Abstract
C–H functionalization chemistry is one of the most vibrant research areas within synthetic organic chemistry. While most researchers focus on the development of small-scale batch-type transformations, more recently such transformations have been carried out in flow reactors to explore new chemical space, to boost reactivity or to enable scalability of this important reaction class. Herein, an up-to-date overview of C–H bond functionalization reactions carried out in continuous-flow microreactors is presented. A comprehensive overview of reactions which establish the formal conversion of a C–H bond into carbon–carbon or carbon–heteroatom bonds is provided; this includes metal-assisted C–H bond cleavages, hydrogen atom transfer reactions and C–H bond functionalizations which involve an SE-type process to aromatic or olefinic systems. Particular focus is devoted to showcase the advantages of flow processing to enhance C–H bond functionalization chemistry. Consequently, it is our hope that this review will serve as a guide to inspire researchers to push the boundaries of C–H functionalization chemistry using flow technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The construction of carbon–carbon and carbon–heteroatom bonds is a key objective for synthetic chemists to build up complex organic molecules. Such bonds are prevalent in many materials, medicinally and biologically active compounds. In the most recent decades, these linkages have been forged through transition metal catalyzed cross coupling between aryl/alkyl halides or pseudo halides and nucleophiles (Fig. 1a) [1, 2]. However, such an approach requires prefunctionalized substrates and coupling partners, often prepared in a multistep reaction sequence, which is time-consuming and inefficient.
Inspired by selective biosynthetic pathways [3,4,5], C–H activation has emerged as a new and promising area for the construction of carbon-carbon and carbon-heteroatom bonds (Fig. 1a) [6,7,8,9,10,11,12,13,14,15,16,17,18,19]. C–H bonds are the fundamental linkage in organic molecules and, consequently, C–H activation strategies would allow for very versatile transformations, even applicable in late-stage functionalizations enabling rapid diversification of hit molecules. This provides an atom-efficient and cost-effective alternative for the traditional cross-coupling strategies. In 2005, the ACS GCI Pharmaceutical Roundtable have ranked C–H activation as the top priority of the aspirational reactions, i.e. reactions which companies would like to use on the proviso that they are available [20, 21]. While C–H activation has indeed been hailed for its use of unfunctionalized starting materials, the applicability of C–H activation chemistry has been limited mainly by the inert nature of the carbon-hydrogen bond (bond dissociation energies of aromatic C–H are around 110 kcal mol−1 and of aliphatic C–H around 105 kcal mol−1). Consequently, in order to cleave the C–H bond, harsh reaction conditions, long reaction times and high catalyst loadings are typically required. Also, stoichiometric amounts of toxic oxidants are often needed to close the catalytic cycle.
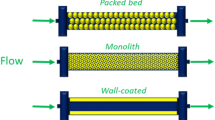
In the past two decades, continuous-flow microreactors have been increasingly used as an interesting new tool to boost chemical reactions. Advantages, such as excellent heat- and mass-transfer, safety of operation and ease of scale-up, have attracted the interest of both synthetic and process chemists and allow to perform reactions under conditions that cannot be easily achieved in conventional batch reactors (Fig. 1) [22,23,24].
Since the emergence of flow chemistry, a lot of research efforts have been devoted to the development of continuous-flow alternatives for cross-coupling chemistry, which even served as a benchmark reaction for early reactor concepts [25, 26]. Notably, despite the fact that C–H activation requires in general harsher reaction conditions than their cross-coupling counterparts, flow approaches for C–H activation have been comparatively rare. However, as shown in Fig. 1b, many of the advantages that popularized flow processing are also of great use to boost C–H functionalization and might deliver a solution to the known shortcomings of the field (Fig. 1a). Herein, we provide an overview of those C–H functionalization processes that have been carried out in flow [20, 27]. The review is structured by highlighting the different bonds that are formed, including carbon–carbon, carbon–nitrogen, carbon–oxygen, carbon–sulfur, carbon–halogen and carbon–hydrogen bonds. We have chosen to include a diverse set of C–H bond functionalizations, including metal-assisted C–H bond cleavages, hydrogen atom transfer (HAT) reactions and radical C–H bond functionalizations, which involve the addition of radicals to unsaturated systems. Especially the inclusion of the latter reaction class is debatable as the cleavage of the C–H bond occurs in the final deprotonation step and thus this class cannot be regarded as a formal C–H activation reaction. However, for reasons of completeness and due to the difficulty in determining the operating C–H scission mechanism [28], we have chosen to incorporate radical-based C–H functionalization reactions in this review. The advantages of flow chemistry have been highlighted when- and wherever appropriate. Hence, we hope this review will serve as a useful guide for those researchers working in C–H functionalization chemistry who aspire to implement flow processing in their experiments.
C − H functionalization in flow
C − C bond formation
C − H alkylation
The insertion chemistry of carbenes represents an interesting and mechanistically distinct case of C − H functionalization reaction [18]. While carbenes can be formed in a number of ways, one of the most well-known approaches is through the thermal, photochemical or metal-catalyzed decomposition of diazo compounds [29]. One of the main advantages of flow chemistry, in particular when looking at industrial applications, is the ability to perform reactions which would ordinarily be considered hazardous in a safe manner, as the reacting volumes at any given time are typically very small [30, 31]. Many reports have appeared on the synthesis and chemistry of the synthetically useful but unstable diazo compounds in continuous-flow microreactors [32,33,34,35,36,37,38,39,40,41,42,43].
Many routes to access diazo compounds exist, e.g. the diazo transfer reaction. While a reliable approach, it suffers from poor atom economy as stoichiometric amounts of sulfonamide waste products are formed. A straightforward approach to access diazo compounds is via oxidation of the corresponding hydrazones. The oxidation can be carried out with insoluble MnO2, or a solid-supported oxidant can be used, such as N-iodo-p-toluenesulfonamide potassium salt (PS-SO2NIK). Using an excess of the supported oxidant PS-NIK (5 equivalents), Davies et al. converted hydrazones to donor-acceptor diazo compounds in flow for subsequent application in C − H functionalization reactions using enantioselective Rh(II) catalysis.
Critical to the success of the transformation is the elimination of water (which would result in the formation of O − H insertion products) and the removal of trace iodine leaching from the oxidant column. By constructing a modular flow set-up consisting first of an oxidant column, followed by a column packed with 4 Å molecular sieves and sodium thiosulfate, both water and iodine were removed from the reagent stream giving access to a series of push-pull diazo compounds. This allowed for the C − H functionalization of p-cymene and methyl tert-butyl ether (MTBE, with Rh2(S-p-BrTCP)4) and n-pentane (with a Rh2(R-3,5-di)p-tBuPh)TPCP)4 catalyst) via C − H insertion of the carbene generated from the parent diazo compound (Scheme 1) in batch [44]. However, both the price of the noble metal rhodium and the catalyst ligands are a non-negligible cost. Provided leaching can be kept to a minimum and high enantioselectivities maintained, catalyst immobilization is an attractive strategy, both from an economical and environmental point of view. The Rh2(S-p-BrTCP)4 catalyst in particular shows enantioselectivity for the challenging functionalization of primary C − H bonds. As demonstrated by Jones, Davies and co-workers, the catalyst can be grafted on silica particles by exchanging one of the ligands of the rhodium catalyst with a ligand bearing an alkyne functionality. The silica particle can be connected with an azide-bearing silane, allowing covalent linking through the azide-alkyne click reaction with no apparent loss of selectivity (Scheme 2) [45].
The inside of the PTFE tube is coated with the fiber containing the silica particles. As such, the liquid is forced to flow through the fiber in an axial direction. Application of the poly(amide-imide) material Torlon represents a notable improvement over the previous reported reactor design, also reported by the groups of Jones and Davies and applied to a C − H functionalization reaction [46], making use of a radial flow through cellulose fibers, which were incompatible with chlorinated solvents. This was an important drawback, as this necessitated the use of hydrocarbon solvents, e.g. hexane, which can undergo C − H insertion with carbenes, thereby functioning as an alkylating agent. Aditionally, the axial hollow-fiber design allows to drastically reduce the amount of chlorinated solvent necessary, reducing the environmental footprint of the reaction, while the turnover number (TON) was increased (Scheme 3 and 4).
After 10 consecutive runs with the same immobilized catalyst to couple 4-methoxytoluene and hydrazone, the yield was only slightly lower, dropping from 74% to 65%, while the enantioselectivity remained unchanged (89 to 86% ee). These results are comparable with the results obtained for the homogeneous catalyst under batch conditions [47].
The polyoxometalate decatungstate (W10O324−) is a useful photocatalyst for the direct activation of hydridic C − H bonds through hydrogen atom transfer (simultaneous abstraction of a proton and an electron). Upon irradiation with near-UV light (λmax TBADT = 323 nm) it undergoes ligand-to-metal charge transfer (LMCT), giving rise to a first excited state [W10O324−]* with a lifetime of 30 ps. It decays into the second excited state known as wO, in which the bridging oxygen atoms of the cluster have partial radical character, with a lifetime of 55 ± 20 ns. The second excited state can be quenched through participation in both single-electron transfer (SET) or hydrogen atom transfer (HAT) events [48]. Acting as electrophilic radicals, the bridging oxygen atoms of the cluster are able to engage in selective hydrogen atom transfer (HAT). Decatungstate is usually prepared as the sodium or tetrabutylammonium salt, the latter showing enhanced solubility in organic media (acetonitrile or DCM). Upon abstraction of the hydrogen atom, the one electron-reduced form of the catalyst is formed, H+[W10O32]5−, which has a deep blue colour, as well as a carbon [49] or silicon-centered [50] radical. The nucleophilic alkyl radicals thus formed can be trapped with suitable electrophilic acceptors, e.g. electron-poor olefins (Giese type reaction) or electron-poor (hetero)arenes (Minisci type reaction). After formation of the adduct, the resulting radical reoxidizes and deprotonates the catalyst to close the catalytic cycle. Several transformations involving the decatungstate catalyst in continuous-flow will be discussed in this review, encompassing the C − H activation of alkane substrates, ethers and aldehydes for the direct alkylation, acylation, fluorination and oxygenation, depending on the radicals and the coupling partners involved.
Building on previous work on C − H alkylation through decatungstate photcatalysis [51,52,53,54,55,56,57], Fagnoni et al. reported the alkylation of a series of electron-poor olefins under flow conditions by construction of a 50 ml mesoscale (i.d. 2.1 mm) photoflow reactor consisting of a 500 W medium pressure Hg vapor lamp and FEP (fluorinated ethylene propylene) tubing, decreasing the required reaction time for the transformation from 6 to 2 h. Alkylating agents include simple cycloalkanes, cyclic ethers, e.g. oxetane, and 1,3-benzodioxole, which form nucleophilic alkyl radicals upon quenching of the wO excited state of decatungstate. They can undergo coupling with electron-poor olefinic partners (e.g. maleates, phenyl vinyl sulfone and diisopropyl azodicarboxylate, the latter allowing the formation of C − N bonds, Scheme 5) [58].
Zuo and co-workers were able to activate light (C1-C4) gaseous alkanes using cerium photocatalysis in flow. Their strategy relies on the formation of Ce(IV) alkoxy complexes which undergo ligand-to-metal charge transfer (LMCT) under 400 nm irradiation, generating highly electrophilic alkoxy radicals (through homolytic fragmentation of the cerium-oxygen bond) and Ce(III). The electrophilic alkoxy radicals can then operate as HAT catalysts by abstracting hydridic hydrogens, such as those found in methane and ethane, to form the corresponding nucleophilic alkyl radical (polarity matching-strategy). The alkyl radical can be trapped for the alkylation of electron-poor olefins, heteroarenes and DBAD (N-di-Boc azadicarboxylate). After addition, the radical adduct reoxidizes Ce(III) to Ce(IV), though an external oxidant, (NH4)2S2O8, is required for the rearomatisation of azine substrates (Scheme 6). A plausible explanation for this is that they are functionalized through oxidation of the radical adduct formed during the C–C bond forming-step (resulting in a cationic intermediate which rearomatises upon loss of a proton, i.e. a Minisci reaction) rather than through reduction of the radical adduct to an anionic species (as in the case of addition of nucleophilic alkyl radicals to DBAD and electron-poor olefins, the Giese reaction) which undergoes protonation to form the alkylated product.
All of the linear gaseous alkanes were successfully employed in radical alkylation reactions of DBAD, electron-poor olefins and azines. The alkylation of the Boc-protected DBAD was performed in less than 15 min residence time, using both gaseous and liquid alkanes (cyclohexane), in a glass microreactor with a volume of 4.5 ml and various alkane pressures in the range of 400–1800 kPa (Scheme 7) [59].
Eosin Y is a common organic photocatalyst in photoredox catalysis because the excited T1 state formed under visible light irradiation (green light) acts as a one-electron transfer agent under basic conditions [60]. Recently, two new reactivity modes of excited state Eosin Y were reported [61]. Wang and co-workers discovered that Eosin Y can act as a photoacid, which allowed the synthesis of 2-deoxyglucosides from glycals [62], while Wu and co-workers discovered Eosin Y can act as a direct hydrogen atom transfer (HAT) catalyst under neutral conditions. Under irradiation with white light, the T1 state of Eosin Y is generated. Analogous to decatungstate (vide supra), as an oxygen-centered radical it acts as an electrophilic HAT catalyst successfully activating hydridic C − H bonds to create nucleophilic alkyl radicals. The scope of the transformation was explored in regard to the substrate undergoing HAT. Ethers, thioethers, amides and alcohols were successfully activated at the α-position. Acyl radicals could be generated from aldehydes (notably, 2-pyrrole carboxaldehyde was successfully activated) while alcohols undergo functionalization at the hydroxyl group-bearing carbon. As carbon-centered radicals are nucleophilic species, the substrate scope was explored with electron-poor coupling partners. Malononitriles are electrophilic olefins and good coupling partners for nucleophilic radicals (Scheme 8). A series of α,β-unsaturated compounds were tested, the reaction proving to be compatible with amide, imide, nitro and sulfone functionalities. An interesting coupling partner for nucleophilic radicals is 2-vinylpyridine, providing the hydroalkylated Giese product with THF as the radical coupling partner in 60% yield. 2-vinylthiophene was also supported in the transformation when the double bond bears the electron-withdrawing phenyl ketone moiety.
The reaction was adapted to flow to enable scale-up, although elevated temperatures (50–70 °C) were required with the alkyl coupling partner (THF, i-PrOH) serving as the solvent. Further, a polar solvent which does not contain a hydridic hydrogen atom is required (t-BuOH was found to be suitable in the Giese reaction of 2-ethyl-propen-3-one with benzaldehyde). The mechanism of the reaction is particularly intriguing (Scheme 9), since earlier reports have demonstrated Eosin Y to be photoactive as an anion (either the anion or dianion) under basic conditions.
