Key summary points
To investigate the performance of an adverse drug reaction (ADR) trigger tool in patients with polypharmacy acutely admitted to our geriatric ward.
AbstractSection FindingsThe ADR trigger tool had a positive predictive value (PPV) of 41.8%. Usual care recognised 83.5% of ADRs considered as possible, probable or certain, increasing to 97.1% when restricted to probable and certain ADRs.
AbstractSection MessageIt is unlikely that implementation of the ADR trigger tool will improve detection of unrecognised ADRs in older patients acutely admitted to our geriatric ward.
Abstract
Purpose
Adverse drug reactions (ADRs) account for 10% of acute hospital admissions in older people, often under-recognised by physicians. The Dutch geriatric guideline recommends screening all acutely admitted older patients with polypharmacy with an ADR trigger tool comprising ten triggers and associated drugs frequently causing ADRs. This study investigated the performance of this tool and the recognition by usual care of ADRs detected with the tool.
Methods
A cross-sectional study was performed in patients ≥ 70 years with polypharmacy acutely admitted to the geriatric ward of the University Medical Centre Utrecht. Electronic health records (EHRs) were screened for trigger–drug combinations listed in the ADR trigger tool. Two independent appraisers assessed causal probability with the WHO-UMC algorithm and screened EHRs for recognition of ADRs by attending physicians. Performance of the tool was defined as the positive predictive value (PPV) for ADRs with a possible, probable or certain causal relation.
Results
In total, 941 trigger–drug combinations were present in 73% (n = 253/345) of the patients. The triggers fall, delirium, renal insufficiency and hyponatraemia covered 86% (n = 810/941) of all trigger–drug combinations. The overall PPV was 41.8% (n = 393/941), but the PPV for individual triggers was highly variable ranging from 0 to 100%. Usual care recognised the majority of ADRs (83.5%), increasing to 97.1% when restricted to possible and certain ADRs.
Conclusion
The ADR trigger tool has predictive value; however, its implementation is unlikely to improve the detection of unrecognised ADRs in older patients acutely admitted to our geriatric ward. Future research is needed to investigate the tool’s clinical value when applied to older patients acutely admitted to non-geriatric wards.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Older people are more susceptible to adverse drug reactions (ADRs) due to comorbidity, polypharmacy, frailty and age-related changes in pharmacokinetics and -dynamics [1,2,3]. It is estimated that ADRs account for approximately 10% of all acute hospital admissions in older people [4, 5]. Despite this high frequency of hospital admissions due to ADRs in older people, studies show that drug-related problems, including ADRs, are missed or misdiagnosed by physicians at the emergency department in approximately 40–60% of the cases [6,7,8]. Consequently, methods to improve detection and management of ADRs are needed [9].
Polypharmacy is one of the most important risk factors for developing ADRs [10]. It is known that a few commonly used drug classes account for the majority of ADRs leading to or developed during hospital admission in the older population [1, 3,4,5, 9]. A meta-analysis found that ADR-induced hospital admissions were most frequently related to non-steroidal anti-inflammatory drugs (NSAIDs) causing upper gastrointestinal bleeding, hypertension, coronary events and renal failure. Other ADRs frequently associated with hospitalisations were hypotension due to beta-blockers, angiotensin-converting enzyme (ACE) inhibitors or calcium antagonists; hypoglycaemia due to oral antidiabetics; bleeding due to oral anticoagulants and bradycardia due to digoxin [4]. The use of a trigger tool focusing on clinical events and drugs frequently associated with such events may, therefore, reduce the problem of undiagnosed ADRs.
Several trigger tools have been developed to increase ADR detection in patient care. The most commonly known trigger tool is the Global Trigger Tool [11, 12], but other trigger tools targeting ADR detection, especially in the older population, have been investigated [13,14,15]. These trigger tools have in common that they comprise lists of either clinical events (e.g. ‘hypotension’), the use of specific drugs or antidotes (e.g. ‘naloxone use’) or abnormal drug or laboratory values (e.g. ‘potassium < 2.9 mEq/L’, ‘digoxin level > 2 ng/L’). However, the positive predictive values (PPVs) of such triggers were generally low, which impedes their implementation in clinical practice to improve ADR detection in older people [12,13,14,15]. Consequently, no ‘gold standard’ to improve ADR detection in older people has yet been established.
The performance of trigger tools in detecting clinically relevant ADRs in older people may be improved by combining clinical events with drug classes frequently associated with such events. The Dutch national geriatric guideline on ‘polypharmacy optimisation in hospitalised older people’ provides a consensus-based trigger tool listing combinations of certain clinical events and associated drugs that frequently result in ADR-related hospital admissions in older people [16]. The guideline strongly recommends screening each patient aged 70 years and older with polypharmacy (≥ 5 drugs) admitted to the emergency department for potential ADRs by using this ADR trigger tool. However, the recommendation has not been substantiated by evidence supporting the use of such a trigger tool in clinical practice. Hence, evaluation of the performance of the ADR trigger tool in the above-mentioned guideline is warranted.
