Key summary points
The aim was to investigate the association of severity of neuropsychiatric symptoms and health-related quality of life and their interaction with dementia severity among institutionalized older people with dementia.
AbstractSection FindingsNeuropsychiatric symptom burden was associated with higher health-related quality of life in residents with severe dementia, whereas among those residents with mild–moderate dementia this association was not seen. Very low functional capacity was linked to both low number of neuropsychiatric symptoms and low health-related quality of life among those with severe dementia.
AbstractSection MessageIn severe dementia, higher neuropsychiatric symptom burden and better health-related quality of life indicate better functioning and higher vitality.
Abstract
Purpose
The primary focus in long-term care is to maintain quality of life. The aim of this study was to investigate the association of severity of neuropsychiatric symptoms (NPS) and health-related quality of life (HRQoL) and their interaction with dementia severity among institutionalized older people with dementia.
Methods
352 long-term care residents aged 65 years or over with dementia participated in this cross-sectional study. NPS were measured with Neuropsychiatric Inventory (NPI). HRQoL was measured with 15D. Dementia severity was measured with Clinical Dementia Rating (CDR).
Results
The severity of NPS was significantly associated with better HRQoL in 15D. Residents with severe dementia (CDR 3) had worse HRQoL than residents with mild–moderate dementia (CDR < 3). There was a significant interaction between NPI and CDR (p = 0.037 for NPI, p < 0.001 for CDR, p < 0.001 for interaction). HRQoL correlated positively with all NPS subgroups in residents with severe dementia, but in residents with mild–moderate dementia, no significant correlation existed. In severe dementia, higher NPI correlated positively with such dimensions of 15D as mobility, vision, eating, speech, excretion, usual activities, mental functions, and vitality, whereas in residents with mild–moderate dementia only with mobility. In mild–moderate dementia, NPI correlated negatively with depression, distress and vitality.
Conclusion
Dementia severity and NPS burden are important determining factors of HRQoL in long-term care. NPS have a distinct impact on HRQoL at different stages of dementia. In severe dementia, higher NPS and better HRQoL indicate better functioning and higher vitality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia is characterized by cognitive decline, loss of autonomy in activities of daily living, and neuropsychiatric symptoms (NPS), all of which increase the risk of institutionalization as the disease progresses [1]. NPS among long-term care residents are very common. According to recent studies, the prevalence is 82–92% in long-term care settings [2, 3]. Longitudinal studies have revealed NPS to be persistent [4,5,6]. NPS include agitation, aggression, depression, anxiety, apathy, delusions, hallucinations, and sleep impairment [7]. These symptoms form clusters and four NPS subsyndromes: hyperactivity, psychosis, affective symptoms, and apathy, have been identified [8]. NPS have been associated with severity of cognitive impairments and declining functional abilities [9]. Several studies have also shown that having NPS impairs quality of life [10,11,12,13,14,15,16].
The primary focus in long-term care facilities is to improve or maintain quality of life. Thus, health-related quality of life (HRQoL) has been increasingly recognized as an important patient-centered outcome [17]. HRQoL is a multidimensional concept that encompasses the physical, emotional, and social components associated with illness or treatment [18]. Factors affecting HRQoL need to be identified to improve HRQoL.
Previous studies of NPS in long-term care have concentrated on the prevalence of NPS and its associations. So far, however, there has been a scarcity of studies focusing on the severity of NPS and its various implications [19]. Severity is important in understanding the burden of a symptom to both the resident and care staff [20]. To our knowledge, no earlier study has investigated the impact of NPS severity on HRQoL. Thus, the aim of this study was to determine whether an association exists between (1) the severity of NPS or (2) specific NPS subsyndromes and HRQoL, and further, (3) whether there is an interaction with NPS, HRQoL, and severity of dementia. In addition, we aimed to examine the characteristics of residents with the highest NPS burden and possible correlations between different dimensions of HRQoL and NPS to advance our understanding of factors associated with HRQoL in institutionalized older people with dementia.