Several experiments were performed to elucidate the mechanism. Under irradiation with different wavelengths of light, the highest conversions (99%) were achieved with blue and white light, which corresponds to the absorption maximum of neutral Eosin Y. The maximum of absorption of the anionic forms is centred on longer wavelengths, i.e. green light, which in this case resulted in lower conversion (75%). The luminescence of neutral excited state Eosin Y was not quenched by THF or phenyl vinyl sulfone, ruling out the operation of sensitization or SET as the quenching mechanism. Additionally, cyclic voltammetry (CV) studies performed in acetone have shown Eosin Y is neither able to oxidise THF nor reduce phenyl vinyl sulfone. The transient intermediates involved in photochemical processes can be studied with laser flash photolysis, where the sample is excited with a very short laser pulse (in this particular case on the microsecond time scale) after which the absorption properties and lifetimes of the excited states generated during the flash can be measured. Moreover, it allows elucidation of photochemical reaction pathways as the absorption of the excited states of transient species such as radicals will be quenched due to the shortening of their lifetimes if a suitable reactant is present. After excitation of a THF solution of Eosin Y with a 470 nm laser, two intermediates were detected with lifetimes of 20.6 (absorbing at 329 nm) and 21 (absorbing at 543 nm) μs, which the authors assigned to the T1 excited state of *Eosin Y. After decay of these intermediates, a new intermediate with a lifetime of several milliseconds was detected, indicating it could be the product formed after HAT to triplet Eosin Y, i.e. H–Eosin Y. Most importantly, the lifetime of this intermediate was shortened to 1 ms in the presence of phenyl vinyl sulfone which is required for the completion of the catalytic cycle. Finally, DFT calculations show that two mechanisms could operate in the final step of the catalytic cycle. One is the reduction and protonation of the radical adduct by H–Eosin Y (Ea = 32.3 kcal mol−1), or an additional THF molecule could be oxidised by the radical adduct, the anion of which then deprotonates Eosin Y (Ea = 19.9 kcal mol−1). Although this indicates the latter pathway to be more likely, on the basis of deuterium labelling studies direct proton – and electron transfer between the adduct and H–Eosin Y could not be ruled out [63].
An increasing amount of reports on dual catalytic strategies have appeared in the scientific literature during the last few years [64,65,66]. Most of these newly developed methodologies seek to combine the strengths of transition metal catalysis for cross-coupling reactions with the advantages offered by photoredox catalysis for the generation of reactive radical intermediates, allowing an unprecedented amount of novel reactions to be developed to forge carbon-carbon bonds. Seminal contributions to the field were made, in particular, by the group of MacMillan. The excited state of the Fukuzumi catalyst, 9-mesityl-10-methylacridinium (Mes-Acr+) perchlorate, is a powerful oxidant (excited state reduction potential of +2.06 eV), with the ability to oxidise the chloride ion to a chlorine radical (Eox (Cl−/Cl·) = +2.03 V vs. SCE). Based on these principles, Wu and co-workers developed a photocatalytic strategy employing chlorine radical as the HAT catalyst in conjuction with Mes-Acr+ as the oxidant. After excitation with 450 nm light (blue LEDs), the excited state of Mes-Acr+ is quenched by chloride delivered to the solution as molecular HCl, forming the chlorine radical. As an electrophilic radical species, it is able to activate hydridic C − H bonds in a variety of substrates, e.g. 3 °C-H and aldehydic C − H bonds, as well as the α C − H bond of alcohols, ethers and amides, while preferring a distal functionalization in ketones.
Similar reactivities are observed with other electrophilic radical species discussed in this article, i.e. the second excited state of the decatungstate photocatalyst, wO, and oxygen radicals generated through fragmentation of a labile oxygen-metal bond (like cerium, Zuo and co-workers, vide supra), although the selectivity of HAT depends on the species (polar and steric effects). The nucleophilic alkyl radicals thus formed were trapped with the electron-poor olefin benzylidenemalononitrile (Scheme 10). Notable is the successful activation of the primary C − H bond in ethane, which was then applied in a series of alkylation reactions. Aside from benzylidenemalononitrile, unsaturated phenyl sulfones also proved to be suitable radical traps, setting the stage for C − H allylation reactions as the phenyl sulfone moiety easily undergoes elimination.
The radical adduct formed after trapping of the nucleophilic alkyl radicals with an unsaturated species reoxidises the reduced form of Mes-Acr+, closing the catalytic cycle, forming a carbanion which is protonated by HCl to release chloride (Scheme 11). The cycle involving phenyl sulfones is slightly different, as addition of the radical to the unsaturated moiety of the sulfone yields an olefin product and a sulfonyl radical, the latter being the species re-oxidising the catalyst and deprotonating hydrochloric acid [67].
Due to the ease with which amines are oxidised, amines are common substrates in oxidative methodologies. After initial oxidation of an amine to form an aminium radical, deprotonation by another equivalent of amine then leads to the formation of an α-amino radical, which is itself easily oxidised to an iminium ion. The oxidation of amines under basic or neutral conditions generally yields α-amino radicals, unless the reaction is kinetically favourable enough to compete with this process, which was demonstrated elegantly in a series of challenging photocatalytic hydroamination methodologies developed by Knowles and co-workers [68,69,70]. Additionally, amines are commonly used as sacrificial oxidants in reductive quenching cycles of transition metal photocatalysts. The reactivity of α-amino radicals in particular has been thoroughly explored, especially in the case of tetrahydroisoquinolines, in which the resulting α-amino radical is benzylic and relatively long-lived. As a consequence, many functionalizations of this position have been reported [71,72,73,74,75]. The properties of these radicals were exploited by the group of Rueping for the development of a series of photoredox catalytic cross-dehydrogenative coupling (CDC) reactions under flow conditions in which tertiary aryl amines can be coupled with a variety of nucleophiles. The organic dye Rose Bengal was identified as the most suitable catalyst for the transformation, rendering the process highly sustainable as H2 is formally the only waste product in a cross-dehydrogenative coupling reaction. A series of substituted N-aryl tetrahydroisoquinolines undergo C − H alkylation with nitroalkane and malonate coupling partners. Coupling with TMSCN produces α-amino nitriles (yielding α-amino acids upon hydrolysis). Phosphonylated products can be accessed through reaction with diethyl phosphonate in 3–5 h residence time, representing an improvement over batch procedures previously reported (Scheme 12) [76]. Following these insights, an oxidative Ugi multicomponent reaction was developed combining N,N-dimethylanilines, isocyanides and water as reaction partners giving access to α-amino amides. Although recirculation of the mixture proved necessary to obtain the α-amino amides in high yield, it represents a drastic improvement over batch conditions, e.g. N-butyl-2-(methyl(phenyl)amino)acetamide was formed in 29% yield after 3 days of reaction time in batch, whereas an isolated yield of 60% was obtained in flow with recirculation after 20 h [77].
The introduction of fluorine-containing substituents in organic compounds is of great importance in the development of pharmaceuticals, as the high electronegativity of fluorine can be applied in the modulation of basicity, lipophilicity and bioavailability, as well as increasing metabolic stability [78]. Methods for fluorination and fluoroalkylation are thus continuously being developed.
A traditional approach to aromatic trifluoromethylation is the Swarts reaction, which is the substitution of a trichloromethyl group (formed after perchlorination of an aromatic methyl group) to trifluoromethyl with SbF5. Modern approaches make use of electrophilic or nucleophilic trifluoromethylating agents, such as Togni’s reagent or Umemoto’s reagent [79]. Radical trifluoromethylation strategies are particularly attractive as C − H functionalization reactions do not require prefunctionalized substrates [80, 81]. A number of trifluoromethylation methodologies have been developed under continuous-flow conditions, making use of different kinds of trifluoromethylating agents, and will be discussed below.
Building on the work completed by the group of MacMillan on the α-trifluoromethylation of carbonyl compounds with CF3I and Ru photocatalysis [82], Kappe and co-workers applied the liquid reagent triflyl chloride (CF3SO2Cl) to the α-trifluoromethylation of ketones via a two-step continuous-flow strategy (Scheme 13). A mixture of the organic photocatalyst Eosin Y, trimethyl silyl triflate (TMSOTf) and ketone is mixed with the base, N,N-diisopropyl ethylamine (DIPEA) in a T-mixer allowing both the formation of a silyl enol ether and deprotonation of Eosin Y (2 min residence time), which is a pH sensitive photocatalyst (basic conditions being required in its use as a single-electron reductant). Using a second T-mixer, triflyl chloride (1.5 eq. in THF) is added to the reagent stream en route to the photoreactor consisting of FEP tubing coiled around a glass beaker irradiated by a compact fluorescent light bulb (CFL) placed inside the beaker. The unsaturated moiety of the silyl enol ether functions as a radical trap towards the trifluoromethyl radical. The resulting radical adduct is then oxidised. Following deprotonation, the trifluoromethylated silyl enol ether then equilibrates to the ketone. Both the formation of the silyl enol ether and the trifluoromethylation step occur in less than 20 min overall residence time. In these conditions, both the use of more expensive silylating agents, gaseous CF3I and transition metal photocatalysts are avoided by employing Eosin Y as an environmentally benign and inexpensive photoorganocatalyst [83].
From the viewpoint of atom economy, CF3I is an attractive trifluoromethyl source, forming only iodide as waste product. Although perfluoroalkylation starting from C-5 chains can be performed under homogeneous reaction conditions as the perfluoroiodoalkanes become liquids at ambient temperatures, CF3I is a gaseous reagent, which is cumbersome to handle under batch conditions, while gases can be conveniently handled under flow conditions [84].
Noël et al. developed a photocatalytic protocol for the trifluoromethylation and perfluoroalkylation of heteroarenes with CF3I under continuous-flow with a [Ru(bpy)3]Cl2 photocatalyst and TMEDA (N,N,N′,N′-tetramethylethane-1,2-diamine) as the base. The trifluoromethylation and perfluoroalkylation can be performed in <1 h of reaction time (Scheme 14) [85]. The scope of the transformation was expanded, and the organic dye Eosin Y also proved a viable photocatalyst for the transformation, providing a greener alternative to Ru photocatalysis [86]. Stern-Volmer kinetics [87] show that the reaction occurs through a reductive quenching cycle of the Ru photocatalyst (Scheme 15) [88].
After activation of the Ru(II) photocatalyst through irradiation with blue light, the excited state is quenched through SET with TMEDA which serves as a sacrificial electron donor, forming a Ru(I) species which can then reduce the trifluoromethyl iodide, generating the iodide ion and the trifluoromethyl radical. After addition of the trifluoromethyl radical to the arene, the resulting adduct is thought to be oxidised by the radical cation of TMEDA and then undergoes rearomatisation through the loss of a proton.
Leaving the realm of Minisci-type reactions (i.e. the radical C − H functionalization of arenes), the conditions were adapted to the functionalization of styrenes, which are challenging substrates in radical reactions due to their tendency towards polymerisation and oxidation. In fact, the low oxidation potentials of styrenes allow their anti-Markovnikov functionalization with nucleophiles, an approach pioneered by the group of Nicewicz [89,90,91,92,93,94,95,96]. Radical trifluoromethylations of styrenes were reported requiring electron-donating groups in the ortho position or β-substitution on the olefin tail [94, 97]. When the trifluoromethylation is performed in the presence of the powerfully reducing photocatalyst fac-Ir(ppy)3, Stern-Volmer kinetics proved the reaction occurs through an oxidative quenching cycle via quenching of the excited state with CF3I. The benzylic radical formed after anti-Markovnikov addition of the trifluoromethyl radical to the styrene substrate can be reoxidized by the Ir(IV) species formed after oxidative quenching. Following deprotonation of the benzylic cation by CsOAc, the olefin functionality is restored (Scheme 16).
Alternatively, if the reaction is performed in the presence of a hydrogen donor, the hydrotrifluoromethylated product is obtained. Thiophenols in particular are frequently applied as hydrogen donors in radical hydrofunctionalization reactions. In this case, 4-hydroxythiophenol proved optimal. A distinct advantage of performing the transformation under continuous-flow is the fact that the Ir(ppy)3 photocatalyst, which has a high triplet energy, promotes olefin isomerisation via sensitization of the olefin (triplet-triplet energy transfer, TTET) [98]. As a diradical, the T1 state of the olefin can undergo rotation leading to the formation of the (Z)-isomer [99]. Due to its higher thermodynamic stability, the (E)-isomer is thought to be kinetically favoured, and accelerating the reaction under flow conditions (24–72 h in batch to 0.5 – 1 h in flow) allows the reaction to occur with high levels of stereocontrol. Indeed, longer reaction times were shown to lead to reduced levels of stereoselectivity (Scheme 17) [100].
A non-photocatalytic approach to trifluoromethylation under flow conditions using CF3I as the trifluoromethylating agent was developed in the group of Kappe by employing Fenton-type conditions [101] (developed in the group of the late Francesco Minisci) for the generation of alkyl radicals from alkyl iodides [102].
By combining catalytic amount of iron(II)sulfate heptahydrate and hydrogen peroxide as the oxidant (generally known as Fenton’s reagent), the trifluoromethylation of heteroarenes can be performed in a residence time of just a few seconds (Scheme 18). The use of DMSO as solvent is key to the success of the transformation, as this allows the reaction to be performed in a more reliable and reproducible way. Fe(II) reduces hydrogen peroxide, forming Fe(III), a hydroxyl anion and a very reactive hydroxyl radical, the latter which forms an adduct with DMSO.
After expelling methylsulfinic acid, a highly reactive methyl radical is formed which then undergoes a thermodynamically favourable halogen atom transfer reaction with the perfluoroalkyl iodide present. This leads to the formation of methyl iodide and the comparatively stable perfluoroalkyl radical. After addition to the arene, the resulting adduct is reoxidized by Fe(III) to form Fe(II) and a cation, which forms the desired product after loss of a proton (Scheme 19).
The reaction shows excellent scalability, as shown by the trifluoromethylation of the pharmaceutically relevant anti-migraine agent, dihydroergotamine. It is a semi-synthetic derivative of ergotamine, a natural product found in the ergot rye fungus, Claviceps purpurea [103, 104]. Due to nausea being a common side effect, dihydroergotamine treatment is being superseded by application of the more selective sulfonamide-bearing tryptamines, known as the triptan drugs, of which sumatriptan is a prominent example, which also have a higher cost associated with their use [105, 106]. However, the trifluoromethylated analog of dihydroergotamine shows promise as a cheap anti-migraine agent with reduced side effects, and is thus an interesting target for the scope of radical trifluoromethylation methodologies [107]. Due to the short residence time of less than 10 s, an impressive amount of 600 g of dihydroergotamine mesylate could be processed in 5 h with 98% conversion and a selectivity of 85–86% (Scheme 20) [108]. As a readily available and low-cost chemical reagent, trifluoroacetic acid (TFA) would be an attractive source of the trifluoromethyl radical. Although the oxidation of TFA has been reported to generate trifluoromethyl radicals by electrochemical means, harsh conditions are required due to the high oxidation potential of TFA, which drastically limits the scope of these methodologies. Hence, trifluoromethylation via an oxidative decarboxylation approach commonly followed in photoredox catalysis [109] to generate alkyl radicals is impractical in the case of TFA. By installing a redox auxiliary group, Stephenson and co-workers managed to bring the oxidation potential of TFA within the electrochemical potential range of [Ru(bpy)3]2+. The redox auxiliary approach relies in this case on the reaction between pyridine N-oxide and trifluoroacetic anhydride (TFAA), forming an adduct in which the weak N-O bond can be cleaved reductively. Stern-Volmer kinetics point to an oxidative quenching cycle in which the excited state of the Ru photocatalyst is quenched by the trifluoroacetoxy pyridinium adduct, delivering the trifluoroacetate radical, forming the trifluoromethyl radical upon decarboxylation (Scheme 21).