This study aimed to investigate the performance of the ADR trigger tool recommended by the Dutch geriatric guideline and the recognition by usual care of ADRs detected with the tool in patients with polypharmacy acutely admitted to our geriatric ward.
Methods
Setting and study population
The study population consisted of patients aged 70 years and older with polypharmacy acutely admitted to the geriatric ward at a 1000 bed tertiary university hospital in the Netherlands (University Medical Centre Utrecht). Admissions of patients to the geriatric ward through the emergency department (ED) in the period between 01-01-2011 and 01-08-2017 were extracted with SAS enterprise guide v7.1 from a pseudonymised hospital database. Based on the consecutive order of randomly assigned numbers for each patient, admission letters were manually screened to include approximately 350 patients aged ≥ 70 years with polypharmacy. Polypharmacy was defined as the chronic use of at least five prescription drugs excluding dermatological preparations at admission [16]. For patients with multiple hospital admissions during the study period, the first admission that met the inclusion criteria was selected. A patient’s first admission was selected to minimise interference of consecutive hospital admissions with the study outcomes. Patients with an incomplete record (i.e. no admission or discharge letter available) were excluded.
Study procedures
Electronic health records (EHRs) from the ED on the day of admission were screened for trigger–drug combinations listed in the ADR trigger tool of the Dutch national geriatric guideline ‘polypharmacy optimisation in hospitalised older people’ (first publication 2017, last revision 2020) [16]. This consensus-based trigger tool was developed in accordance with literature listing ten clinical events (i.e. triggers) and their associated drug classes frequently resulting in ADR-related admissions in older people [16,17,18]. Next, a causality assessment was performed for all detected trigger–drug combinations. The admission and discharge letters were also screened for ADR recognition by the attending physicians.
Screening for trigger–drug combinations
For this study, the original ADR trigger tool from the Dutch guideline was explicated to reduce undesirable variations in interpretation when applied to EHRs. Modifications to the original ADR trigger tool were implemented at three levels prior to screening for trigger–drug combinations:
-
1)
Triggers were specified if they represented clinical events which could be linked to different drug classes (e.g. specification of ‘disturbed serum glucose levels’ into ‘hypoglycaemia’ and ‘hyperglycaemia’).
-
2)
Drug classes were further specified following the ATC classification system (e.g. specification of ‘diuretics’ into ‘thiazide diuretics’, ‘loop diuretics’ and ‘potassium sparing diuretics’).
-
3)
Triggers were merged for clinical events that are difficult to distinguish and are used interchangeably in clinical practice. For instance, ‘fall’ was merged with the triggers ‘collapse/(orthostatic) hypotension/dizziness/syncope’. Especially in older patients, it is difficult to distinguish falls and syncope, because falls can be preceded by temporarily loss of consciousness due to cerebral hypoperfusion [19].
Modifications to the original ADR trigger tool were performed by two researchers with clinical experience in medical practice (WL, NN) and reviewed by a senior geriatrician/clinical pharmacologist (WK) with the intention to follow the original ADR trigger tool as closely as possible. Table 1 illustrates the original ADR trigger tool as published in the Dutch national geriatric guideline and the explicated ADR trigger tool used for this research.
Two researchers (WL, NN) screened EHRs for the presence of trigger–drug combination. The trigger had to be either documented as a symptom, or listed by the physician as a diagnosis or health problem. Trigger–drug combinations were regarded as discrete events if the prescribed drugs were related to different drug classes according to the explicated trigger tool. However, if multiple drugs from the same drug class were linked to the same trigger, this was counted as one trigger–drug combination. For example, oxycodone and morphine linked to constipation were considered as one trigger–drug combination (constipation-opioids), while hydrochlorothiazide (thiazide diuretics) and furosemide (loop diuretics) linked to hyponatraemia were considered as two separate trigger–drug combinations.
Causality assessment
A causality assessment was performed to establish the likelihood of an ADR for all trigger–drug combinations detected with the ADR trigger tool. Data from the admission and discharge letters were taken into account, because both letters could contain relevant information for causality assessment (e.g. to establish a potential time-relationship). A geriatrician (NN) and a clinical pharmacist (BS) independently assessed all trigger–drug combinations. The WHO-UMC system was used for causality assessment, which differentiates between the categories certain, probable, possible, unlikely and unclassifiable [20, 21]. Trigger–drug combinations with a causality score of certain, probable and possible were considered ADRs. Before the causality assessment, both appraisers trained with a previously published, Delphi-based chart review method developed to detect drug-related admissions by Thevalin et al.[22]. The level of agreement between the two appraisers was measured with the Cohen’s kappa test statistic (poor: κ < 0.00; slight: κ = 0.00–0.20; fair: κ = 0.21–0.40; moderate: κ = 0.41–0.60; substantial: κ = 0.61–0.80; almost perfect: κ = 0.81–1.00) [23]. If ratings differed ≥ 1 WHO-UMC category for causality between the two appraisers, the appraisers discussed each case to reach consensus. The appraisers consulted a third expert (WK, senior geriatrician-clinical pharmacologist) for a final consensus round in case no consensus was reached.