Methods
Participants
The participants were recruited to this cross-sectional cohort study from institutional settings in Helsinki in 2017. The study was offered to all 54 nursing homes in Helsinki. Of them, the first 18 to volunteer were included. We recruited and assessed consecutive participants from each of these nursing homes until we reached a targeted sample of 544. Residents without dementia were excluded (n = 192). The participants with dementia (n = 352) were assessed between February 2018 and August 2018.
Measures
Data on demographic factors (age, sex, and education), diagnoses, and medication use were collected from medical records. We trained study nurses to collect data and perform the assessments. One of the researchers (HMR) participated and supported the nurses in their assessments. The study nurses performed Mini Mental State Examination (MMSE) [21] and Clinical Dementia Rating (CDR) [22] to assess the severity of dementia. The Charlson Comorbidity Index was used to calculate each resident’s burden of comorbidity [23]. The nurses used Mini Nutritional Assessment (MNA) [24] to assess and grade each resident’s nutritional state and Barthel Index [25] for functional evaluation. The phenotypic frailty status was defined by modified Fried criteria [26], with four criteria as follows: (1) shrinking was based on weight loss of ≥ 5% in the preceding year, (2) physical weakness was based on self-reported or care staff evaluation of difficulty in carrying a grocery bag, (3) exhaustion was based on self-reported or care staff evaluation of low energy during the preceding 4 weeks, (4) physical inactivity was based on the question: “Do you/does the resident exercise regularly weekly?” A negative response meant physical inactivity. The sum of fulfilled criteria classified the person as “not frail” (0 criteria), “pre-frail” (1–2 criteria), or “frail” (3–4 criteria).
Data on use of medications were retrieved from medical records on the assessment day. All medications were classified using the Anatomical Therapeutic Chemical (ATC) classification system [27]. Psychotropic medications included antipsychotics (N05A), antidepressants (N06A), anxiolytics (N05B), and hypnotics and sedatives (N05C). Anticholinergic medications were classified according to the Anticholinergic Risk Scale (ARS) [28], which is a list of commonly prescribed medications with anticholinergic potential. The use of Alzheimer medication (N06D) included cholinesterase inhibitors (N06DA) and/or memantine (N06DX01). Only regularly used medications were considered. Medication use was considered regular if there was a documented regular sequence of administration.
HRQoL was assessed using the 15D instrument, which is a generic 15-dimensional measure, internationally validated in various population samples [18]. It correlates well with other HRQoL measures such as SF-36 (RAND-36) and EQ-5 [29]. 15D includes the following 15 dimensions: mobility, vision, hearing, breathing, sleeping, eating, speech (communication), excretion, usual activities, mental function, discomfort and symptoms, depression, distress, vitality, and sexual activity. Each dimension is divided into five levels. The single index score of 0–1 represents the total HRQoL. The maximum score is 1 (no problems on any dimension) and the minimum score is 0. Usually the 15D is filled in by the participant being assessed, but it may also be filled in by the interviewer of the participant or his/her proxy. In our study, all the participants were interviewed by a study nurse and the 15D was filled in by study nurse based on the interview and observation of the participant. The 15D shows good discriminant validity among various aged populations and also prognostic validity [30]. In our study, all participants responded to the question about sexuality with “The state of my health makes sexual activity almost impossible”. Thus, this question was excluded from the partial correlation analysis.
To evaluate NPS, care staff from the long-term care units were interviewed by study nurses using the Neuropsychiatric Inventory (NPI) [31]. The original NPI includes 10 common dementia NPS (delusions, hallucinations, agitation, dysphoria, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behavior). For each symptom, the severity is multiplied by the frequency, and the sum score provides the total NPI score (range 0 to 120). Subsyndromes of “Psychosis” (delusion, hallucinations), “Hyperactivity” (agitation, euphoria, disinhibition, irritability, aberrant motor behavior), “Affective symptoms” (depression and anxiety), and “Apathy” (apathy) were calculated separately, as earlier described [8]. We grouped the residents according to the total score on NPI into three groups: no relevant NPS (NPI 0–3), low NPS burden (NPI 4–12), and high NPS burden (NPI > 12). According to previous studies, a score > 3 is taken to indicate the presence of clinically relevant symptoms [32,33,34]. The cutoff point of 12 was chosen as it was the median.