As is common for photochemical transformations, the reaction showed limited scalability in batch. When scaling up the trifluoromethylation of N-Boc pyrrole to 100 g scale, the product was obtained in a modest yield of 35% after a reaction time of 62 h.
As discussed previously, one of the main advantages of flow photochemistry is its inherent scalability [110,111,112,113,114,115]. Performing the reaction under flow conditions yielded 71% of the trifluoromethylated N-Boc pyrrole (20 g scale, 10 min residence time, Scheme 22), compared to 57% yield on 20 g scale in batch (15 h reaction time) [116]. A later report elaborating on the mechanistic aspects of the reaction includes scale-up of the trifluoromethylation of an N-Boc protected pyrrole bearing a methyl ester functionality to 1 kg scale (Vr = 150 mL), in which a productivity of 20 g h−1 was achieved. Additionally, evidence for the formation of EDA (electron donor-acceptor) complexes between the pyridine-N-oxide and arenes is presented, opening up another possibility for the fragmentation of the trifluoroacetylated pyridines, although this is limited to a few specific cases, e.g. mesitylene [117].
Sodium trifluoromethanesulfinate (CF3SO2Na), commonly known as the Langlois reagent, has found wide application in radical trifluoromethylation reactions, including in the Minisci-type functionalization of heteroarenes [118,119,120,121,122]. Multiple equivalents of an external oxidant, such as t-BuOOH, are usually required to oxidise the sulfinate and effect SO2 extrusion to form the trifluoromethyl radical [123,124,125]. As a bench-stable solid, it is one of the most convenient to handle trifluoromethylation agents [119]. Long reaction times and multiple additions of the sulfinate salt are usually required. The conditions originally reported by Langlois and co-workers make use of a Cu(I) additive to promote cleavage of the peroxide bond, which initiates the reaction. Duan et al. adapted the conditions of the original Langlois trifluoromethylation reaction to continuous-flow (60 °C, 40 min residence time) to generate a library of trifluoromethyl-substituted coumarins (Scheme 23) [126].
Rueping and co-workers applied the Langlois reagent to the hydrotrifluoromethylation of maleimides (representing electron-poor olefins) and heteroarenes with 4,4′-dimethoxybenzophenone as an organic HAT catalyst under near-UV irradiation (350 nm) (Scheme 24).
The reaction requires the presence of hexafluoroisopropanol (HFIP) as an additive, a reaction medium with high polarity known to stabilize polar intermediates such as radicals [127,128,129]. The authors propose a mechanism based on the oxidation of the sulfinate by the T1 state of 4,4′-dimethoxybenzophenone forming the trifluoromethyl radical (vide supra) and a ketyl radical which deprotonates the acidic HFIP. The radical adduct formed after addition of the trifluoromethyl radical to the double bond of the maleimide is then proposed to abstract a hydrogen atom from the alcohol, resulting in the hydrotrifluoromethylation of the olefin and regenerating the ketone functionality of the catalyst closing the catalytic cycle (Scheme 25). The strongly oxidising iridium photocatalyst [Ir(dF(CF3)ppy)2](dtbpy)]PF6 was also found to be a suitable visible light photocatalyst for this transformation, improving the yields under similar reaction conditions [130].
Through application of the luminescence screening approach developed in the group of Glorius [131], Noël, Alcazar and co-workers confirmed the [Ir(dF(CF3)ppy)2](dtbpy)]PF6 photocatalyst is quenched efficiently by the Langlois reagent. On the basis of these findings, a continuous-flow trifluoromethylation of highly functionalized heteroarenes was developed. Although the reaction proceeds in 30 min of residence time, 1 equivalent of an external oxidant, (NH4)2S2O8, is required for the reaction to occur in synthetically useful yields, since two consecutive oxidations are required (Scheme 26) [132].
Stephenson and co-workers reported the alkylation of heteroarenes by quenching of excited state [Ru(bpy)3]2+ by bromomalonate through an oxidative Ru(III)/Ru(II) quenching cycle, forming bromide ion and the malonyl radical [133, 134]. The transformation was also reported using organic photoredox catalysts (e.g., Th-BT-Th) [135]. By calculating the spectroscopic properties and reduction potentials with DFT at the M06-2X / 6-31G+(d) level of theory using the continuum solvation model PCM, a series of alkynylated bithiophenes were synthesized for their evaluation as potential photocatalysts by Alcazar, Noël and co-workers, the alkynes functionalized with phenyl substituents bearing both electron-releasing and electron-withdrawing groups. To benchmark their performance, the thiophene photocatalysts were applied in the C − H alkylation of heteroarenes with bromomalonate and Ph3N as the base, proving viable alternatives to [Ru(bpy)3]2+. Five-membered heterocycles such as pyrrole, thiophene and furan derivatives can be alkylated with 1 mol% of the photocatalyst (PC) through irradiation with purple LEDs (400 nm), as well as benzofurans and indole derivatives, including N-Boc-Trp-OMe (55% isolated yield, Scheme 27). The alkylation of 3-methylindole was performed in continuous flow and afforded the alkylated product in 70% yield in 7 min residence time. The catalysts were also applied to other C − H alkylations of heteroarenes, ie. trifluoromethylation (with CF3I and TMEDA, 20 min residence time, 450 nm LEDs) and difluoromethylation (using BrCF2CO2Et as the reagent) [136].
C − H acylation
Aldehydes are excellent hydrogen atom donors in decatungstate HAT photocatalysis [137, 138]. By irradiation with near UV light (λmax TBADT = 323 nm), acyl radicals are formed via HAT with the decatungstate catalyst (we refer to Scheme 4 for the catalytic cycle, R· = acyl radical) (Scheme 28).
Fagnoni and co-workers used α,β-unsaturated esters as radical traps to form γ-ketoesters. The reaction was performed in 2 h of residence time in a photoflow reactor constructed from PTFE tubing (12 ml reactor volume), a medium pressure Hg lamp and a HPLC pump. By introducing a second reagent stream containing 0.4 M of NaBH4 in EtOH as a reductant to the product stream exiting the photoflow reactor, the ketone functionality is reduced to an alcohol and undergoes intramolecular cyclisation to form a γ-lactone product, e.g. the lactone condensation product obtained from heptan-2-one and diethyl maleiate is formed in a 65% overall yield [139].
Ravelli and co-workers trapped the acyl radicals with phenyl vinyl sulfone under flow conditions to form the hydroacylated product. Using a modular set-up, the sulfonyl moiety can be removed in a second flow module with base (tetramethylguanidine, TMG or triazabicyclododecene supported on polystyrene, TBD-PS) to form an α,β-unsaturated ketone, which can then undergo conjugate addition. This was applied to the synthesis of γ-nitroketones and β-(3-indolyl)ketones with nitroalkanes and indole as nucleophiles, respectively. Starting from O-Boc protected salicylaldehyde followed by acylation of phenyl vinyl sulfone, both the sulfonyl and the carbonate protecting group are cleaved under basic conditions, after which the product undergoes an intramolecular cyclization to afford chromanone (35% overall yield, Scheme 29) [140].
In contrast to the C-3 formation of β-(3-indolyl)ketones accomplished through decatungstate photocatalysis, the C-2 acylation of indoles to yield α-(2-indolyl)ketones in flow via a dual catalytic cycle with Pd and Ir involving acyl radicals at room temperature was reported by Noël, Van der Eycken, and co-workers. The acyl radicals are formed from aldehydes through HAT with the tert-butoxyl radical. The tert-butoxyl radical is formed by engaging t-BuOOH in an oxidative Ir(III)/Ir(IV) quenching cycle with the iridium photocatalyst (i.e. photocatalytic Fenton initiation). Pd(OAc)2 activates the C-2 position of the indole assisted by a pyrimidine directing group and is hypothesized to react with the acyl radical to form an acylated Pd(III) species, which is oxidised by Ir(IV) to a Pd(IV) species, closing the photocatalytic cycle. Upon reductive elimination, the C-2 acylated product is formed and the Pd(II) center is regenerated, closing the “dark” catalytic cycle (Scheme 30).
A range of aromatic, heteroaromatic (including furfural, a biomass-derived feedstock) and aliphatic aldehydes undergo acylation with several substituents being tolerated on the indole ring, although the absence of a directing group (e.g. pym, py) was not tolerated (Scheme 31). By performing the reaction in continuous flow, both the catalytic loadings and the reaction time could be decreased (20 min residence time in flow from 20 h under batch conditions) improving the sustainability of the process [141].
C − H carboxylation and C − H cyanation
The selective activation of carbon dioxide has long stood as one of the holy grails of chemistry [142,143,144,145]. The reduction of CO2 using the organic photoredox catalyst p-terphenylene under UV irradiation was achieved by Jamison and co-workers and applied to the synthesis of amino acids in flow (Scheme 32). The synthesis occurs through radical-radical coupling of the carbon dioxide radical anion with a benzylic α-amino radical, which is formed from the single-electron oxidation and deprotonation of a benzylic amine in a segmented flow regime, compellingly combining the advantages of flow chemistry in gas-liquid and photochemical transformations. To increase the selectivity of the reaction, a UV filter was applied with a cut-off at 280 nm (λmaxp-terphenylene = 283 nm) (Scheme 33).
Stern-Volmer kinetics show the S1 excited state of p-terphenylene is quenched by the amine, forming the radical anion of p-terphenylene and an aminium radical, which forms an α-amino radical upon deprotonation by the base potassium trifluoroacetate (CF3CO2K). The radical anion of the photocatalyst is then able to reduce CO2, closing the catalytic cycle, while the resulting carboxylate is formed by radical-radical coupling with the stabilized (and hence, somewhat persistent) α-amino benzylic radical [146].
Using magnetite (Fe3O4) nanoparticles as a heterogeneous catalyst, Varna et al. were able to activate the methyl group of a series of N,N-dimethylanilines in an oxidative cyanation reaction with H2O2 and NaCN in aqueous methanol (1:1) as the solvent. The reaction is performed in a stainless steel microreactor with a reactor volume of 5 ml and i.d. of 0.8 mm.
The coil was submerged in an oil bath to keep the reactor at a temperature of 50 °C allowing a series of N-methylanilines to be monocyanated in less than 10 min residence time (Scheme 35). After exiting the reactor, the nanoferrites can be separated conveniently from the product stream with a magnet. The mechanism suggested by the authors occurs through the formation of an Fe(IV) oxo-species from Fe(II) through oxidation with hydrogen peroxide. The Fe(IV) species oxidises the amine to an iminium ion, which reacts with HCN to form the α-aminonitrile product (Scheme 34) [147].
C − H alkenylation
The Fujiwara-Moritani reaction or oxidative Heck reaction represents one of the earliest examples of Pd-catalysed C − H activation [148, 149]. In contrast to the Mizoroki-Heck reaction, in which prefunctionalization of the vinyl or arene coupling partner is required to allow oxidative addition to Pd(0) to occur, the Fujiwara-Moritani reaction starts from Pd(II). While the production of stoichiometric amounts of halide waste can be avoided by employing diazonium salts as the coupling partners in the Heck-Matsuda reaction, the Fujiwara-Moritani reaction as a cross-dehydrogenative coupling reaction represents a more atom-economical approach [150].
Because of its higher reactivity, Pd(II) is frequently used as the catalyst in combination with an external oxidant to close the catalytic cycle and prevent the formation of Pd(0) particles. The reactions are performed in the presence of acid, as they are able to coordinate to the Pd(II) center, rendering it more electron-poor, which favours the C-H activation step and allows for the deprotonation of the targeted C-H bond (Scheme 36) [151].
From an environmental point of view, one of the attractive aspects of the Fujiwara-Moritani reaction is the possibility to use oxygen as an external oxidant. The use of oxygen in batch processes can be hazardous and is limited in its efficiency due to the poor solubility of oxygen in organic media. The use of gases in continuous flow offers a number of distinct advantages including precise control of pressure and flow rate (i.e., the stoichiometry of the gaseous reagents) [152, 153]. Moreover, the establishment of a segmented flow regime provides a high gas-liquid interfacial area and assists in preventing precipitation of Pd0. Due to better heat dissipation, the reaction can be run at higher temperatures without catalyst degradation. Using Pd(OAc)2 in combination with TFA and O2 as the oxidant, these principles were applied for the development of a C-3 vinylation of indoles, yielding alkenylated indoles in a residence time of 10–20 min (Scheme 37) [154].
A variant of the Fujiwara-Moritani reaction allowing the ortho-functionalization of acetanilides with Pd/C as a reusable heterogeneous catalyst and benzoquinone as an external oxidant in the presence of TsOH as the acid was reported by the group of Vaccaro making use of the acetamide directing group.
The use of a green solvent derived from lignocellulosic biomass, γ-valerolactone (GVL), is notable, as it is both a less toxic and more sustainable alternative to traditional polar aprotic solvents, e.g. DMF. Although leaching is a requirement for the reaction to occur with Pd(II) as the active catalytic species, Pd(0) formed at the end of the catalytic cycle is hypothesized to be redeposited on the support, overall resulting in minimal amounts of palladium leaching (4 ppm). Apart from this, leaching is also decreased when using GVL compared to DMF or NMP. Under batch conditions, the catalyst can be reused for five runs with little decrease of performance. Performing the reaction in a continuous-flow packed bed reactor allows scaling up the reaction to a productivity of 4 g h−1 (Scheme 38) [155].
Combining Brønsted acid catalysis and C − H activation with Mn(I), Ackermann and co-workers performed a C − H alkenylation of indoles, thiophenes, pyrroles, tryptophans and pyridones through the use of a pyridine directing group. The alkene functionality is furnished by the hydroarylation of an alkyne bearing a carbonate leaving group.
The carbonate is left untouched, in contrast to previous reports where hydroarylation occurred with β-elimination. Using a cheap and air-stable manganese carbonyl catalyst, MnBr(CO)5, allylic carbonates and ethers can be synthesized from alkynes bearing these functionalities through C − H activation of the heterocyclic ring directed by pyridine. The reaction is accelerated under flow conditions, from 16 h in batch to 1–20 min residence time in flow, which can be coupled with an in-line catalyst separation. The scalability of the method was demonstrated by the production of 2.24 g of the alkenylated product using indole as the coupling partner in 1 h (Scheme 39) [156].
C − H arylation
Li and co-workers reported a direct intramolecular C − H arylation of aryl bromides using Pd(OAc)2 under ultrasonication without requiring the presence of any additional ligands. By immersing the coil in an ultrasonic cleaning bath, as in this case, ultrasound irradiation can be applied to prevent microreactor clogging and accelerate the reaction [157]. Starting from functionalized phenyl ethers and N-functionalized indoles, the method allows the construction of polycyclic ether systems and fused indolines (Scheme 40) [158].