ADR recognition by usual care
In addition to the causality assessment, EHRs were screened for recognition of ADRs by usual care. Recognition was defined as an explicit documented trigger–drug combination by the attending physician (i.e. a geriatric resident, supervised by a geriatrician) in the admission and/or discharge letter, implying that the trigger–drug combination was identified as an ADR. In addition, explicit documentation of the trigger combined with medication changes in associated drugs (i.e. withdrawal, discontinuation or a dose adjustment) was also considered as being recognised by usual care.
Outcomes
The performance of the ADR trigger tool was operationalised by calculating the overall PPV for detecting ADRs in general and for each trigger separately. The PPV was defined as the total number of detected trigger–drug combinations divided by the number of ADRs with a causality score of possible, probable or certain. The recognition by usual care was calculated for both ADRs with a causal relationship considered to be possible, probable or certain and for those with a probable or certain causal relationship.
Data analysis
Descriptive data analysis and Cohen’s kappa test statistic was performed with IBM SPSS Statistics v.26.0.0.1.
Results
Study population
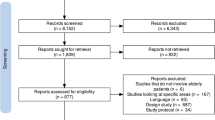
A random selection of 589 out of all 1366 patient admissions to the geriatric department through the ED between 01-01-2011 and 01-08-2017 was screened for eligibility. From this selection, 378 admissions met our inclusion criteria (i.e. age ≥ 70 and polypharmacy), of which 33 admissions were excluded because they were not a patient’s first admission within the study period. The study population of 345 patients had a median age of 84 (IQR 79–88). The median number of drugs at admission was 10 (IQR 8–13), and 61% of the patients were female. Subsequently, admission letters of these patients were screened for the presence of trigger–drug combinations according to the ADR trigger tool. Out of 345 eligible patients, 253 (73%) had at least one trigger–drug combination present. In 52% (178/345) of the total study population, at least one ADR with a causal relationship considered possible, probable or certain was present.
Number of trigger–drug combinations
The total number of trigger–drug combinations was 941, with a median of 3 (IQR 2–5) and a maximum of 16 trigger–drug combinations per patient. Fall (32.4%), delirium (24.0%), renal insufficiency/dehydration (16.2%) and hyponatraemia (13.5%) were the most frequent clinical events and covered 86.3% of all identified trigger–drug combinations (Table 2).
Causality assessment and PPV
Of the 941 identified trigger–drug combinations, 41.8% (n = 393) were adjudicated as an ADR by the two appraisers in 178 patients. More than a quarter (27.0%) of all 941 trigger–drug combinations were considered as possible ADRs, 12.3% were adjudicated as probable ADRs, and 2.4% as certain ADRs. In 57.0% of the trigger–drug combinations, an ADR was considered as unlikely, and the other 1.3% of the combinations were unclassifiable (Table 2). Inter-rater agreement for causality assessment of ADRs was substantial (κ = 0.61–0.80) with a Cohen’s kappa of 0.76 [23]. In total, causality scores of 163/941 trigger–drug combinations differed between the adjudicators, with a difference of only one WHO-UMC category in 91.1% of the cases (n = 149). The two appraisers reassessed and discussed all discrepancies and reached a consensus without consulting a third expert.
Overall, the PPV of the ADR trigger tool was 41.8%. The PPV varied considerably across triggers. The PPV related to the triggers fall (28.2%) and delirium (23.0%) were the lowest, whereas the mean number of drugs associated with these triggers was highest with a large range (fall: mean 3.1, min–max 1–8; delirium: mean 2.3, min–max 1–6). Although numbers were relatively small, the PPVs related to the triggers hypokalaemia (100%), supratherapeutic INR (100%) and vomiting/diarrhoea (88.9%) were highest (Table 2).
Drugs related to ADRs
More than half of the 941 trigger–drug combinations detected by the ADR trigger tool were associated with three drug classes: diuretics (25.4%), agents acting on the renin–angiotensin system (16.7%) and psychotropic agents (12.2%). The top three drug classes most frequently associated with the 393 ADRs were diuretics (35.4%), agents acting on the renin–angiotensin system (13.5%) and analgesics (11.2%), covering 60% of all drugs that caused an ADR.