Statistics
Data are presented as means with range or standard deviations (SD) or as counts with percentages. Statistical significances for the unadjusted hypothesis of linearity across categories of NPI levels were evaluated using the Cochran–Armitage test for trend and analysis of variance with an appropriate contrast. Adjusted hypothesis of linearity (orthogonal polynomial) was evaluated using analysis of co-variance (ANCOVA): age, sex, and the Charlson Comorbidity Index were added to the model as covariates. In the case of violation of the assumptions (e.g. non-normality), a bootstrap-type test was used (5000 replications). Adjusted correlation (partial) coefficients were calculated by the Pearson method with bootstrapped 95% confidence intervals. The normality of the variables was tested using the Shapiro–Wilk W test. Stata 15.1 (StataCorp LP, College Station, TX, USA) was used for the analysis.
Results
The three NPI groups were similar in basic demographic characteristics such as age, sex, and education (Table 1). Residents’ mean age was 83 years, 80% were women, and two in three had less than 8 years of education. The mean number of comorbidities according to the Charlson Comorbidity Index was 2.1. No significant differences were present between the groups in nutrition or frailty status. According to the MNA, 68% of the residents were at risk of malnutrition and 81% were pre-frail according to the modified Fried Frailty Criteria, and only two residents were robust. Mean MMSE was rather low, 6.8. Two in three residents suffered from severe dementia (CDR 3). The NPI groups did not differ in severity of dementia according to MMSE or CDR.
The groups differed significantly in medication use. The mean number of medications in the high NPS burden group was 8.8, compared with 7.9 in the group with no significant NPS. Residents with high NPS burden were also administered more often anticholinergics (p < 0.001) and Alzheimer medication (p = 0.041). The use of psychotropics was very high (85–90%) in all NPI groups. Significant differences between the groups were also detected in functional capacity according to Barthel Index and in HRQoL according to 15D. The severity of NPS was significantly associated with HRQoL in 15D. The mean 15D score was 0.58 in the group with no clinically significant NPS, 0.61 in the group with low NPS burden, and 0.63 in the group with high NPS burden.
Interaction effects of CDR and NPI score on HRQoL
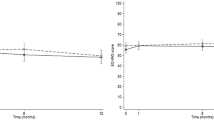
Residents with severe dementia (CDR 3) had worse HRQoL than residents with mild–moderate dementia (CDR < 3). Residents with severe dementia and with higher NPI score had better HRQoL according to 15D than the respective residents with lower NPI score. In addition, there was a significant interaction between NPI and CDR (p = 0.037 for NPI, p < 0.001 for CDR, p < 0.001 for interaction adjusted for age, sex, and Charlson Comorbidity Index) (Fig. 1). HRQoL was worst for residents with severe dementia and low NPI score (0–3) and best for residents with mild–moderate dementia and low NPI score (0–3).
In residents with severe dementia, HRQoL correlated positively with all NPS subgroups. Thus, the higher the HRQoL, the more the NPS in each subsyndrome (Fig. 2). In residents with mild–moderate dementia, HRQoL was not significantly correlated with any of the NPS subsyndromes.
In residents with severe dementia, higher NPI correlated positively with several dimensions of 15D: mobility, vision, eating, speech, excretion, usual activities, mental function, and vitality. In residents with mild–moderate dementia, higher NPI correlated positively only with mobility. On the other hand, among residents with mild–moderate dementia lower NPI score correlated with lower levels of distress and depression and vitality. Among those with severe dementia, lower NPI score correlated with lower level of distress (Fig. 3).