Mihovilovic and co-workers reported the first intermolecular C − H activation process under continuous-flow conditions. Starting from aryl bromides and aryl boronic acids, they performed the Suzuki-Miyaura reaction in flow using Pd(PPh3)4 catalyst and K2CO3 as the base allowing for the synthesis of 2-phenylpyridines in 20 min residence time. Notably, they found the reaction was sensitive to the material of the flow reactor coil used, with PFA performing better than stainless steel. With pyridine acting as the directing group, the ortho-position to the pyridyl substituent in 2-phenylpyridine can be selectively targeted for C − H activation in a second step using dichloro(p-cymene)ruthenium(II) as the precatalyst, PPh3 and DBU as the base under an atmosphere of air allowing the synthesis of bis – or trisarylated products in 1 h residence time (Scheme 41) [159].
Another class of cross-dehydrogenative coupling (CDC) reaction in which two unfunctionalized aryl rings are coupled via C − H activation under aerobic oxidation was extensively investigated by Stahl and co-workers [160,161,162]. The coupling of o-xylene, specifically, holds promise in the preparation of monomers used in the production of the polyimide resin Upilex [163] and metal-organic frameworks [164].
Mechanistically, two separate Pd(II) metal centers allow the C − H activation of two xylene molecules, followed by a transmetalation step leading to a single Pd(II) center with two arene ligands. Upon reductive elimination, the biphenyl moiety and Pd(0) are formed, which is then reoxidized with O2 (Scheme 42). Although the reaction shows remarkable selectivity for the desired product, it is limited by low yields (8%) and the requirement for long reaction times.
Noël and co-workers increased the yield of the homocoupled product to 41% (60% selectivity) by applying 40 bar of O2 pressure in a stainless steel capillary microreactor requiring a reaction time of 40 min, compared to 17 h under batch conditions (Scheme 43) [165].
The meta-selective arylation of anilines under copper catalysis was reported by Phipps and Gaunt, offering an orthogonal approach to functionalizations of arenes targeting mainly the ortho positions (as in many palladium-catalyzed C − H activation methodologies making use of a directing group) – and para positions (i.e. the more traditional electrophilic aromatic substitution reactions) of the arene moiety [166]. For this transformation, diaryliodonium salts, a class of hypervalent iodine compounds, are used concomitantly as arylating agents and for the oxidation of Cu(I) to Cu(III). Mechanistic studies indicate the first step is an oxidative addition to copper followed by a C − H activation step in which deprotonation of the adduct by triflate occurs. Reductive elimination forges the aryl-aryl bond to yield the meta-functionalized amide and regenerates Cu(I) (Scheme 44).
Although diaryliodonium salts are now commonly used as arylating reagents [167], their synthesis requires both the use of the superacid TfOH and m-CPBA as an oxidant, rendering the transformation highly exothermic and potentially hazardous. For these reasons, Noël and co-workers developed a continuous-flow synthesis of diaryliodonium triflates. As mentioned earlier, heat transfer to the environment is more efficient and the active volume of reactants reacting in a microreactor is typically very small greatly decreasing the potential explosive hazard of the reaction. By submerging the reactor coil into an ultrasonic bath, clogging of the microreactor is prevented. A variety of diaryliodonium triflates were synthesized on a gram-scale in a residence time of a few seconds [168]. Apart from the availability of diaryliodonium salts, the synthesis of meta-arylated anilines makes use of amides as protected amines, resulting in the necessity of a deprotection step. For pharmaceutical production, Cu levels are required to be below a threshold of 25 ppm, requiring the removal of copper. The different steps were integrated in a modular flow set-up by Noël and co-workers using a copper tubular flow reactor (CTFR) for the arylation reaction, where copper leaching from the reactor walls acts as a catalyst for the transformation, based on the initial observation that the reaction was accelerated with powdered copper as the catalyst, compared to the initially reported copper(II)triflate. Copper leaching from the coil can be removed from the mixture by a membrane separator in a continuous liquid-liquid extraction with aqueous NH3. Finally, the resulting pivalanilides can be deprotected in continuous flow with a 1:1 mixture of HCl:1,4-dioxane. Due to the modularity of the process, the meta-arylated anilines can be obtained in <1 h without the necessity of chromatographic separation, reducing downstream processing (Scheme 45) [169].
During the last decade, many reports on C − H activation processes using transient directing groups as a strategy to selectively target C − H bonds have appeared in the scientific literature [170,171,172]. A notable transient directing group is the bicyclic olefin norbornene [173,174,175]. Catellani and co-workers first reported the use of norbornene and Pd(II) for an ortho di – or trifunctionalization of aryl iodides in 1997 [176].
Starting from Pd(0), an oxidative addition of the aryl iodide occurs. This is followed by a carbopalladation step, inserting norbornene via its double bond between the arene moiety and the metal center, bringing the Pd(II) in proximity to the ortho C − H bond, which is then softly deprotonated to form a palladacycle intermediate. A second oxidative addition to the palladacycle occurs, bringing palladium to its tetravalent oxidation state. After reductive elimination, the ortho position to the original site of the oxidative addition has been selectively functionalized. This is followed by norbornene extrusion. Although norbornene, in principle, acts as an organocatalyst, stoichiometric amounts are necessary for the reaction to occur at a reasonable rate [177]. The resulting ArPd(II)X species can then undergo further functionalization (e.g. through another cross-coupling cycle), with many possibilities having been reported [178,179,180,181,182]. This brings the catalytic Pd species back to its original zerovalent oxidation state and closes the catalytic cycle (Scheme 46). A common termination pathway is the Heck reaction. In this case, competition between coupling of the desired olefin and norbornene is a known issue which can be addressed by controlling the stoichiometry of the olefinic reagents. In principle, the transformation can also be performed using gaseous olefins.
Due to the difficulties arising from the volatility of norbornene and poor control of the stoichiometry of the gaseous reagents, these transformations can be challenging to perform under batch conditions, in particular when attempting to perform the reaction using two distinct arene coupling partners. In the first report on gas-liquid Catellani reactions, Noël, Della Ca′ and co-workers reported an increase in selectivity for a heterocoupling from 12% yield in batch to 66% under flow conditions, due to the accurate control over the stoichiometry of the gaseous reagents and the high gas-to-liquid mass transfer under flow conditions (Scheme 47) [183].
C − H activation chemistry employing Earth-abundant metals such as Ni, Mn and Co is becoming more commonplace as alternatives are sought to the precious metals traditionally employed in C − H activation catalysis [184]. Ackermann and co-workers reported the ortho C − H arylation of substituted azines with an amide directing group under continuous-flow conditions with MnCl2 salt combined with a neocuproine ligand, a Grignard species as the arylating agent (Kumada-type coupling), TMEDA as the base and DCIB as an oxidant.
After forming the complex and coordination of Mn(II) by the amide functionality, the Grignard reagent undergoes transmetalation. The amide directing group brings the metal center in proximity of the ortho C − H bond for the C − H activation step occurring through deprotonation with the Grignard reagent. After ligand exchange, Mn(II) undergoes a single-electron oxidation to Mn(III) which is arylated by the Grignard reagent in a transmetalation step. Reductive elimination from Mn(III) forges the C-C bond, generating Mn(I). The external oxidant DCIB (1,2-dichloro-2-methylpropane), is required to form the Mn(III) species and close the catalytic cycle, reoxidising Mn(I) to Mn(II).
Using this methodology, both azines and diazines can be arylated with arenes, thiophene and the sterically hindered mesityl moiety. A variety of substituents on the amide nitrogen were also tolerated. The reaction can be performed in flow in 100 min compared to 16 h under batch conditions, which can be scaled-up with a productivity of 1.12 g h−1 (Scheme 48).
The Pd-catalyzed intermolecular C − H activation of the C-5 position in 1,2,3-triazoles to give arylated products with aryl halides as the coupling partners was reported in 2016 by Vaccaro and Ackermann, using the green solvent γ-valerolactone (GVL) and palladium on charcoal (Pd/C) as a heterogeneous catalyst [185]. The methodology was also adapted to flow conditions, mainly for the intramolecular C − H arylation to yield triazole-fused chromanes and triazole-fused isoindolines. As in their report on a Fujiwara-Moritani reaction in flow (vide supra), Pd/C was immobilized in a flow reactor and combined with GVL resulting in minimal amounts of leaching, making the transformation highly sustainable.
Starting from Pd(0), oxidative addition to the aryl halide occurs, followed by the C − H activation of the triazole through deprotonation. Reductive elimination then delivers the final product (Scheme 49). As well as intramolecular cyclization products, intermolecular C − H arylation with aryl bromides is also possible under these conditions. The reaction was scaled to 100 mmol scale under flow conditions, yielding 24 g of product after 44 h, corresponding to a productivity of 0.55 g h−1 (Scheme 50).
Several reports have been published on the application of copper complexes as photoredox catalysts for C − H functionalization reactions [186].
Collins et al. applied the in situ formed copper photoredox catalyst [Cu(Xantphos)(dmp)]BF4 in conjunction with I2 as an oxidant for the oxidative cyclization of diaryl – and triarylamines to form carbazoles (Scheme 51).
Surprisingly, the copper catalyst was found to outperform Ru(bpy)32+, however, a reaction time of 14 days was required to obtain the N-phenylcarbazole in 85% yield. For this reason, a flow set-up was constructed allowing the reaction to be performed in a residence time of 20 h. The authors propose a mechanism based on the oxidative quenching of the Cu(I) catalyst by molecular iodine. The Cu(II) species then formed oxidises the carbazole which allows the cyclization to occur through homolytic aromatic substitution. After oxidation, the aromaticity of the carbazole is restored (Scheme 52) [187].
In the Meerwein-type C − H arylation reaction, aryl diazonium salts are used to generate aryl radicals via one-electron reduction [188]. Several photocatalytic variants of this reaction have been developed for selective C − H arylation, including the use of the inorganic photocatalyst TiO2 (Scheme 53).
The behaviour of semiconductor nanoparticles as photocatalysts is well-described, representing one of the earliest and most important examples of photocatalysis [189, 190]. Due to the relatively small band gap of semiconductor materials, electrons can be excited from the valence band to the conduction band, creating an electron-hole pair, in which the electron can effect one-electron reduction, and the hole can act as a one-electron oxidant [191]. For this reason, TiO2 is frequently used for the oxidative solar decontamination of waste water [192]. As an abundant, non-toxic and heterogeneous photocatalyst, it is a very attractive material for sustainable chemistry [193], the downside being the band gap of unmodified TiO2 is too large for the creation of electrons and holes through visible light irradiation. This problem is generally solved through the use of modified TiO2 [194], e.g. employing Pt and Pd as co-catalysts, bringing the absorption of the material from the ultraviolet into the visible range through the creation of intra-bandgap states in which the size of the electronic transitions fall within the energetic range of visible light [195]. Rueping et al. have shown that the combination of TiO2 with aryl diazonium salts results in the formation of a TiO2 azoether with a strong absorption at 450 nm (corresponding to the blue part of the visible spectrum) to create aryl radicals. To allow the use of heterogeneous titania in flow, a falling film microreactor (FFMR) was constructed by Rehm, Rueping and co-workers. An open stainless steel flow cartridge, equipped with a quartz window, is coated with TiO2 nanoparticles and irradiated on one side with blue light. The performance of the FFMR markedly improved the reaction compared to batch conditions, and was successfully applied to the C − H arylation of heteroarenes such as pyridine, thiophene and furfural with a range of aryl diazonium salts (Scheme 54) [196].
The group of Ackermann used another 3d earth-abundant metal, manganese, to perform a visible light-photocatalyzed C − H arylation of heteroarenes with diazonium salts under flow conditions. Inexpensive CpMn(CO)3 proved to be the most effective catalyst for the transformation.
On the basis of mechanistic studies, the authors suggest a radical mechanism starting with the exchange of one CO ligand for the arene substrate, followed by coordination of the aryl diazonium salt to the Mn(I) center. After irradiation with blue light (450 nm LEDs), metal-to-ligand charge transfer (MLCT) leads to electron transfer from Mn(I) to the diazonium ligand, forming Mn(II) and an aryl radical with extrusion of N2. The aryl radical adds to the arene substrate to forge the aryl-aryl bond, whereupon the radical adduct formed is oxidised to form a cation, either by reaction with the oxidised Mn(II) complex or another equivalent of diazonium salt. Deprotonation then restores the aromaticity of the system (Scheme 55 and 56).
Flow conditions proved to be highly beneficial for the reaction, accelerating the reaction time to 60 min residence time and drastically improving the yield. For p-trifluoromethylbenzenedizonium tetrafluoroborate and benzene, by switching to flow, an improvement in yield from 25% to 64% was noted with a productivity of 1.42 g h−1. The methodology was also applied to the synthesis of a precursor of the hyperthermia drug Dantrolene, starting from furfural derived from biomass [197].
Apart from the photocatalytic C − H arylations of (hetero)arenes which have been described using Eosin Y [198], dual catalysis by [Ru(bpy)3]2+ in conjunction with Pd(OAc)2 [199], TiO2 [200] and Mn photocatalysis (vide supra), a catalyst-free arylation would be attractive from the viewpoint of sustainability and atom-economy. Exploiting the advantages offered by the implementation of microreactor technology for the generation of highly reactive, explosive intermediates, Kappe and co-workers developed a catalyst-free radical C − H arylation through photochemical means. The so-called diazo anhydrides (Ar-N=N-O-N=N-Ar), which are nitrosamine dimers, fragment homolytically under irradiation with near-UV light (> 300 nm) to yield aryl radicals which can be applied in the C − H arylation reaction of (hetero)arenes. Although these intermediates are highly unstable, they can be safely generated and consumed in situ by performing the reaction in a microreactor. Nitrosamines are formed through the nitrosation reaction of anilines with tert-butyl nitrite (t-BuONO, Scheme 57).
To decrease the formation of byproducts formed from the spontaneous decomposition of diazo compounds, t-BuONO and the arene coupling partners are fed via two separate reagent streams to a T-mixer before entering the photoreactor, equipped with a medium pressure Hg lamp (125 W, although a reduction to 75 W was found to be suitable for certain substrates) and UV-filter (cut-off at 300 nm). The substrate scope includes thiophenes, furan and N-protected pyrroles, as well as electron-rich phenyl derivatives and azines (including pyridine N-oxide) which were arylated in a residence time of 45 min, producing only N2, H2O and t-BuOH as waste in a metal-free process (Scheme 58) [201].
Another application of α-amino radicals was reported by Vega, Trabanco and co-workers at Janssen Pharmaceuticals for the C − H arylation of the α-position in N,N-dialkylhydrazones.
The authors propose an oxidative quenching cycle based on the reductive abilities of the Ir(ppy)3 photocatalyst (Scheme 59). Upon excitation with blue light (455 nm), excited Ir(III) undergoes SET with an electron-poor arene substrate (e.g., 2,4-dicyanobenzene), forming Ir(IV) and the one-electron reduced form of the arene (radical anion). Ir(IV) is then reduced to Ir(III) to restart the cycle by oxidising the hydrazone, generating a hydrazinium radical, which readily undergoes deprotonation by LiOAc to form the stabilized α-amino radical. The α-amino radical forms an anionic adduct through radical-radical coupling with the radical anion of the electron-poor arene. With cyanide acting as the leaving group in the adduct, the product is then formed. Through the use of continuous-flow, the arylation could be accelerated in 20–40 min allowing the gram-scale synthesis of arylated dialkylhydrazones. Repeating the reaction after the first arylation with another flow reactor allowed for a regioselective second arylation step on the same substrate, though more forcing conditions (increase of catalyst loading, residence time and reaction temperature) were required. Apart from cyanobenzenes, azines were also successfully employed as electron-poor arene coupling partners (Scheme 60) [202].