ADR recognition by usual care
Usual care recognised 51.8% (481/929) of the trigger–drug combinations detected by the trigger tool and for which a causality classification could be determined. 42.3% (393/929) were considered ADRs with at least possible causality, of which 83.5% (328/393) were recognised by usual care according to information in the admission and discharge letters (Table 3). 16.5% (65/393) of ADRs were not recognised by usual care, of which 93.9% (n = 61) had a causal relationship considered to be possible. The majority of these possible ADRs not recognised by usual care were related to the top three most common events (fall, n = 29; delirium, n = 13; renal insufficiency, n = 10). Three probable ADRs were not recognised (furosemide–hyponatraemia; fentanyl–constipation; fentanyl–delirium) and one certain ADR was not recognised by usual care (bumetanide–renal insufficiency/dehydration). Recognition by usual care increased to 97.1% (135/139) when only ADRs considered to be probable or certain ADRs were included (Table 3).
In 75.6% of possible, probable or certain ADRs and in 85.6% of probable or certain ADRs, the suspected drug was discontinued, or the dosage was reduced by usual care. The top three most frequently discontinued drugs related to ADRs were thiazides, opioids and high-ceiling diuretics. Table 3 provides a detailed overview of the number of ADRs per trigger and their associated drug classes in relation to their recognition by usual care. ADRs were stratified for a causal relationship considered to be possible, probable or certain and for those considered to be probable or certain.
Discussion
Main findings
ADRs were highly prevalent in older patients with polypharmacy acutely admitted to the geriatric ward. The ADR trigger tool detected one or more trigger–drug combinations at admission in almost three quarters (73%) of all screened patients, and more than half (52%) of these patients had at least one confirmed ADR after causality assessment. The overall PPV of the ADR trigger tool was 41.8%, indicating that less than half of the trigger–drug combinations were considered to be ADRs. Usual care recognised the majority of ADRs (83.5%), increasing to 97.1% when restricted to possible and certain ADRs.
Performance
The performance of the ADR trigger tool recommended by the Dutch geriatric guideline was not previously studied. Using an ADR trigger tool may be a helpful and efficient strategy to increase ADR detection in older people, especially in cases of low recognition by usual care. A high PPV is important for a positive balance between reviewing signals and detecting actual ADRs. Although there is no generally accepted definition to distinguish ‘good’ from ‘poor’ trigger tool performance—which also depends on its intended use—a PPV ≥ 20% is often considered good [23, 24]. In our study, the PPV per trigger of the investigated ADR trigger tool was highly variable, ranging from 0–100%. However, if triggers with a frequency of only one were excluded, all triggers had a PPV ≥ 20%, of which the PPVs for the triggers ‘fall/…/dizziness’ (PPV 28%) and ‘delirium/…/drowsiness’ (PPV 23%) were lowest. These clinical events often have multiple possible causes related to comorbidity, drugs and drug combinations, impeding the confirmation of a clear causal relationship. The mean number of drugs related to these two events at a patient’s level were highest. In contrast, trigger–drug combinations based on clinical events related to a single drug class (e.g. vitamin K antagonist–supratherapeutic INR) or for which a dechallenge usually results in a direct improvement (e.g. diuretics–hypokalaemia) were more likely considered to be ADRs.
The low PPV for triggers related to fall and delirium are in line with other findings. Carnevali et al. found a PPV for the triggers ‘fall’ and ‘emergence of confused state’ of 19% and 9%, respectively, in hospitalised adults [12]. In addition, a French retrospective cohort study in acutely admitted geriatric patients investigated the triggers ‘fall’ and ‘delirium’ from the Global Trigger Tool [11, 25]. The mean number of suspected drugs per patient related to these clinical events was comparable with our results, as well as the PPV for delirium (21% vs 23%). However, the PPV for falls was much higher (54% vs. 28%), which is likely due to differences in the ADR causality method used; the relationship between the suspected drug and the identified ADRs in this French study was uncertain in over 80%. Removing the triggers for falls and delirium from the ADR trigger tool will increase the overall PPV of the ADR trigger tool from 41.8% to 62.2% (n = 255/410, Table 2). Nevertheless, we would not recommend excluding falls and delirium as triggers because these clinical events are often associated with drug-related admissions in older patients with polypharmacy [24]. In addition, a large proportion of ADRs would be excluded (35%, n = 138/393), and recognition by usual care for these triggers was lowest for ADRs of at least possible causality (Table 3). To increase the PPV, we would rather suggest to explore strategies for excluding drugs with a relatively low risk on the clinical event. A recent observational study compared the association of potentially inappropriate medication on inpatient falls listed in the explicit screening tools STOPP v2, STOPP v2 section K, and STOPPFall [26,27,28] Although all screening tools were independently associated with falls, the strongest effect was identified for STOPP section K [28]. This is plausible because STOPP section K is the most restrictive tool, including only four drug classes with highest risk of falls (i.e. benzodiazepines, hypnotic z-drugs, vasodilator drugs, and neuroleptic drugs). For delirium, selecting drugs with the highest anticholinergic burden will likely increase the PPV. However, a disadvantage of excluding drugs from the ADR trigger tool is that less ADRs may be detected.
