et al (2021) Impact of a comprehensive geriatric assessment on decision-making in older patients with hematological malignancies. Eur J Haematol 106(5):616–626
Corre R, Greillier L, Le Caër H et al (2016) Use of a comprehensive geriatric assessment for the management of elderly patients with advanced non-small-cell lung cancer: the phase III randomized ESOGIA-GFPC-GECP 08–02 study. J Clin Oncol 34(13):1476–1483
Presley CJ, Mohamed MR, Culakova E et al (2022) A geriatric assessment intervention to reduce treatment toxicity among older adults with advanced lung cancer: a subgroup analysis from a cluster randomized controlled trial. Front Oncol 12:835582
Hamaker M, Lund C, Te Molder M et al (2022) Geriatric assessment in the management of older patients with cancer - a systematic review (update). J Geriatr Oncol 13(6):761–777
Acknowledgements
Thanks to all the physicians of the department of Hematology at Hospital Universitario Fundación Jimenez Diaz for making teamwork easy.
Funding
One of the co-authors was supported by the Pfizer grant no. 56565519. This grant did not provide financial support for performing research, including the study design, data collection, data analysis and interpretation.
Author information
Authors and Affiliations
Contributions
AIHS literature search, conception and design, data collection, analysis and interpretation of data, manuscript preparation and writing, tables, approval of final document. ALG Conception and design, data collection, data analysis and interpretation of data, approval of final document. RC conception and design, interpretation data analysis, approval of final document. IMG statistical analysis and approval of final document. JIGM writing tables, analysis and interpretation of data, approval of final document. DM interpretation data analysis, approval of final document. EA interpretation data analysis, approval of final document. MAPS interpretation data analysis, approval of final document. CUR interpretation data analysis, approval of final document. MRO interpretation data analysis, approval of final document. FJMP interpretation data analysis, approval of final document. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests related to this work.
Ethics approval
The study was approved by the Hospital Clinical Research Ethics Committee of the Fundación Jiménez Díaz University Hospital (EO121-21_FJD).
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hormigo-Sanchez, A.I., Lopez-Garcia, A., Mahillo-Fernandez, I. et al. Frailty assessment to individualize treatment in older patients with lymphoma. Eur Geriatr Med 14, 1393–1402 (2023). https://doi.org/10.1007/s41999-023-00870-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-023-00870-2