C − N bond formation
Activation of the beta C − H bond in hindered aliphatic amines to yield aziridines (or beta-lactams in the presence of carbon monoxide) was reported by Gaunt and co-workers in 2014 [203] and was later adapted to a continuous-flow process by Lapkin and co-workers [204]. Starting from palladium(II) acetate, the hindered amine coordinates to the metal. This can happen a second time to form a Pd(II) center coordinated by two amines. The steric bulk on the amine is critical to achieve activation of the C − H bond, since the C − H activation step requires dissociation of this complex to the less stable monocoordinated variant. This dissociation is more favoured when hindered amines are used. The monocoordinated complex can then undergo the C − H activation step (rate-determining), i.e. deprotonation of the beta C − H bond through a concerted metalation-deprotonation (CMD) mechanism to yield a cyclometalated Pd(II) complex. This can undergo oxidation by the hypervalent iodine oxidant PhI(OAc)2 resulting in a Pd(IV) complex. The nitrogen is then deprotonated to form a four-membered Pd(IV) intermediate which undergoes reductive elimination, liberating the aziridine and Pd(II), thereby closing the catalytic cycle.
The mechanism of the reaction was investigated in detail (Scheme 61). The rate of the reaction was shown to increase over time, which could have two causes: either the rate was affected negatively by increasing the concentration of the amine, or the reaction rate was increased by one of the byproducts of the reaction (autoinduction). The latter was ruled out by starting the reaction at 20% conversion. Essentially, the reaction is performed with the same equivalent ratios, but with a smaller amount of the reagents. Because less byproducts are formed, an autocatalyzed reaction should then be slower. When factoring in the different starting concentrations, the rates were shown to be identical, suggesting instead that the rate was negatively affected by the concentration of amine (its consumption during the reaction then leads to an increase in the reaction rate). The effect of adding reaction products as additives to the reaction (PhI, aziridine, and HOAc) was investigated as well. In this case, increasing the amount of HOAc showed a small increase in the rate. The rate at t0 was shown by comparison to t1/2 to be inversely proportional to the concentration of amine. Apart from this, the reaction was shown to be zeroth order in PhI(OAc)2 and first order in Pd(OAc)2.
The kinetic isotope effect (KIE) can be applied to the study of reaction mechanisms by isotopic labeling. In C − H activation, deuterium labeling of the target C − H bond can change the rate of the reaction (a primary kinetic isotope effect) which shows the C − H activation to be the rate-limiting step (k3), which proved to the case for the C − H aziridination, explaining the first order dependence on Pd(OAc)2. The oxidative addition of PhI(OAc)2 following this step then does not alter the rate. The negative first order dependence on the amine can be rationalized by the increased reversible formation of the square planar bisaminated Pd(II) complex (k2), which is unproductive, since the C − H activation step requires a vacant coordination site [205, 206]. As the protonated amine cannot coordinate to Pd(II), increasing the amount of HOAc (k0) decreases the effective concentration of amine available to coordinate the metal which shifts the k2 equilibrium towards the desired monoaminated complex. The optimal amount of HOAc was determined to be 20 equiv., since higher concentrations of acid lead to degradation of the aziridine product.
Another piece of evidence in the puzzle is the rate with which more sterically congested amines react. Increasing the steric bulk makes the formation of the bisaminated complex less favorable, increasing the rate, which was shown by a competition experiment between two amines of which one was more substituted. The selectivity and the mechanism of C − H activation were further probed by DFT calculations. Data from the mechanistic investigations were combined in a kinetic model and applied to the design of a process model for an ideal plug flow reactor, with the limitation that Tmax = 120 °C (stability limit of Pd(OAc)2) and that the space-time yield be large enough (full conversion within 10 min). Optimization experiments were performed and yielded conditions with 0.5 mol% loading of Pd catalyst for which tr = 10 min.
Next, further development of the flow process for gram-scale production of aziridines was carried out by incorporating modules to remove the catalyst as well as the desired aziridine product. Separation of the homogeneous catalyst was accomplished through the use of an amine-functionalized QuadraSil AP column (which coordinates and retains the Pd(II) catalyst), leaving the eluent with <1 ppm of Pd. The aziridine was removed from the mixture by incorporating a column packed with Isolute SCX-3 gel (sulfonic acid-functionalized silica gel). Washing the column with a basic eluent subsequently elutes the aziridine yielding the product without further purification required. Performing the reaction in a commercial Vapourtec R-Series allowed a productivity of 0.77 g h−1 (space-time yield 0.463 kg Vr−1 h−1, Scheme 62).
A derivatization in flow of the resulting aziridines by reaction with nucleophiles was also developed. In the case of non-activated aziridines and weak nucleophiles, this generally requires activation by (Lewis) acids. Hence, aziridines retained on the acidic column are susceptible to nucleophilic attack and these principles were applied to the in-line derivatization with MeOH, H2O and in-situ generated HN3, yielding the functionalized amine products in good yield (Scheme 63) [204].
The direct C − H amination of arenes (Minisci-type amination) is a challenging transformation, requiring strongly oxidizing conditions [207, 208] or the installment of a redox auxiliary or electrophoric group [209]. The group of Leonori recently reported the application of O-aryl hydroxylamines as a redox auxiliary to generate aminium radicals for late stage C − H amination [210, 211]. Building on this work while eliminating the need for prefunctionalization, a visible light photocatalytic approach to generate aminium radicals from amines directly via the in situ formation of an N-chloroamine with N-chlorosuccinimide (NCS) was developed (Scheme 64).
Under acidic conditions, the protonated N-chloroamines can engage in an oxidative quenching cycle with the photocatalyst [Ru(bpy)3]Cl2, generating the chloride ion and an electrophilic aminium radical, which undergoes addition to an arene. The radical adduct formed after addition is then oxidised to a carbocation by Ru(III), closing the catalytic cycle and generating the aminofunctionalized arene after loss of a proton. Key to the para-selectivity of the aromatic amination is the polarity of the medium combine with the highly polarized aminium radical. Under these conditions, the aromatic chlorination which is a competing pathway when using N-chloroamine reagents is suppressed in favour of the aromatic C − H amination. Using HFIP as a solvent also allowed expanding of the substrate scope to more electron-poor arenes, e.g. fluorobenzene, and improves selectivity towards the para-position. The scope of the reaction is very broad, including the direct functionalization of the organometallic compound [Ru(ppy)(bpy)2]PF6, polystyrene (degree of functionalization: 19%) and a tetrapeptide, the latter undergoing selective amination in the para-position of the phenyl moiety in a Phe residue, which is underexplored in bioconjugation chemistry.
The method was adapted to flow in collaboration with AstraZeneca with piperidine and iodobenzene as reacting partners to furnish the para-substituted building block 1-(4-iodophenyl)piperidine in a productivity of 3.8 g h−1 (Scheme 65) [212]. A different photochemical approach was followed by Marsden and co-workers, performing an intramolecular C − H amination in a mixture of acetic acid and sulfuric acid and fragmenting the protonated N-chloroamine homolytically using UV light [213].
The reaction was performed in flow, in which the N-chloroamine could be synthesized from the amine and NCS followed by cleaving the nitrogen-chlorine bond homolytically under irradiation of a 125 W UV lamp. This was then applied to intramolecular C − H amination reactions in flow to synthesize a range of tetrahydroquinolines (Scheme 66). Although higher isolated yield was obtained in both batch and flow conditions when both steps were performed separately, integrating the chlorination of the amine and the intramolecular C − H amination reaction in a modular fashion gave N-methyltetrahydroquinoline in an overall yield of 34% [214].
A myriad of reports on reactions involving a 1,5-hydrogen atom transfer step have recently appeared in the literature [215,216,217]. The first 1,5-HAT reaction, the classic Hoffmann-Löffler-Freytag reaction, originated in 1883 and involves photolysis of a protonated N-chloroamine after which the resulting aminium radical abstracts a hydrogen from a carbon five atoms away, resulting in δ-chlorination. The 1,5-radical translocation is thermodynamically favourable because the abstraction of the hydridic hydrogen atom is exergonic and occurs through a six-membered transition state, although 1,6- and 1,7-translocations involving sulfamates and sulfamides have also been reported by the group of Roizen [218, 219]. Following the translocation of the halide, an intramolecular nucleophilic substitution reaction then furnishes pyrrolidine products. Recently, different electrochemical variations of the Hoffmann-Löffler-Freytag reaction were reported. Lei and co-workers reported a halide-free HLF reaction of tosylamides in the presence of acetate and HFIP involving amidyl radical intermediates. Different pathways (involving ionic or radical reactivity) were proposed to arrive at the amidyl radical, which generates a carbon-centered radical after the intramolecular remote hydrogen atom transfer. The carbon-centered radical is then oxidised to a carbocation, which cyclizes to form pyrrolidines [220]. A complementary approach involving both the use of electrochemistry and light to cleave the N-haloamides was reported by Stahl and co-workers [221]. Iodide ion is oxidised to iodine at the anode, which reacts with amides to generate the photolabile N-iodoamides leading to amidyl radicals [222,223,224,225]. Using imines, iminyl radicals can be accessed as well. This was applied to the synthesis of oxazolines and 1,2-amino alcohols. With bromide as the mediator, Rueping and co-workers developed an electrochemical cross-dehydrogenative approach to the Hoffmann-Löffler-Freytag reaction for the synthesis of pyrrolidines under both batch and flow conditions. The mechanistic pathways through which the reaction occurs are again complicated, since several species and pathways (both occuring at the anode or cathode in the undivided cell) can be involved in the generation of the key amidyl radical intermediate (Scheme 67).
The amidyl radical undergoes intramolecular HAT to yield the carbon-centered radical which can form the pyrrolidine in a number of different ways. First, the carbon radical can trap bromine (generated under oxidative conditions from bromide) which can undergo an intramolecular nucleophilic substitution. Alternatively, the carbon-centered radical can undergo direct oxidation to the carbocation followed by cyclization. The presence of base (methoxide, which is also formed from MeOH through the cathodic reduction of protons to hydrogen in a divided cell) was found necessary for the reaction to occur, as the amidyl radical can be accessed through the deprotonated tosylamide. The reaction was scaled under both batch (50 g scale) and flow conditions. Flow chemistry is an attractive option for the scale-up of electrochemical reactions due to the fast reaction rates and decrease of the necessary amount of supporting electrolyte (a non-neglible factor during scale-up). A commercial Asia Flux module manufactured by Syrris was used in conjuction with a graphite anode and 316 alloy stainless steel cathode. The desired pyrrolidines were formed in high yields under both conditions of constant current (5 mA cm−2) and constant potential (2.8 V). Accurate control of residence time in flow reactions generating gases is difficult, since the formation of gas bubbles (slugs) decreases the residence time during the reaction. Hence, a back-pressure regulator was included in the system to solubilize the majority of hydrogen formed at the cathode (1 to 5 bar), allowing the synthesis of the tosylpyrrolidine compound methyl 4-methyl-1-tosylpyrrolidine-2-carboxylate in 76% yield with a productivity of 0.37 mmol h−1 (Scheme 68) [226].
Another reaction of historical importance involving such a translocation is the Barton reaction. It can be classified as a photochemical C − H oximation occuring through the homolytic photolysis of a nitrite functional group in which a nitroxy - and alkoxy radical are formed. The electrophilic alkoxy radical then engages in a 1,5-HAT leading to the radical translocation of a nitroxide functionality which equilibrates to an oxime (Scheme 69, right part).
The group of Ryu reported a Barton reaction under flow conditions. An alcohol is transformed into a nitrite by reaction with nitrosyl chloride (NOCl) and the labile N-O bond is cleaved by irradiation with near-UV light (365 nm proving sufficient). The reaction was performed in a stainless steel microreactor with a glass cover to allow irradiation of the reaction mixture (Scheme 68, left part). Different parameters of the reaction were investigated, e.g. light source combinations with different glasses (cut-offs being at different wavelengths depending on the material). Out of these combinations, the application of a 15 W black light in combination with Pyrex glass performed best for small-scale reactions. While the Barton reaction is usually run in acetone as the solvent, the limited solubility of the steroid in acetone necessitated switching to DMF. The reaction was scaled-up to gram scale, combining two microreactors and 8 × 20 W black lights, yielding 3.1 g of the oxime after 20 h (32 min residence time), an intermediate in the synthesis of myriceric acid A [227].
C − O bond formation
Pasau and co-workers at UCB developed a benzylic photochemical C − H oxidation in continuous flow (Scheme 70). Oxygen gas is used as the oxidant in conjunction with the organic photocatalyst riboflavin tetraacetate (RFT) under UV light irradiation with an Fe(III) salt, the latter being necessary for the reduction of hydrogen peroxide which causes decomposition of the riboflavin [228]. After irradiation with UV light, the riboflavin can abstract a hydrogen atom from the benzylic position to generate a benzylic radical. Since riboflavin is a known photosensitizer for oxygen, the authors propose the formation of singlet oxygen, 1O2, which would then react with the benzylic radical to give a peroxo radical, which can engage in HAT forming a hydroperoxide. The hydroperoxides can form either ketone or alcohol products. The reduced riboflavin can then be reoxidised by 1O2, returning to its ground state, while the H2O2 formed during the oxidation is reduced by the catalytic amount of the iron(II)perchlorate additive (Scheme 71).
To synthesize a series of acetals, Kappe and co-workers used Cu(OAc)2 and t-BuOOH as the oxidant for the oxidative coupling of ethers and enols (from phenols and β-ketoesters), in which a bond is formed between the enol oxygen and the α-carbon of an ether (Scheme 72). To avoid the dangers associated with heating a mixture of peroxides and ethers, a feed containing a non-aqueous solution of t-BuOOH in n-decane is premixed in a glass static mixer with a feed containing the copper catalyst, the ether and the substrate.
The reaction is performed in a stainless steel reactor coil with a volume of 20 ml and an internal diameter of 1 mm leading to the formation of the acetal products in less than 20 min at 130 °C compared to 3 h reflux under batch conditions [229].
Flow chemistry offers several advantages when dealing with exothermic reactions (e.g., oxidations) due to excellent heat transfer to the environment, while the small reacting volumes decrease explosion and combustion hazards [31, 230, 231]. Thus, it becomes possible to operate in novel process windows, i.e. conditions of high temperature and pressure not safely accessible in batch. The MC system is a highly effective mixture of Co, Mn and bromide salts for the synthesis of carbonyl compounds through aerobic oxidation [232, 233]. Kappe and co-workers studied the oxidation of ethylbenzene to acetophenone or benzoic acid under flow conditions using 2.5 mol% of CoBr2 and Mn(OAc)2 and air as the oxidant (Scheme 73).