The difficulties in achieving a high PPV in ADR detection were illustrated in a systematic review on methods to detect drug-related problems. This systematic review identified 28 studies, three of which used a trigger tool to detect ADRs [29]. The PPVs of these ADR trigger tools ranged from 1.8% to 32% [30,31,32]. The study with the lowest PPV (1.8%) was the only one performed in a geriatric population (rehabilitation ward) using a commercially available database grounded on potential ADRs extracted from a drug’s product information [30]. The highest PPV was reported in patients (age 16–90 years) admitted to a gastroenterology department using a trigger tool solely based on laboratory signals [32]. The use of trigger tools appeared to be the most labor-efficient method; however, incident report review generally showed a higher specificity compared to other methods.
More recently, Zerah et al. evaluated the PPV of a trigger tool to detect adverse drug events (ADEs) and drug-related admissions (DRAs) in older people based on chart review [24]. The DRA trigger tool comprised 26 triggers and associated drugs frequently involved in ADEs. The DRA trigger tool was more comprehensive than the ADR trigger tool used in our study and included triggers to detect ADEs, including both ADRs and medication errors (i.e. underuse, overuse and misuse of drugs). The overall PPV for the detection of ADEs of the DRA trigger tool was 87% [24]. The better performance of the DRA trigger tool compared with the ADR trigger tool may be explained by the inclusion of medication errors, which had a large impact on the PPV. For instance, 11.8% (n = 76) of all ADEs with a causal relationship were related to the trigger ‘heart failure’, with the majority of these ADEs being adjudicated as underuse of beta-blockers, ACE-inhibitors, and diuretics [24]. For this reason, the PPVs of these two tools are difficult to compare.
ADR recognition by usual care
In addition to aiming for a high PPV, an ADR trigger tool needs to be of clinical value to usual care and increase the detection of unrecognised ADRs. Previous studies reported that drug-related problems are missed or misdiagnosed in approximately 40–60% of the cases by physicians at the ED; however, we found a much higher recognition by usual care of ADRs identified with the use of the ADR trigger tool [6,7,8]. There are several explanations for this discrepancy. First, we investigated a subset of most frequent and serious ADRs in older people targeted by the ADR trigger tool, which cannot be compared with the broader definition of ‘drug-related problems’ in previous studies. In addition, our study was performed in an academic, teaching hospital and all patients were under geriatric care. Compared to other specialists, geriatric residents are well trained in detecting drug-related problems in their patients under the direct supervision of experienced geriatricians [33]. The high recognition of ADRs found in our study was comparable with the results of Klopotowska et al. who found that 80% of ADRs of at least possible causality in older hospitalised patients admitted to an internal medicine ward were recognised by usual care during the hospital stay [34]. Similar to our results, the majority of unrecognised ADRs were those with a possible causality score [34].
Strengths and limitations
If implemented in daily practice, the PPV as a measure for performance is an important outcome to assess the relevance of triggers. The reported ADR recognition by usual care is highly relevant in deciding whether implementation of such a tool would add clinical value to usual patient care.
To ensure that ADR recognition by usual care was not biased, we selected patients who were admitted before publication of the tool in the national guideline. Two independent clinicians thoroughly and manually screened admission letters for trigger–drug combinations, followed by causality assessment by a geriatrician and a clinical pharmacist revealing substantial inter-rater agreement (κ = 0.76).
There are, however, several limitations to this research. First, EHRs were only screened for trigger–drug combinations listed in the ADR trigger tool. Therefore, the negative predictive value, sensitivity or specificity of the tool could not be calculated. Second, retrospective studies based on chart review rely on documented information by attending physicians. The introduction of information bias by physician’s notes and actions cannot be fully ruled out. For instance, the screening of trigger–drug combinations was based on information documented in admission letters and laboratory results were not examined as a primary source of triggers. A mild hyponatraemia with concomitant use of diuretics could potentially have been missed as trigger–drug combination if it was not mentioned as a clinical problem by the attending physician. However, the triggers listed in the ADR trigger tool are serious and admission letters were comprehensive, which makes underreporting of these triggers unlikely.
Third, the definition of ‘recognition by usual care’ was not very specific since a documented event combined with discontinuation or a dose adjustment of the associated drug was also considered as being ‘recognised’ without explicit mention. However, this does not necessarily correspond with ADR recognition because drugs could be discontinued for other reasons (e.g. a lack of indication). In addition, the persistence of drug changes after hospital discharge was not evaluated in our study. A discontinuation or dose adjustment of the suspected drug was implemented by the attending physician in three quarters of ADRs, but previous research illustrated that a quarter of drugs discontinued because of an ADR were re-prescribed after admission [35].