Discussion
Our study found that among institutionalized older people both severity of NPS and severity of dementia were significant factors determining HRQoL and they had a significant interaction. Surprisingly, a higher total NPI score was associated with better HRQoL in residents with severe dementia, whereas among those residents with mild–moderate dementia this association was not seen. In severe dementia, higher HRQoL correlated with higher points in all subsyndromes of NPI. In severe dementia, higher score in NPI correlated positively with functional dimensions of 15D (mobility, usual activities, eating, speech, excretion, and mental function) as well as vitality, whereas in mild–moderate dementia lower NPI score correlated with lower levels of distress and depression as well as vitality.
These findings highlight the importance of both severity of dementia and burden of NPS in HRQoL. NPS seem to have a distinct impact on HRQoL at different stages of dementia. Low functional capacity seems to be linked to both low number of NPI and low HRQoL among those with severe dementia. A possible explanation for these results may be that when dementia progresses to a severe stage with a high number of disabilities the residents have less capacity to present NPS, leading to a low NPI score but worse HRQoL. Several dimensions of 15D measure functioning. Therefore, it is understandable that those residents with severe dementia and slightly better capabilities of functioning score also better in 15D. Interestingly, vitality in severe dementia indicated higher NPI score, whereas it was associated with lower NPI score in mild–moderate dementia. Among residents with mild–moderate dementia, lower NPI scores logically correlated with less depression and distress.
Furthermore, in severe dementia higher HRQoL correlated positively with all subsyndromes of NPI. In mild–moderate dementia, there was no such correlation. Our study cohort comprised only 109 long-term care residents with mild–moderate dementia, so the results should be interpreted with caution for patients in earlier stages of dementia.
NPI scores among our residents were rather low, mean NPI total score being 12, but this is consistent with other studies from long-term care [4, 15, 35, 36]. Studies of home-dwelling people with dementia have found higher NPI scores [14, 16, 37]. This difference may be partly explained by the properties of NPI as an assessment tool. The assessment comes from a third-party perspective. The care staff in long-term care or the caregivers in the case of home-dwelling people with dementia may have different perceptions of the severity of symptoms encountered [38]. Care staff might minimize the burden of symptoms, accepting the behavior as part of the dementia disorder and emphasizing their professionalism in being able to take care of the various symptoms of their residents, whereas an informal caregiver might find him/herself in a stressful situation without any formal education, resulting in the same symptoms causing more distress.
The multiple different instruments used in previous studies assessing quality of life in people with dementia complicate comparison between studies. Most of these instruments are disease specific and measure mainly mood, behavior, social relations, and well-being. In our study, we used the 15D instrument in which various dimensions measure functioning. The generic 15D instrument has been compared with the disease-specific QoL-AD and has been reported to evaluate different aspects of quality of life, for example general health correlated with 15D but not with the QoL-AD scores, whereas depressive symptoms correlated inversely with QoL-AD but not with 15D [12].
Our study found dementia severity to be a significant factor determining HRQoL. This is in concordance with two systematic reviews that noted a negative association between proxy-rated quality of life and dementia severity [39, 40]. The association between dementia severity and quality of life has also been reported by a recent cross-sectional cohort study in long-term care in the Netherlands [15]. In previous studies, dementia severity has also been associated with a higher prevalence of NPS in both long-term care and home-dwelling people with dementia [16, 19, 41, 42]. In our data, NPS burden was not associated with dementia severity according to MMSE or CDR. This difference might be partly explained by the different study groups. The participants of our study had overall a more severe stage of dementia, the mean MMSE being only 6.8.
In our study, a higher NPS burden was associated with better HRQoL. This result is contradictory to most previous studies showing that having NPS impairs quality of life [10,11,12,13,14,15,16]. To our knowledge, only one earlier study on quality of life in nursing home residents found that a higher NPI had a positive influence on the course of quality of life [43]. In this study, the cognition of the residents was also rather low, mean MMSE being 7.1, which is similar to our study population. Thus, one explanation for why our results differ from most previous ones seems to be partly due to characteristics of the study population. The other studies have examined earlier stages of dementia and have had less participants with severe dementia.