Air, delivered from a gas bottle connected to a mass-flow controller is mixed with the liquid reagent stream (1 M ethylbenzene, 2.5 mol% CoBr2 and 2.5 mol% Mn(OAc)2 in HOAc) pumped with a HPLC pump to the reactor, a 50 m (Vr = 25 mL) PFA coil placed inside a GC oven (100–150 °C). The system is kept under pressure through the use of a 12 bar back-pressure regulator (BPR). Acetophenone can be obtained in a residence time of 4–8 min in 66% yield without chromatography. 2-bromoacetophenone present in the reaction mixture can be removed by reduction through treatment of the crude with Zn metal. Alternatively, doubling the residence time to 16 min allows the production of benzoic acid in 71% yield (purification with acid-base extraction, no chromatography necessary) [234].
The oxidation of unactivated sp3 C − H bonds is a highly challenging transformation [235]. Several approaches to the oxidation of methylene or methine groups exist, including metal catalysis (e.g., the Chen-White oxidation or the use of metal porphyrins, vide infra), biocatalysis, photocatalysis and electrochemistry [236]. Examples of these oxidation chemistries performed in flow will be discussed below.
Schultz and co-workers at Merck Sharpe and Dohme (MSD) reported the remote C − H oxidation of amines using sodium decatungstate (NaDT) as a HAT photocatalyst (Scheme 74). The reaction is carried out in a 1:1 mixture of acetonitrile and water in the presence of 1.5 equivalents of H2SO4 to protonate the amine (neutral amines being incompatible with the decatungstate photocatalyst [237]) and 2.5 equivalents of H2O2 as the oxidant. Acidic conditions are important to the remote functionalization, as the radical is preferentially formed on a distal position to the protonated amine, as exemplified by the β-selective functionalization of pyrrolidine, and the γ-selective functionalization of piperidine and azepane. These building blocks are useful but costly in drug discovery. The reaction was performed in a 10 ml flow reactor with an FEP coil using oxygen as the oxidant under 4.5–5 bars of pressure with 365 nm LEDs. As >1 h of residence time was required, recirculating the mixture during 22 h was necessary for the scale-up of a pyrrolidine oxidation on 5 g scale [238].
Noël and co-workers employed tetrabutylammonium decatungstate (TBADT) with a mixture (2.5:1) of acetonitrile and 1 M aqueous HCl for the oxidation of activated and unactivated C − H bonds in flow using oxygen gas (2.5 equivalents) as the oxidant (Scheme 75). The reaction was carried out in a 5 ml PFA capillary reactor at atmospheric pressure in 45 min residence time under 365 nm LED irradiation. The methodology was applied to the oxidation of terpenoid natural products, including the gram scale oxidation (1.5 h residence time, 10 ml reactor volume) of the sesquiterpene antimalarial, artemisinin [239]. Due to the electrophilic nature of the catalyst, both methods allow the site-selective oxidation of a methylene group to a ketone [240, 241]. The alkyl radical formed after HAT with decatungstate is trapped by 3O2 to form an alkyl hydroperoxide intermediate, which leads to alcohol and ketone products, though ketones are the major product (alcohols further being oxidised to ketones having previously been described) [242]. The reduced form of the catalyst is then re-oxidised by a second equivalent of oxygen, closing the catalytic cycle [243, 244].
Another approach to C(sp3)–H hydroxylation involves the use of strong oxidants, e.g. hypervalent iodine reagents or dioxiranes (DMDO or TFDO). While dioxiranes such as TFDO are highly effective oxidants, they are cumbersome to work with: TFDO, for instance, is a gaseous reagent which decomposes above 10 °C. As such, despite its potential, its use is limited to small-scale batch reactions. As discussed previously, one of the main advantages offered by flow chemistry is the potential to generate and consume small quantities of hazardous intermediates in situ, improving both the safety of the process and opening the doors to scale-up.
Ley, Pasau and co-workers developed an in situ formation of the oxidant TFDO under flow conditions, starting from 1,1,1-trifluoromethylacetone (Scheme 76). A mixture of the substrate and 1,1,1-trifluoromethylacetone in DCM (feed 1) are mixed with an aqueous solution of sodium bicarbonate (feed 2), followed by the addition of a third reagent (feed 3) stream containing the oxidant (aqueous solution of Oxone) which are then pumped to a microreactor filled with glass beads and kept at 25 °C. The system is kept under pressure through the use of a back-pressure regulator (75 psi), allowing both the oxidation of unactivated and activated C − H bonds in a residence time of 80 s.
The formation of TFDO was detected by 19F NMR and 1H NMR spectroscopy, after in-line phase separation of the organic phase with a liquid-liquid membrane separator (Zaiput), proving the yield to be higher in flow than batch (5% in flow, compared to 2% in batch). The method also proved scaleable, as exemplified by the production of 2.7 g 1-adamantanol from adamantane using two 20 mL reactors equipped with static mixer coils (96% yield, productivity 1.17 g h−1) [245].
Aside from the applications of C–H oxidation to deliver oxygenated building blocks or as a biomimetic strategy in total synthesis, another interesting application of C–H oxidation can be found in the practice of drug discovery. One of the key aspects in the development of a new drug is the study of its metabolic stability. The first step in the metabolism of drugs in vivo is first pass hepatic oxidation by Cytochrome P450 oxidase liver enzymes, which contain an Fe porphyrin. Oxidative methodologies which are able to mimic drug metabolism are thus in high demand [246, 247].
The field of organic electrochemistry has recently seen a resurgence in attention [248] and can benefit greatly from the implementation of microreactor technology [249]. Electrochemical processes require long reaction times as they are typically highly mass-transfer limited, being limited both by the dimensions of the electrode surface available for the reaction to occur, as well as the fact that electron-transfer processes are absent in the bulk of the solution as they only occur in a very thin layer near the surface of the electrode (Helmholtz layer). While the use of electrons as green and traceless reagents justifies the classifcation of electrochemical reactions under the moniker ‘green chemistry’, an important drawback of organic electrochemistry is the poor conductivity of organic solvents necessitates the use of large (commonly stoichiometric) amounts of supporting electrolyte, which can be either reduced or eliminated entirely in a microreactor due to the very small inter-electrode spacing [250,251,252,253,254]. Aditionally, the potential applied can be used to perform oxidations in a in a more reliable and reproducible way and several electrochemical C–H oxidation methodologies have already been reported [255, 256].
Although electrochemistry has already seen application in the mimicry of drug metabolism through the use of coupled techniques such as electrochemical-mass spectrometry (EC-MS), these methods typically do not allow the delivery of useful amounts of drug metabolites, which can be problematic, in case their full characterization by NMR spectroscopy is required [257, 258]. Hence, flow electrochemical oxidation is an attractive tool for the preparation of drug metabolites.
Stalder and Roth studied the oxidation of five drugs (diclofenac, tolbutamide, primidone, albendazole, and chlorpromazine) by electrochemical means (Scheme 77). While diclofenac, tolbutamide and primidone undergo C–H oxidation, albendazole and chlorpromazine are sulfide drugs which undergo facile electrochemical oxidation to sulfoxides [259]. Diclofenac, a non-steroidal anti-inflammatory drug (NSAID), undergoes first-pass hepatic oxidation via aromatic hydroxylation. At 8 F mol−1 with the use of reducing sodium bisulfite as supporting electrolyte, the metabolite 5-hydroxyldiclofenac was isolated in 46% yield, a notable improvement compared to earlier syntheses requiring both multiple transformations and expensive reagents. An interesting quinone by-product was also isolated which can undergo conjugation with glutathione, one of the strategies followed by the liver to eliminate toxic electrophiles. This can be easily implemented in a modular flow set-up by connecting the output of the electrochemical microreactor to a T-junction and introducing a second stream containing glutathione to synthesize conjugates. Unsurprisingly (vide supra), tolbutamide underwent oxidation at the α-position to the amide nitrogen yielding a product which is not a known tolbutamide metabolite. Primidone was oxidised electrochemically to phenobarbital, a barbiturate drug known to be one of the two first-pass metabolites of primidone. Phenobarbital was isolated in 24% yield, although its productivity was limited (7 mg h−1) by solubility issues. Albendazole (ABZ), a sulfide drug, has two first-pass metabolites corresponding to the sulfoxide and the sulfone, both of which can be accessed electrochemically. The sulfoxide was isolated in 38% yield (productivity 65 mg h−1) while chlorpromazine, an antipsychotic drug, also underwent S-oxidation to yield a sulfoxide (83% yield, 33 mg h−1), which is one of its hepatic metabolites [259].
C − S bond formation
The heterocyclic motifs thiazole and thiazine account for 15% of all sulfur-containing drugs approved by the US Food and Drug Administration (FDA) [260]. These compounds are traditionally accessed from the parent N-aryl thioamides through the use of chemical oxidants, while complementary catalytic approaches have also been developed involving the use of palladium [261] and photoredox catalysis [262, 263]. As mentioned before, performing oxidative reactions in an electrochemical cell can avoid both the necessity of using highly oxidising conditions and the use of transition metals. Although the first report on electrochemical oxidation for the synthesis of benzothiazoles was published in 1979 [264], due to renewed interest in the field of electrochemistry more methodologies are being reported in literature, including improvements in the electrosynthesis of benzothiazoles via dehydrogenative C-S coupling [265, 266]. The group of Wirth reported the synthesis of thiazoles using flow electrochemistry. As before, the advantages associated with the use of flow electrochemistry are exploited. In this case, the reaction could be performed without mediator and without supporting electrolyte. Taking into account electrons are the only reagents in the reaction, requiring only a mixture of acetonitrile and methanol as the solvent and a C anode/Pt cathode couple, the methodology is highly sustainable. The electrochemical method is also compatible with thioamide-substituted pyridines, leading to thiazolopyridine products. The method is tolerant of a large variety of functional groups including free alkyl alcohols. The synthesis of 2-phenylbenzothiazole was performed on gram-scale with a productivity of 0.3 g h−1, yielding 2.4 g after 7.2 h (Scheme 78). Due to the presence of methanol, hydrogen evolution is observed at the counter-electrode. The authors propose a mechanism based on the one-electron oxidation of the (deprotonated) thioamide to a thiamidyl radical in which the sulfur atom has partial radical character. This intermediate can then undergo a dimerisation (observed in some cases) or undergo an intramolecular cyclization with the arene. Following oxidation of the radical adduct after intramolecular cyclization, a carbocation is formed yielding the aromatic thiazole after deprotonation (Scheme 79) [267].
Apart from thiazoles, six-membered heterocycles (e.g., thiazines) incorporating a sulfur atom can also be accessed through oxidative methodologies, although their electrochemical synthesis was hitherto underexplored. Again through an oxidative dehydrogenative C-S coupling, Xu and co-workers developed the synthesis of 1,4-thiazines and 1,4-benzoxathiins using a flow electrolysis cell (Scheme 80).
The reaction is run in the presence of the Lewis acid Sc(OTf)3 with a mixture of acetonitrile and TFA (9:1) as the solvent with a Pt cathode (the reduction of protons being the reaction occuring at the counter electrode) and a carbon-filled polyvinylidene fluoride anode. The mechanism of the reaction is similar to the cyclization to form thiazoles (vide supra), analogously occuring via oxidation of the thioamide to a thiamidyl radical intermediate. Particular to this transformation is the use of a catalytic amount of the Lewis acid Sc(OTf)3 and the strong Bronsted acid TFA. The use of acids is important to promote the formation of the cyclic products with good selectivity, as protonation allows for the formation of the thiamidyl radical at lower voltages and protonation of the cyclized product prevents it from undergoing further oxidation, as demonstrated by cyclic voltammetry studies [268]. Because the protonated thiamidyl radical is more electrophilic, its polarity matches the arene coupling partner more closely promoting the desired intramolecular cyclization reaction and allowing for the synthesis of substituted 1,4-benzothiazines and 1,4-benzoxathiins bearing both electron-withdrawing and electron-donating groups [211].
C − X bond formation
Transforming a carbon-hydrogen bond to a carbon-halogen bond is a key transformation in organic synthesis. As many dangers are associated with the use of elemental halogens, for the C − H halogenation of organic compounds the application of microreactor technology is an obvious choice. Moreover, C − H halogenations are frequently initiated by the homolytic cleavage of a halogen-halogen bond using light, with the advantages of performing photochemistry in flow having been previously highlighted, while elemental halogens (or equivalent sources of electrophilic halogens, “X+”) can be safely generated under flow conditions. The halogenation of organic compounds was comprehensively reviewed by Cantillo et al. (2017) [269].
C − H fluorination
Although the carbon-fluorine bond has many attractive properties, due to the difficulties associated with working with fluoride salts (low solubility in organic media), elemental fluorine and hydrofluoric acid, the development of novel fluorinating agents is an active field. Depending on their reactivity, the reagents can be classified as being either electrophilic or nucleophilic. One such nucleophilic reagent is diethylaminosulfur trifluoride (DAST). A downside of its use is the fact that it decomposes above 90 °C, which makes applying microreactor technology an attractive choice when working with this reagent. Aside from the explosive aspect, perfluorinated tubing is not affected by the hydrofluoric acid formed during the reaction, which can be quenched by introducing a bicarbonate solution as the reagent stream exits the reactor. Seeberger et al. applied DAST to the fluorination of aldehydes to form acyl fluorides [270].
A different approach to acyl fluorides was followed by Britton et al. using the decatungstate photocatalyst and the electrophilic fluorinating agent N-fluorobenzenesulfonimide (NFSI) [271]. Through decatungstate photocatalysis, site-selective C − H fluorination was achieved for both unactivated and activated C − H bonds, the methodology also being applied to the synthesis of tracers for Positron Emission Tomography (PET) studies by the labelling of amino acids and peptides with [18F]NFSI [272,273,274]. Notable is the difference in selectivity obtained when using photocatalysis when compared to a thermal radical chain reaction initiated with AIBN, exemplified by the functionalization of 4-ethyltoluene bearing two distinct benzylic positions, with AIBN favouring methyl functionalization (kinetic control). The reaction was performed under flow conditions for the fluorination of ibuprofen methyl ester (Scheme 81) [275].
In collaboration with Merck, the methodology was applied to the fluorination of leucine methyl ester, a key component of Odanacatib, an osteoporosis drug candidate [276]. Under flow conditions, the reaction could be accelerated from 16 h to 2 h, allowing the production of γ-fluoroleucine in 90% yield (> 20 g scale, Scheme 82) [237].
An organocatalytic approach towards benzylic fluorination was followed by Kappe and co-workers by application of xanthone as a photoorganocatalyst, Selectfluor as the fluorinating agent and irradiation with a 105 W CFL bulb (Scheme 83).
The T1 excited state of xanthone functions as a HAT catalyst to generate benzylic radicals [277, 278]. This allowed the fluorination of a variety of benzylic substrates at room temperature in a residence time of less than 30 min, although for more challenging substrates, an elevated reaction temperature of 60 °C was required. Ibuprofen methyl ester was fluorinated at the benzylic position with >90% selectivity in 80% isolated yield, while the natural product celestolide, a common fragrance component, was fluorinated in 9 min in 88% isolated yield. Processing of 100 ml of solution allowed the production of 2.3 g of product [279].