In addition, this study was performed in a specific population of older patients with polypharmacy acutely admitted to a geriatric ward. The admission to a geriatric ward in an academic, teaching hospital could have biased the type and prevalence of certain trigger–drug combinations. For instance, patients presenting with fall and delirium are likely to be admitted to a geriatric ward; these clinical events were most prevalent in our population comprising more than half of all identified trigger–drug combinations. Consequently, these two triggers had the largest impact on the overall PPV of the ADR trigger tool. In contrast, the clinical event ‘intracranial bleeding’ was absent in our population and, thus, had no impact on the overall PPV. Acutely admitted patients with an intracranial bleeding are more likely to be admitted to a neurosurgical ward instead of a geriatric ward. Furthermore, geriatric residents and their supervisors in an academic, teaching hospital may be more focused on ADR recognition compared to other medical specialties. For these reasons, the generalisation of ADR prevalence and ADR recognition are limited. Lastly, the PPV was not stratified for different patient populations because the availability of baseline patient characteristics was limited.
Implications
The ADR trigger tool detected ADRs in more than half (52%) of all patients with polypharmacy acutely admitted to the geriatric ward. Combining the ADR trigger tool with ADR risk-prediction models may be a good future strategy to identify older patients at highest risk of ADRs, potentially increasing the predictive value of the tool. However, currently available ADR risk-prediction models for use in older people, such as the GerontoNet ADR risk scale and the Adverse Drug Reaction Risk in Older Persons (ADRROP) prediction scale, failed to predict ADRs well, and the most important risk factor for the occurrence of ADRs—polypharmacy—was already included in our study [10, 36,37,38].
ADR recognition by geriatric residents/geriatricians was very high for ADRs detected with the trigger tool in the setting of a tertiary university teaching hospital. Therefore, implementation of this trigger tool is not likely to improve care for older patients acutely admitted to our geriatric ward. However, ADR recognition by physicians less experienced in ADR detection in older people may be lower. Future research could focus on the clinical value of the tool if used in older patients acutely admitted to non-geriatric wards. In addition, it would be interesting to investigate if the ADR trigger tool could decrease the time to ADR detection, for example, when integrated with electronic healthcare systems. The use of clinical decision support systems to improve in-hospital fall and delirium care (e.g. reminders for patient screening and support to review medication) was identified as a facilitator in a recent interview study among Dutch healthcare professionals [39]. However, the risk of alert fatigue was also addressed as a potential barrier for this strategy [39]. In view of our results, we highly recommend conducting performance and feasibility studies before recommending ADR trigger tools as a standard of care.
Conclusion
The ADR trigger tool has predictive value (PPV 41.8%), but implementation of this tool is not likely to improve ADR recognition in older patients acutely admitted to our geriatric ward because the majority of ADRs were recognised by usual care.
References
Davies EA, O’Mahony MS (2015) Adverse drug reactions in special populations—the elderly. Br J Clin Pharmacol. https://doi.org/10.1111/bcp.12596
Mangoni AA, Jackson SHD (2004) Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. https://doi.org/10.1046/j.1365-2125.2003.02007.x
Zazzara MB, Palmer K, Vetrano DL, Carfì A, Graziano O (2021) Adverse drug reactions in older adults: a narrative review of the literature. Eur Geriatr Med 12:463–473. https://doi.org/10.1007/s41999-021-00481-9
Oscanoa TJ, Lizaraso F, Carvajal A (2017) Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur J Clin Pharmacol. https://doi.org/10.1007/s00228-017-2225-3
Alhawassi TM, Krass I, Bajorek B, Pont LG (2014) A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interv Aging. https://doi.org/10.2147/CIA.S71178
Roulet L, Ballereau F, Hardouin JB, Chiffoleau A, Potel G, Asseray N (2014) Adverse drug event nonrecognition in emergency departments: an exploratory study on factors related to patients and drugs. J Emerg Med 46:857–864. https://doi.org/10.1016/j.jemermed.2013.11.124