Interestingly, in our study the use of both anticholinergic medication and Alzheimer medication was associated with a higher severity of NPS. Due to the cross-sectional nature of our study, we do not know whether this is due to adverse effects of the medication or the fact that the residents using these medications had had even more severe symptoms before drug initiation. The use of Alzheimer medication is very high due to Current Care Guidelines which in Finland recommend Alzheimer medication as the first-line drugs for NPS. The use of psychotropic medication was alarmingly high in all NPI groups, but there was no difference between the groups.
Limitations of our study include the cross-sectional design, which limits the possibilities of drawing conclusions about causal relationships. Care staff rating of residents’ HRQoL may also be considered a limitation. However, this method was intentionally chosen because of the high prevalence of severe dementia, which could have compromised self-reporting. It is known from previous research that there are differences between caregiver and self-rated quality of life [10, 16, 39]. Residents tend to consider their quality of life as significantly higher than caregivers. Assessments of residents were performed by the member of staff who knew each particular resident best in order to increase the validity of the data. In addition, 15D can also be rated by a proxy [18]. The study population was long-term care residents with advanced dementia and, therefore, the results cannot be generalized to other populations with dementia. Even though CDR scale is one of the most well-known and well-studied dementia staging instruments, it is however not without limitations. CDR score addresses both cognition and physical functioning but it may also be influenced by physical comorbidities. Another limitation is that pain, a possible confounder, was not assessed in our study.
An important strength of our study is the large sample size and the use of a large number of well-validated variables. Residents were assessed by well-trained study nurses using the same data collection instruments and methodology, resulting in high validity of the data. Eighteen of the 54 nursing homes in Helsinki were included in this study. The baseline characteristics and HRQoL measured in 15D were similar to those of the total long-term care population in Helsinki; thus, our study cohort was representative [44]. Another important strength is that, to our knowledge, no other study has previously examined the impact of the severity of NPS on HRQoL, nor have the interaction effects of dementia severity and NPS on HRQoL been investigated. Thus, these results make an important contribution to our understanding of the factors associated with HRQoL in institutionalized older people with dementia.
Conclusions
Severity of NPS and dementia are important determining factors of HRQoL. NPS seem to have a distinct impact on HRQoL at different stages of dementia. In severe dementia, higher NPS and better HRQoL indicate better functioning and higher vitality. These results may help clinicians and care staff to detect persons with dementia at risk for a lower HRQoL. The data are also important for developing personalized interventions to improve HRQoL in persons with dementia in long-term care.