C − H chlorination
The C − H photochlorination in continuous flow reported by Jähnisch and co-workers using Cl2 gas in a FFMR is one of the earliest examples of continuous-flow radical C − H functionalization in the chemical research literature. Apart from the obvious hazards associated with storing chlorine gas in its elemental form, one of the downsides of using Cl2 for radical chlorinations is the occurrence of electrophilic aromatic substitution as a side-reaction, especially when working with electron-rich substrates. Through the use of a quartz window, irradiation of the reaction mixture in the FFMR with short wavelength UV light (190–250 nm) coming from a 1000 W Xenon lamp became possible, supressing the ionic reactivity and providing the desired chlorinated product in a spacetime yield of 400 mol L−1 h−1 (Scheme 84) [280].
Ryu et al. developed a photochlorination of alkanes with a 15 W CFL black light (Scheme 85). Sulfuryl chloride was also used as a chlorinating agent. Due to the dangers associated with chlorine gas, in situ generation of molecular chlorine is highly desirable. Several reports have appeared on photochemical oxidative chlorination. Hydrochloric acid is then mixed with bleach to form chlorine in situ, which is then homolytically cleaved in a photoreactor, as demonstrated by the groups of Ryu and Kappe (Scheme 86) [281, 282].
C − H bromination
Although elemental bromine is easier to handle than chlorine and bromine radicals are more selective when performing HAT, bromination is electronically less deactivating than chlorination. For this reason, photochemical C − H bromination suffers more frequently from polybromination in unbiased substrates such as cycloalkanes [283]. Through the use of microreactor technology, Ryu et al. performed the C − H bromination of cycloalkanes in a residence time of a few minutes using a glass microreactor and irradiation with 352 nm light through the use of a 15 W black light (Scheme 87) [284].
An additional benefit of microreactor technology is the fact that the product stream leaves the reactor, hence, the formation of polybrominated side products is avoided. The use of solar light as a sustainable energy source for photochemistry is of interest [285,286,287], and was applied to photobromination by Park and co-workers [288]. The photochemical bromination is frequently performed in CCl4, a toxic solvent which use being phased out due to health and environmental concerns [289]. By using the electrophilic brominating agent N-bromosuccinimide, the photobromination can be performed in acetonitrile as the solvent as demonstrated by Kappe and co-workers who developed a continuous-flow C − H benzylic bromination with NBS using FEP tubing and CFL bulb [290]. This was also applied to the synthesis of 5-bromomethylpyrimidine by Casar and co-workers, applied in the synthesis of Rosuvastatin [291]. O’Brien et al. solved the problem of possible microreactor clogging due to the formation of succinimide by performing the reaction in a slug flow with an aqueous phase, allowing in-line separation with a liquid-liquid separator [292].
C − D bond formation
Deuteration and tritiation of pharmaceutical drug candidates is of great importance in the field of drug discovery for the study of drug metabolism and pharmacokinetics [293, 294]. Specifically, the synthesis of stable isotopically labelled standards for detection with mass spectrometry techniques is of interest. Stable isotopically labelled standards are usually prepared via a hydrogen isotope exchange reaction in which C − H activation leads to a H − D or H − T exchange. The benefits of using gases in flow chemistry have been highlighted extensively in this review. The mass-transfer and safety aspects are of particular importance when considering the use of D2. Noël, Vliegen and co-workers developed a continuous-flow approach to the deuteration of a model compound (N-4-methoxyphenyl)-N-methylbenzamide using an immobilized iridium catalyst (Scheme 88). Starting from polystyrene beads functionalized with a diphenylphosphine group, ligand exchange with Crabtree’s catalyst allowed for the immobilization the catalyst. Several reactor designs were tested, including a CSTR, packed bed reactor and the commercial H-Cube Pro™. In the CSTR, the catalyst particles are suspended as a fluidized bed but only allowed deuterium incorporation up to M + 2. In the micro-packed bed reactor, deuteration up to M + 7 was obtained up to 64% conversion after two runs under 40 bars of pressure. M + 7 only for normal pressure with 54% conversion, 64% conversion has only M + 3 product Employing the commercial H-Cube Pro™ system allows for the direct generation of deuterium gas from deuterated water, as well as deuteration up to M + 7 [295].
Conclusion and outlook
The main limitations of C–H functionalization chemistry arise from the selectivity and reactivity of C–H bonds. Herein, we have shown that continuous-flow processing is able to address at least in part those aspects. Selectivity problems can be addressed by increasing the mixing efficiency and by controlling the reaction temperature carefully. Moreover, the reaction mixture can be quenched efficiently in flow, limiting the time that sensitive molecules need to be exposed to harsh reaction conditions and thus offering options to avoid overreaction due to multiple, consecutive C–H functionalization events. The reactivity of C–H bonds can be enhanced by selecting the right catalyst and use of high reaction temperatures. In flow, such conditions can be easily reached due to so-called superheating of the reaction mixture (heating above the boiling point) through use of back pressure regulators. In some cases, such boosting of reaction kinetics allows to even lower the catalyst loading which is another common hurdle associated with batch C–H functionalization chemistry. Moreover, multiphase reaction mixtures can be easily carried out in flow allowing to explore new chemical space, e.g. use of gaseous reagents and safe use of oxygen as a cheap and green oxidant. Also, photochemical HAT reactions can be substantially accelerated in flow due to the homogeneous irradiation of the entire reaction mixture.
Due to these apparent advantages of flow C–H functionalization, we anticipate that microreactor technology will be more frequently used in the future. While some notable advances have been made in recent years, we believe that the field has barely scratched the surface of what is technologically possible. Moreover, new flow technologies are currently developed in academic and industrial settings, which should allow to provide new and unanticipated opportunities to this promising field. Thus, it is our hope that this review will serve as a useful starting point for those aspiring to carry out their C–H functionalization chemistry in flow.
References
Colacot ET (2015) New Trends in Cross-Coupling: Theory and Applications. R Soc Chem
de Meijere A, Diederich F (2004) Metal-catalyzed cross-coupling reactions, Second Edition ed., WILEY-VCH Verlag GmbH & Co. KGaA
Nazor J, Schwaneberg U (2006). Chem Bio Chem 7:638–644
Clardy J, Walsh C (2004). Nature 432:829–837
Zhou J, Kelly WL, Bachmann BO, Gunsior M, Townsend CA, Solomon EI (2001). J Am Chem Soc 123:7388–7398
Sambiagio C, Schönbauer D, Blieck R, Dao-Huy T, Pototschnig G, Schaaf P, Wiesinger T, Zia MF, Wencel-Delord J, Besset T (2018). Chem Soc Rev 47:6603–6743
Karimov RR, Hartwig JF (2018). Angew Chem Int Ed 57:4234–4241
Hartwig JF, Larsen MA (2016). ACS Central Sci 2:281–292
Gensch T, Hopkinson MN, Glorius F, Wencel-Delord J (2016). Chem Soc Rev 45:2900–2936
Dey A, Agasti S, Maiti D (2016). Org Biomol Chem 14:5440–5453
Dey A, Maity S, Maiti D (2016). Chem Commun 52:12398–12414
Hartwig JF (2015). J Am Chem Soc 138:2–24
Lyons TW, Sanford MS (2010). Chem Rev 110:1147–1169
Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O (2010). Chem-Eur J 16:2654–2672
Chen X, Engle KM, Wang DH, Yu JQ (2009). Angew Chem Int Ed 48:5094–5115
Collet F, Dodd RH, Dauban P (2009). Chem Commun:5061–5074
Chen MS, White MC (2007). Science 318:783–787
Godula K, Sames D (2006). Science 312:67–72
Crabtree RH (2001). J Chem Soc-Dalton Trans:2437–2450
Santoro S, Ferlin F, Ackermann L, Vaccaro L (2019). Chem Soc Rev 48:2767–2782
Constable DJ, Dunn PJ, Hayler JD, Humphrey GR, Leazer Jr JL, Linderman RJ, Lorenz K, Manley J, Pearlman BA, Wells A (2007). Green Chem 9:411–420
T. Noël, Y. Su, V. Hessel (2015) In Organometallic Flow Chemistry, Springer, pp. 1–41
Hessel V, Kralisch D, Kockmann N, Noël T, Wang Q (2013). Chem Sus Chem 6:746–789
Hartman RL, McMullen JP, Jensen KF (2011). Angew Chem Int Ed 50:7502–7519
Cantillo D, Kappe CO (2014). Chem Cat Chem 6:3286–3305
Noel T, Buchwald SL (2011). Chem Soc Rev 40:5010–5029
G. Laudadio, T. Noël (2017) In Strategies for Palladium-Catalyzed Non-Directed and Directed CH Bond Functionalization, Elsevier, pp. 275–288
Gemoets HPL, Kalvet I, Nyuchev AV, Erdmann N, Hessel V, Schoenebeck F, Noel T (2017). Chem Sci 8:1046–1055
de Frémont P, Marion N, Nolan SP (2009). Coord Chem Rev 253:862–892
Elvira KS, Casadevall i Solvas X, Wootton RCR, de Mello AJ (2013). Nat Chem 5:905–915
Movsisyan M, Delbeke EIP, Berton JKET, Battilocchio C, Ley SV, Stevens CV (2016). Chem Soc Rev 45:4892–4928
Hock KJ, Koenigs RM (2018). Chem Eur J 24:10571–10583
Hock KJ, Mertens L, Metze FK, Schmittmann C, Koenigs RM (2017). Green Chem 19:905–909
Pieber B, Kappe CO (2016). Org Lett 18:1076–1079
Mueller ST, Wirth T (2015). Chem Sus Chem 8:245–250
Deadman BJ, Collins SG, Maguire AR (2015). Chem Eur J 21:2298–2308
Roda NM, Tran DN, Battilocchio C, Labes R, Ingham RJ, Hawkins JM, Ley SV (2015). Org Biomol Chem 13:2550–2554
Poh JS, Tran DN, Battilocchio C, Hawkins JM, Ley SV (2015). Angew Chem Int Ed 54:7920–7923
Nicolle SM, Hayes CJ, Moody CJ (2015). Chem Eur J 21:4576–4579
Mueller ST, Smith D, Hellier P, Wirth T (2014). Synlett 25:871–875
Mastronardi F, Gutmann B, Kappe CO (2013). Org Lett 15:5590–5593
Bartrum HE, Blakemore DC, Moody CJ, Hayes CJ (2011). Chem Eur J 17:9586–9589
Martin LJ, Marzinzik AL, Ley SV, Baxendale IR (2010). Org Lett 13:320–323
Rackl D, Yoo C-J, Jones CW, Davies HML (2017). Org Lett 19:3055–3058
Chepiga KM, Feng Y, Brunelli NA, Jones CW, Davies HM (2013). Org Lett 15:6136–6139
Moschetta EG, Negretti S, Chepiga KM, Brunelli NA, Labreche Y, Feng Y, Rezaei F, Lively RP, Koros WJ, Davies HML, Jones CW (2015). Angew Chem Int Ed 54:6470–6474
Yoo C-J, Rackl D, Liu W, Hoyt CB, Pimentel B, Lively RP, Davies HML, Jones CW (2018). Angew Chem Int Ed 57:10923–10927
De Waele V, Poizat O, Fagnoni M, Bagno A, Ravelli D (2016). ACS Catal 6:7174–7182
Tzirakis MD, Lykakis IN, Orfanopoulos M (2009). Chem Soc Rev 38:2609–2621
Qrareya H, Dondi D, Ravelli D, Fagnoni M (2015). Chem Cat Chem 7:3350–3357
Ryu I, Tani A, Fukuyama T, Ravelli D, Montanaro S, Fagnoni M (2013). Org Lett 15:2554–2557
Montanaro S, Ravelli D, Merli D, Fagnoni M, Albini A (2012). Org Lett 14:4218–4221
Ravelli D, Albini A, Fagnoni M (2011). Chem-Eur J 17:572–579
Ravelli D, Montanaro S, Zema M, Fagnoni M, Albini A (2011). Adv Synth Catal 353:3295–3300
Dondi D, Ravelli D, Fagnoni M, Mella M, Molinari A, Maldotti A, Albini A (2009). Chem-Eur J 15:7949–7957
Dondi D, Fagnoni M, Albini A (2006). Chem-Eur J 12:4153–4163
Dondi D, Fagnoni M, Molinari A, Maldotti A, Albini A (2004). Chem-Eur J 10:142–148
Bonassi F, Ravelli D, Protti S, Fagnoni M (2015). Adv Synth Catal 357:3687–3695
Hu A, Guo J-J, Pan H, Zuo Z (2018). Science 361:668–672
Majek M, Filace F, von Wangelin AJ (2014). Beilstein J Org Chem 10:981–989
Yan DM, Chen JR, Xiao WJ (2019). Angew Chem Int Ed 58:378–380
Zhao G, Wang T (2018). Angew Chem Int Ed 57:6120–6124