Nickel CH, Ruedinger JM, Messmer AS, Maile S, Peng A, Bodmer M et al (2013) Drug—related emergency department visits by elderly patients presenting with non-specific complaints. Scand J Trauma Resusc Emerg Med 21:1. https://doi.org/10.1186/1757-7241-21-15
Hohl CM, Zed PJ, Brubacher JR, Abu-Laban RB, Loewen PS, Purssell RA (2010) Do emergency physicians attribute drug-related emergency department visits to medication-related problems? Ann Emerg Med 55:493-502.e4. https://doi.org/10.1016/j.annemergmed.2009.10.008
Nair NP, Chalmers L, Peterson GM, Bereznicki BJ, Castelino RL, Bereznicki LR (2016) Hospitalization in older patients due to adverse drug reactions—the need for a prediction tool. Clin Interv Aging 11:497–505. https://doi.org/10.2147/CIA.S99097
Onder G, Petrovic M, Tangiisuran B, Meinardi MC, Markito-Notenboom WP, Somers A et al (2010) Development and validation of a score to assess risk of adverse drug reactions among in-hospital patients 65 years or older: the GerontoNet ADR risk score. Arch Intern Med 170:1142–1148. https://doi.org/10.1001/archinternmed.2010.153
Griffin F, Resar R (2007) IHI global trigger tool for measuring adverse events. IHI Innov Ser White Pap. pp.1–44. http://www.ihi.org/resources/Pages/Tools/IntrotoTriggerToolsforIdentifyingAEs.aspx. Accessed 24 May 2022
Carnevali L, Krug B, Amant F, Van Pee D, Gerard V, de Bethune X et al (2013) Performance of the adverse drug event trigger tool and the global trigger tool for identifying adverse drug events: experience in a belgian hospital. Ann Pharmacother 47:1414–1419. https://doi.org/10.1177/1060028013500939
Toscano Guzmán MD, Galván Banqueri M, Otero MJ, Sánchez Fidalgo S, Font Noguera I, Pérez Guerrero MC (2018) Validating a trigger tool for detecting adverse drug events in elderly patients with multimorbidity (TRIGGER-CHRON). J Patient Saf 2:1–7. https://doi.org/10.1097/pts.0000000000000552
Otero MJ, Toscano Guzmán MD, Galván-Banqueri M, Martinez-Sotelo J, Santos-Rubio MD (2020) Utility of a trigger tool (TRIGGER-CHRON) to detect adverse events associated with high-alert medications in patients with multimorbidity. Eur J Hosp Pharm. https://doi.org/10.1136/ejhpharm-2019-002126
Singh R, McLean-Plunckett EA, Kee R, Wisniewski A, Cadzow R, Okazaki S et al (2009) Experience with a trigger tool for identifying adverse drug events among older adults in ambulatory primary care. Qual Saf Heal Care 18:199–204. https://doi.org/10.1136/qshc.2007.024406
Dutch society for geriatric medicine. Multidisciplinary guideline for polpharmacy in older people. Addendum: polypharmacy optimisation in hospitalised older people. 2017:1–166. https://richtlijnendatabase.nl/richtlijn/polyfarmacie_bij_ouderen/polyfarmacie_bij_ouderen_2e_lijn.html (last Accessed: 16-03-2022)
Warlé-van Herwaarden MF, Valkhoff VE, Herings RMC, Engelkes M, Van Blijderveen JC, Rodenburg EM et al (2015) Quick assessment of drug-related admissions over time (QUADRAT study). Pharmacoepidemiol Drug Saf. https://doi.org/10.1002/pds.3747
Warlé-van Herwaarden MF, Kramers C, Sturkenboom MC, van den Bemt PMLA, De Smet PAGM (2012) Targeting outpatient drug safety. Drug Saf 35:245–259. https://doi.org/10.2165/11596000-000000000-00000
Shaw FE, Kenny RA (1997) The overlap between syncope and falls in the elderly. Postgrad Med J. https://doi.org/10.1136/pgmj.73.864.635
Agbabiaka T, Savovic J, Ernst E (2008) Methods for causality assessment. Drug Saf 31:21–37
Center WHO-UMC (2009) WHO Causality assessment. Good Pharmacovigil Pract Guid. p. 39. https://www.who.int/publications/m/item/WHO-causality-assessment. Accessed 24 May 2022
Thevelin S, Spinewine A, Beuscart JB, Boland B, Marien S, Vaillant F et al (2018) Development of a standardized chart review method to identify drug-related hospital admissions in older people. Br J Clin Pharmacol. https://doi.org/10.1111/bcp.13716
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics. https://doi.org/10.2307/2529310
Zerah L, Henrard S, Wilting I et al (2021) Prevalence of drug-drug interactions in older people before and after hospital admission: analysis from the OPERAM trial. BMC Geriatr 21(1):571. https://doi.org/10.1186/s12877-021-02532-z
Marseau F, Prud’Homm J, Bouzillé G, Polard E, Oger E, Somme D et al (2021) The trigger tool method for routine pharmacovigilance. J Patient Saf. https://doi.org/10.1097/pts.0000000000000820
O’mahony D, O’sullivan D, Byrne S, O’connor MN, Ryan C, Gallagher P (2015) STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 44:213–218. https://doi.org/10.1093/ageing/afu145