References
Toot S, Swinson T, Devine M et al (2017) Causes of nursing home placement for older people with dementia: a systematic review and meta-analysis. Int Psychogeriatr 29:195–208
Selbæk G, Engedal K, Bergh S (2013) The prevalence and course of neuropsychiatric symptoms in nursing home patients with dementia: a systematic review. J Am Med Dir Assoc 14:161–169
Björk S, Juthberg C, Lindkvist M et al (2016) Exploring the prevalence and variance of cognitive impairment, pain, neuropsychiatric symptoms and ADL dependency among persons living in nursing homes; a cross-sectional study. BMC Geriatr 16:154
Wetzels R, Zuidema S, De Jonghe JF et al (2010) Course of neuropsychiatric symptoms in residents with dementia in nursing homes over 2-year period. Am J Geriatr Psychiatry 18:1054–1065
Selbæk G, Engedal K, Benth JŠ et al (2014) The course of neuropsychiatric symptoms in nursing-home patients with dementia over a 53-month follow-up period. Int Psychogeriatr 26:81–91
Helvik A, Selbæk G, Benth JŠ et al (2018) The course of neuropsychiatric symptoms in nursing home residents from admission to 30-month follow-up. PLoS ONE 13:e0206147
Lyketsos CG, Lopez O, Jones B et al (2002) Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 288:1475–1483
Aalten P, Verhey FR, Boziki M et al (2007) Neuropsychiatric syndromes in dementia. Results from the European Alzheimer Disease Consortium: part I. Dement Geriatr Cogn Disord 24:457–463
Kolanowski A, Boltz M, Galik E et al (2017) Determinants of behavioral and psychological symptoms of dementia: a scoping review of the evidence. Nurs Outlook 65:515–529
Hurt C, Bhattacharyya S, Burns A et al (2008) Patient and caregiver perspectives of quality of life in dementia. Dement Geriatr Cogn Disord 26:138–146
Wetzels RB, Zuidema SU, de Jonghe JF et al (2010) Determinants of quality of life in nursing home residents with dementia. Dement Geriatr Cogn Disord 29:189–197
Karttunen K, Karppi P, Hiltunen A et al (2011) Neuropsychiatric symptoms and quality of life in patients with very mild and mild Alzheimer’s disease. Int J Geriatr Psychiatry 26:473–482
Mjørud M, Røsvik J, Rokstad AM et al (2014) Variables associated with change in quality of life among persons with dementia in nursing homes: a 10 months follow-up study. PLoS ONE 9:e115248
Conde-Sala JL, Turró-Garriga O, Piñán-Hernández S et al (2016) Effects of anosognosia and neuropsychiatric symptoms on the quality of life of patients with Alzheimer’s disease: a 24-month follow-up study. Int J Geriatr Psychiatry 31:109–119
Klapwijk MS, Caljouw MAA, Pieper MJC et al (2016) Characteristics associated with quality of life in long-term care residents with dementia: a cross-sectional study. Dement Geriatr Cogn Disord 42:186–197
Hongisto K, Hallikainen I, Selander T et al (2018) Quality of life in relation to neuropsychiatric symptoms in Alzheimer’s disease: 5-year prospective ALSOVA cohort study. Int J Geriatr Psychiatry 33:47–57
Sullivan M (2003) The new subjective medicine: taking the patient’s point of view on health care and health. Soc Sci Med 56:1595–1604
Sintonen H (2001) The 15D instrument of health-related quality of life: properties and applications. Ann Med 33:328–336
Helvik A, Engedal K, Wu B et al (2016) Severity of neuropsychiatric symptoms in nursing home residents. Dement Geriatr Cogn Dis Extra 6:28–42
Shin I, Carter M, Masterman D et al (2005) Neuropsychiatric symptoms and quality of life in Alzheimer disease. Am J Geriatr Psychiatry 13:469–474
Folstein MF, Folstein SE, Mchugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Hughes CP, Berg L, Danziger WL et al (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140:566–572
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Guigoz Y, Lauque S, Vellas BJ (2002) Identifying the elderly at risk for malnutrition: the mini nutritional assessment. Clin Geriatr Med 18:737–757
Mahoney FI, Barthel DW (1965) Functional evaluation: the Barthel index. Md State Med J 14:61–65
Perttila NM, Pitkala KH, Kautiainen H et al (2017) Various diagnostic measures of frailty as predictors for falls, weight change, quality of life, and mortality among older finnish men. J Frailty Aging 6:188–194
WHO Collaborating Centre for Drug Statistics Methodology. The anatomical therapeutic chemical classification system. ATC/DDD index 2019. https://www.whocc.no/atc_ddd_index/. Accessed 23 January 2019
Rudolph JL, Salow MJ, Angelini MC et al (2008) The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 168:508–513
Hawthorne G, Richardson J, Day NA (2001) A comparison of the assessment of quality of life (AQoL) with four other generic utility instruments. Ann Med 33:358–370
Strandberg T, Pitkälä K, Sintonen H et al (2006) Usability, discriminant and prognostic validity of 15D instrument for health related quality of life in older population samples. In: Huusko T, Strandberg T, Pitkala K (eds) Can older people’s quality of life be measured?. The Central Union for the Welfare of the Aged, Helsinki, pp 42–61 (in Finnish)
Cummings JL (1997) The neuropsychiatric inventory: assessing psychopathology in dementia patients. Neurology 48:S10–S16
Schneider LS, Tariot PN, Lyketsos CG et al (2001) National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE): Alzheimer disease trial methodology. Am J Geriatr Psychiatry 9:346–360
Steinberg M, Tschanz JT, Corcoran C et al (2004) The persistence of neuropsychiatric symptoms in dementia: the cache county study. Int J Geriatr Psychiatry 19:19–26
Aalten P, Verhey FR, Boziki M et al (2008) Consistency of neuropsychiatric syndromes across dementias: results from the European Alzheimer disease consortium. Part II. Dement Geriatr Cogn Disord 25:1–8
Ballard C, Corbett A, Orrell M et al (2018) Impact of person-centred care training and person-centred activities on quality of life, agitation, and antipsychotic use in people with dementia living in nursing homes: a cluster-randomised controlled trial. PLoS Med 15:e1002500
Husebø BS, Ballard C, Aarsland D et al (2019) The effect of a multicomponent intervention on quality of life in residents of nursing homes: a randomized controlled trial (COSMOS). J Am Med Dir Assoc 20:330–339
Lechowski L, Dieudonné B, Tortrat D et al (2003) Role of behavioural disturbance in the loss of autonomy for activities of daily living in Alzheimer patients. Int J Geriatr Psychiatry 18:977–982
Lai CK (2014) The merits and problems of neuropsychiatric inventory as an assessment tool in people with dementia and other neurological disorders. Clin Interv Aging 9:1051–1061
Beerens HC, Zwakhalen SMG, Verbeek H et al (2013) Factors associated with quality of life of people with dementia in long-term care facilities: a systematic review. Int J Nurs Stud 50:1259–1270
Martyr A, Nelis SM, Quinn C et al (2018) Living well with dementia: a systematic review and correlational meta-analysis of factors associated with quality of life, well-being and life satisfaction in people with dementia. Psychol Med 48:2130–2139
Zuidema SU, Jonghe JF, Verhey FR et al (2009) Predictors of neuropsychiatric symptoms in nursing home patients: influence of gender and dementia severity. Int J Geriatr Psychiatry 24:1079–1086
Siafarikas N, Selbaek G, Fladby T et al (2018) Frequency and subgroups of neuropsychiatric symptoms in mild cognitive impairment and different stages of dementia in Alzheimer’s disease. Int Psychogeriatr 30:103–113
van der Zon A, Wetzels RB, Bor H et al (2018) Two-year course of quality of life in nursing home residents with dementia. Am J Geriatr Psychiatry 26:754–764
Salminen K, Suominen M, Soini H et al (2019) Associations between nutritional status and health related quality of life among long term care residents in Helsinki. J Nutr Health Aging 23:474–478
Acknowledgements
Open access funding provided by University of Helsinki including Helsinki University Central Hospital.
Funding
This work was supported by a grant from the City of Helsinki. The sponsor had no role in study design, participant recruitment, methods, data collection, analysis, interpretation of results, preparing the paper, or in the decision to submit for publication. The authors were independent researchers not associated with the sponsor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study protocol was approved by the Ethics Committee of the University of Helsinki. All procedures performed were in accordance with the ethical standards of the Ethics Committee of the University of Helsinki and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from each participant and in case of significant cognitive decline (CDR 2 or 3) from their closest proxy.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Roitto, HM., Kautiainen, H., Laurila, J. et al. Severity of both neuropsychiatric symptoms and dementia is associated with quality of life in nursing home residents. Eur Geriatr Med 10, 793–800 (2019). https://doi.org/10.1007/s41999-019-00213-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-019-00213-0