Seppala LJ, Petrovic M, Ryg J, Bahat G, Topinkova E, Szczerbinska K et al (2021) STOPPFall (screening tool of older persons prescriptions in older adults with high fall risk): a delphi study by the EuGMS task and finish group on fall-risk-increasing drugs. Age Ageing 50:1189–1199. https://doi.org/10.1093/ageing/afaa249
Damoiseaux-Volman BA, Raven K, Sent D, Medlock S, Romijn JA, Abu-Hanna A et al (2022) Potentially inappropriate medications and their effect on falls during hospital admission. Age Ageing 51:1–8. https://doi.org/10.1093/ageing/afab205
Meyer-Massetti C, Cheng CM, Schwappach DLB, Paulsen L, Ide B, Meier CR et al (2011) Systematic review of medication safety assessment methods. Am J Heal Pharm 68:227–240. https://doi.org/10.2146/ajhp100019
Egger T, Dormann H, Ahne G, Runge U, Neubert A, Criegee-Rieck M et al (2003) Identification of adverse drug reactions in geriatric inpatients using a computerised drug database. Drugs Aging 20:769–776. https://doi.org/10.2165/00002512-200320100-00005
Dormann H, Muth-Selbach U, Krebs S, Criegee-Rieck M, Tegeder I, Thomas Schneider H et al (2000) Incidence and costs of adverse drug reactions during hospitalization. Computerised monitoring versus stimulated spontaneous reporting. Drug Saf. https://doi.org/10.2165/00002018-200022020-00007
Dormann H, Criegee-Rieck M, Neubert A, Egger T, Levy M, Hahn EG et al (2004) Implementation of a computer-assisted monitoring system for the detection of adverse drug reactions in gastroenterology. Aliment Pharmacol Ther 19:303–309. https://doi.org/10.1111/j.1365-2036.2004.01854.x
Egger SS, Bachmann A, Hubmann N, Schlienger RG, Krähenbühl S (2006) Prevalence of potentially inappropriate medication use in elderly patients: comparison between general medical and geriatric wards. Drugs Aging. https://doi.org/10.2165/00002512-200623100-00005
Klopotowska JE, Wierenga PC, Smorenburg SM, Stuijt CCM, Arisz L, Kuks PFM et al (2013) Recognition of adverse drug events in older hospitalized medical patients. Eur J Clin Pharmacol 69:75–85. https://doi.org/10.1007/s00228-012-1316-4
Van Der Linden CMJ, Kerskes MCH, Bijl AMH, Maas HAAM, Egberts ACG, Jansen PAF (2006) Represcription after adverse drug reaction in the elderly: a descriptive study. Arch Intern Med. https://doi.org/10.1001/archinte.166.15.1666
Petrovic M, Van Der Cammen T, Onder G (2012) Adverse drug reactions in older people: detection and prevention. Drugs Aging 29:453–462. https://doi.org/10.2165/11631760-000000000-00000
Mangin D, Bahat G, Golomb BA, Mallery LH, Moorhouse P, Onder G et al (2018) International group for reducing inappropriate medication use & polypharmacy (IGRIMUP): position statement and 10 recommendations for action. Drugs Aging 35:575–587. https://doi.org/10.1007/s40266-018-0554-2
O’Mahony D, O’Connor MN, Eustace J, Byrne S, Petrovic M, Gallagher P (2018) The adverse drug reaction risk in older persons (ADRROP) prediction scale: derivation and prospective validation of an ADR risk assessment tool in older multi-morbid patients. Eur Geriatr Med 9:191–199. https://doi.org/10.1007/s41999-018-0030-x
Damoiseaux-Volman BA, Medlock S, van der Eijk MD, Romijn JA, Abu-Hanna A, van der Velde N (2021) Falls and delirium in older inpatients: Work-as-imagined, work-as-done and preferences for clinical decision support systems. Saf Sci 142:105355. https://doi.org/10.1016/j.ssci.2021.105355
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Authorship eligibility is based on the four ICMJE authorship criteria. The authors certify that they have participated in the aspects conception and design (NN, WK), acquisition of data (NN, BS, WL), interpretation of data (NN, BS, TE, EvP, IW, WK), drafting the article (BS, NN) and revising it critically for important intellectual content (NN, BS, WL, TE, EvP, IW, WK). All authors have approved the final article. NMFN and BTGM. Sallevelt are joint-first authors.
Corresponding author
Ethics declarations
Conflict of interests
The authors declared no conflicts of interest.
Ethics approval
The Research Ethics Committee of University Medical Centre Utrecht confirmed that the Medical Research Involving Human Subjects Act was not applicable to this study, and a waiver was granted (no. WAG/mb/17/024864).
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Noorda, N.M.F., Sallevelt, B.T.G.M., Langendijk, W.L. et al. Performance of a trigger tool for detecting adverse drug reactions in patients with polypharmacy acutely admitted to the geriatric ward. Eur Geriatr Med 13, 837–847 (2022). https://doi.org/10.1007/s41999-022-00649-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-022-00649-x