Fan XZ, Rong JW, Wu HL, Zhou Q, Deng HP, Tan JD, Xue CW, Wu LZ, Tao HR, Wu J (2018). Angew Chem Int Ed 57:8514–8518
Twilton J, Zhang P, Shaw MH, Evans RW, MacMillan DW (2017). Nat Rev Chem 1:0052
Skubi KL, Blum TR, Yoon TP (2016). Chem Rev 116:10035–10074
Hopkinson MN, Sahoo B, Li JL, Glorius F (2014). Chem-Eur J 20:3874–3886
Deng H-P, Zhou Q, Wu J (2018). Angew Chem Int Ed 57:12661–12665
Miller DC, Ganley JM, Musacchio AJ, Sherwood TC, Ewing WR, Knowles RR (2019). J Am Chem Soc 141:16590–16594
Musacchio AJ, Lainhart BC, Zhang X, Naguib SG, Sherwood TC, Knowles RR (2017). Science 355:727–730
Musacchio AJ, Nguyen LQ, Beard GH, Knowles RR (2014). J Am Chem Soc 136:12217–12220
Yi H, Zhang G, Wang H, Huang Z, Wang J, Singh AK, Lei A (2017). Chem Rev 117:9016–9085
Espelt LR, Wiensch EM, Yoon TP (2013). J Organomet Chem 78:4107–4114
Shi L, Xia W (2012). Chem Soc Rev 41:7687–7697
McNally A, Prier CK, MacMillan DWC (2011). Science 334:1114–1117
Campos KR (2007). Chem Soc Rev 36:1069–1084
Rueping M, Zhu SQ, Koenigs RM (2011). Chem Commun 47:8679–8681
Rueping M, Vila C, Bootwicha T (2013). ACS Catal 3:1676–1680
Purser S, Moore PR, Swallow S, Gouverneur V (2008). Chem Soc Rev 37:320–330
Charpentier J, Früh N, Togni A (2015). Chem Rev 115:650–682
Alonso C, de Marigorta EM, Rubiales G, Palacios F (2015). Chem Rev 115:1847–1935
Nagib DA, MacMillan DWC (2011). Nature 480:224–228
Pham PV, Nagib DA, MacMillan DW (2011). Angew Chem Int Ed 50:6119–6122
Cantillo D, de Frutos O, Rincon JA, Mateos C, Kappe CO (2014). Org Lett 16:896–899
Mallia CJ, Baxendale IR (2015). Org Process Res Dev 20:327–360
Straathof NJW, Gemoets HPL, Wang X, Schouten JC, Hessel V, Noël T (2014). Chem Sus Chem 7:1612–1617
Straathof N, Osch D, Schouten A, Wang X, Schouten J, Hessel V, Noël T (2014). J Flow Chem 4:12–17
Kuijpers KPL, Bottecchia C, Cambie D, Drummen K, Konig NJ, Noel T (2018). Angew Chem Int Ed 57:11278–11282
Su Y, Kuijpers KPL, König N, Shang M, Hessel V, Noël T (2016). Chem Eur J 22:12295–12300
Margrey KA, Nicewicz DA (2016). Acc Chem Res 49:1997–2006
Nguyen TM, Manohar N, Nicewicz DA (2014). Angew Chem Int Ed 53:6198–6201
Romero NA, Nicewicz DA (2014). J Am Chem Soc 136:17024–17035
Wilger DJ, Grandjean JMM, Lammert TR, Nicewicz DA (2014). Nat Chem 6:720–726
Nicewicz DA, Hamilton DS (2014). Synlett 25:1191–1196
Wilger DJ, Gesmundo NJ, Nicewicz DA (2013). Chem Sci 4:3160–3165
Nguyen TM, Nicewicz DA (2013). J Am Chem Soc 135:9588–9591
Hamilton DS, Nicewicz DA (2012). J Am Chem Soc 134:18577–18580
Luo H-Q, Zhang Z-P, Dong W, Luo X-Z (2014). Synlett 25:1307–1311
Lin Q-Y, Xu X-H, Qing F-L (2014). J Organomet Chem 79:10434–10446
Zhou Q-Q, Zou Y-Q, Lu L-Q, Xiao W-J (2019). Angew Chem Int Ed 58:1586–1604
Straathof NJW, Cramer SE, Hessel V, Noël T (2016). Angew Chem Int Ed 55:15549–15553
Koppenol W (1993). Free Radic Biol Med 15:645–651
Fontana F, Minisci F, Vismara E (1988). Tetrahedron Lett 29:1975–1978
Colman I, Brown MD, Innes GD, Grafstein E, Roberts TE, Rowe BH (2005). Ann Emerg Med 45:393–401
Bigal ME, Tepper SJ (2003). Curr Pain Headache Rep 7:55–62
Johnston MM, Rapoport AM (2010). Drugs 70:1505–1518
Tfelt-Hansen P, De Vries P, Saxena PR (2000). Drugs 60:1259–1287
Xie J, Yuan X, Abdukader A, Zhu C, Ma J (2014). Org Lett 16:1768–1771
Monteiro JL, Carneiro PF, Elsner P, Roberge DM, Wuts PGM, Kurjan KC, Gutmann B, Kappe CO (2017). Chem Eur J 23:176–186
Patra T, Maiti D (2017). Chem-Eur J 23:7382–7401
Bottecchia C, Noël T (2019). Chem Eur J 25:26–42
Sambiagio C, Noel T (2020). Trends in Chemistry 2:92-106
Noel T (2017). J Flow Chem 7:87–93
Cambié D, Bottecchia C, Straathof NJW, Hessel V, Noël T (2016). Chem Rev 116:10276–10341
Su Y, Straathof NJW, Hessel V, Noël T (2014). Chem Eur J 20:10562–10589
Noel T, Wang X, Hessel V (2013). Chemistry Today 31:10–14
Beatty JW, Douglas JJ, Cole KP, Stephenson CRJ (2015). Nat Commun 6:–7919
Beatty JW, Douglas JJ, Miller R, McAtee RC, Cole KP, Stephenson CR (2016). Chem 1:456–472
Demaerel J, Govaerts S, Paul RR, Van Gerven T, De Borggraeve WM (2018). Ultrason Sonochem 41:134–142
Zhang C (2014). Adv Synth Catal 356:2895–2906
O'Hara F, Blackmond DG, Baran PS (2013). J Am Chem Soc 135:12122–12134
Ji Y, Brueckl T, Baxter RD, Fujiwara Y, Seiple IB, Su S, Blackmond DG, Baran PS (2011). Proc Natl Acad Sci U S A 108:14411–14415
Langlois BR, Laurent E, Roidot N (1991). Tetrahedron Lett 32:7525–7528
Dubbaka SR, Nizalapur S, Atthunuri AR, Salla M, Mathew T (2014). Tetrahedron 70:2118–2121
Dubbaka SR, Salla M, Bolisetti R, Nizalapur S (2014). RSC Adv 4:6496–6499
Presset M, Oehlrich D, Rombouts F, Molander GA (2013). J Organomet Chem 78:12837–12843
Zhang X, Huang P, Li Y, Duan C (2015). Org Biomol Chem 13:10917–10922
Shida N, Imada Y, Nagahara S, Okada Y, Chiba K (2019). Commun Chem 2:24
D'Amato EM, Börgel J, Ritter T (2019). Chem Sci 10:2424–2428
Colomer I, Chamberlain AE, Haughey MB, Donohoe TJ (2017). Nat Rev Chem 1:0088
Lefebvre Q, Hoffmann N, Rueping M (2016). Chem Commun 52:2493–2496
Hopkinson MN, Gómez-Suárez A, Teders M, Sahoo B, Glorius F (2016). Angew Chem Int Ed 55:4361–4366
Abdiaj I, Bottecchia C, Alcazar J, Noёl T (2017). Synthesis 49:4978–4985
Furst L, Matsuura BS, Narayanam JM, Tucker JW, Stephenson CR (2010). Org Lett 12:3104–3107
Tucker JW, Nguyen JD, Narayanam JM, Krabbe SW, Stephenson CR (2010). Chem Commun 46:4985–4987
Wang L, Huang W, Li R, Gehrig D, Blom PW, Landfester K, Zhang KA (2016). Angew Chem Int Ed 55:9783–9787
Bottecchia C, Martín R, Abdiaj I, Crovini E, Alcazar J, Orduna J, Blesa MJ, Carrillo JR, Prieto P, Noël T (2018). Adv Synth Catal 361:945–950
Ravelli D, Zema M, Mella M, Fagnoni M, Albini A (2010). Org Biomol Chem 8:4158–4164
Esposti S, Dondi D, Fagnoni M, Albini A (2007). Angew Chem Int Ed 46:2531–2534
Fagnoni M, Bonassi F, Palmieri A, Protti S, Ravelli D, Ballini R (2014). Adv Synth Catal 356:753–758
Garbarino S, Protti S, Gabrielli S, Fagnoni M, Palmieri A, Ravelli D (2018). ChemPhotoChem 2:847–850
Sharma UK, Gemoets HP, Schröder F, Noël T, Van der Eycken EV (2017). ACS Catal 7:3818–3823
Liu Q, Wu LP, Jackstell R, Beller M (2015). Nat Commun 6:5933
Appel AM, Bercaw JE, Bocarsly AB, Dobbek H, DuBois DL, Dupuis M, Ferry JG, Fujita E, Hille R, Kenis PJA, Kerfeld CA, Morris RH, Peden CHF, Portis AR, Ragsdale SW, Rauchfuss TB, Reek JNH, Seefeldt LC, Thauer RK, Waldrop GL (2013). Chem Rev 113:6621–6658
Cokoja M, Bruckmeier C, Rieger B, Herrmann WA, Kühn FE (2011). Angew Chem Int Ed 50:8510–8537
Sakakura T, Choi JC, Yasuda H (2007). Chem Rev 107:2365–2387
Seo H, Katcher MH, Jamison TF (2017). Nat Chem 9:453–456
Tadele K, Verma S, Nadagouda MN, Gonzalez MA, Varma RS (2017). Sci Rep 7:16311
Liu C, Zhang H, Shi W, Lei AW (2011). Chem Rev 111:1780–1824
Yeung CS, Dong VM (2011). Chem Rev 111:1215–1292
Zhou LH, Lu WJ (2014). Chem-Eur J 20:634–642
Le Bras J, Muzart J (2011). Chem Rev 111:1170–1214
Hone CA, Roberge DM, Kappe CO (2017). Chem Sus Chem 10:32–41
Gemoets HP, Su Y, Shang M, Hessel V, Luque R, Noël T (2016). Chem Soc Rev 45:83–117
Gemoets HP, Hessel V, Noël T (2014). Org Lett 16:5800–5803
Ferlin F, Santoro S, Ackermann L, Vaccaro L (2017). Green Chem 19:2510–2514
Wang H, Pesciaioli F, Oliveira JCA, Warratz S, Ackermann L (2017). Angew Chem Int Ed 56:15063–15067
Noël T, Naber JR, Hartman RL, McMullen JP, Jensen KF, Buchwald SL (2011). Chem Sci 2:287–290
Zhang L, Geng M, Teng P, Zhao D, Lu X, Li J-X (2012). Ultrason Sonochem 19:250–256
Christakakou M, Schön M, Schnürch M, Mihovilovic MD (2013). Synlett 24:2411–2418
Wang D, Stahl SS (2017). J Am Chem Soc 139:5704–5707
Wang D, Izawa Y, Stahl SS (2014). J Am Chem Soc 136:9914–9917
Izawa Y, Stahl SS (2010). Adv Synth Catal 352:3223–3229
Álvarez-Casao Y, van Slagmaat CA, Verzijl GK, Lefort L, Alsters PL, Fernández-Ibáñez MÁ (2018). ChemCatChem 10:2620–2626
Hylland KT, Øien-Ødegaard S, Lillerud KP, Tilset M (2015). Synlett 26:1480–1485
Erdmann N, Su Y, Bosmans B, Hessel V, Noël T (2016). Org Process Res Dev 20:831–835
Phipps RJ, Gaunt MJ (2009). Science 323:1593–1597
Merritt EA, Olofsson B (2009). Angew Chem Int Ed 48:9052–9070
Laudadio G, Gemoets HPL, Hessel V, Noël T (2017). J Organomet Chem 82:11735–11741
Gemoets HPL, Laudadio G, Verstraete K, Hessel V, Noël T (2017). Angew Chem Int Ed 56:7161–7165
Gandeepan P, Ackermann L (2018). Chem 4:199–222
Bhattacharya T, Pimparkar S, Maiti D (2018). RSC Adv 8:19456–19464
Zhao Q, Poisson T, Pannecoucke X, Besset T (2017). Synthesis 49:4808–4826
Della Cá N, Fontana M, Motti E, Catellani M (2016). Acc Chem Res 49:1389–1400
Ye J, Lautens M (2015). Nat Chem 7:863–870
A. Martins, B. Mariampillai, M. Lautens, in CH Activation, Springer, 2009, pp. 1–33
Catellani M, Frignani F, Rangoni A (1997). Angew Chem Int Ed Eng 36:119–122
Dong Z, Wang J, Dong G (2015). J Am Chem Soc 137:5887–5890
Cheng HG, Chen S, Chen R, Zhou Q (2019). Angew Chem Int Ed 58:5832–5844
Chen S, Liu ZS, Yang T, Hua Y, Zhou Z, Cheng HG, Zhou Q (2018). Angew Chem Int Ed 57:7161–7165
Zhou PX, Ye YY, Liu C, Zhao LB, Hou JY, Chen DQ, Tang Q, Wang AQ, Zhang JY, Huang QX, Xu PF, Liang YM (2015). ACS Catal 5:4927–4931
Candito DA, Lautens M (2010). Org Lett 12:3312–3315
Candito DA, Lautens M (2009). Angew Chem Int Ed 48:6713–6716
Casnati A, Gemoets HPL, Motti E, Della Ca N, Noël T (2018). Chem Eur J 24:14079–14083
Gandeepan P, Müller T, Zell D, Cera G, Warratz S, Ackermann L (2019). Chem Rev 119:2192-2452
Tian X, Yang F, Rasina D, Bauer M, Warratz S, Ferlin F, Vaccaro L, Ackermann L (2016). Chem Commun 52:9777–9780
Paria S, Reiser O (2014). Chem Cat Chem 6:2477–2483
Hernandez-Perez AC, Collins SK (2013). Angew Chem Int Ed 52:12696–12700
Kindt S, Heinrich MR (2016). Synthesis 48:1597–1606
Bajorowicz B, Kobylański MP, Gołąbiewska A, Nadolna J, Zaleska-Medynska A, Malankowska A (2018) Adv. Colloid Interface Sci 256:352–372
Beydoun D, Amal R, Low G, McEvoy S (1999). J Nanopart Res 1:439–458
Fujishima A, Rao TN, Tryk DA (2000). J Photochem Photobiol C 1:1–21
Lazar MA, Varghese S, Nair SS (2012). Catalysts 2:572–601
Riente P, Noël T (2019). Catal Sci Technol 9:5186–5232
Sakthivel S, Kisch H (2003). Angew Chem Int Ed 42:4908–4911
Kisch H, Macyk W (2002). Chem Phys Chem 3:399–400
Fabry DC, Ho YA, Zapf R, Tremel W, Panthöfer M, Rueping M, Rehm TH (2017). Green Chem 19:1911–1918
Liang Y-F, Steinbock R, Yang L, Ackermann L (2018). Angew Chem Int Ed 130:10785–10789
Hari DP, König B (2013). Angew Chem Int Ed 52:4734–4743
Neufeldt SR, Sanford MS (2012). Adv Synth Catal 354:3517–3522
Zoller J, Fabry DC, Rueping M (2015). ACS Catal 5:3900–3904
Cantillo D, Mateos C, Rincon JA, de Frutos O, Kappe CO (2015). Chem Eur J 21:12894–12898
Vega JA, Alonso JM, Méndez G, Ciordia M, Delgado F, Trabanco AA (2017). Org Lett 19:938–941
McNally A, Haffemayer B, Collins BSL, Gaunt MJ (2014). Nature 510:129
Zakrzewski J, Smalley AP, Kabeshov MA, Gaunt MJ, Lapkin AA (2016). Angew Chem Int Ed 55:8878–8883
Davies DL, Donald SM, Macgregor SA (2005). J Am Chem Soc 127:13754–13755
Ryabov AD (1990). Chem Rev 90:403–424
Romero NA, Margrey KA, Tay NE, Nicewicz DA (2015). Science 349:1326–1330
Morofuji T, Shimizu A, Yoshida J-i (2013). J Am Chem Soc 135:5000–5003
Davies J, Morcillo SP, Douglas JJ, Leonori D (2018). Chem-Eur J 24:12154–12163
Davies J, Angelini L, Alkhalifah MA, Sanz LM, Sheikh NS, Leonori D (2018). Synthesis 50:821–830
Svejstrup TD, Ruffoni A, Julia F, Aubert VM, Leonori D (2017). Angew Chem Int Ed 56:14948–14952
Ruffoni A, Juliá F, Svejstrup TD, McMillan AJ, Douglas JJ, Leonori D (2019). Nat Chem 11:426–433
Cosgrove SC, Plane JM, Marsden SP (2018). Chem Sci 9:6647–6652
Cosgrove SC, Douglas GE, Raw SA, Marsden SP (2018). ChemPhotoChem 2:851–854
Hu A, Guo J-J, Pan H, Tang H, Gao Z, Zuo Z (2018). J Am Chem Soc 140:1612–1616
Morcillo SP, Dauncey EM, Kim JH, Douglas JJ, Sheikh NS, Leonori D (2018). Angew Chem Int Ed 57:12945–12949
Dauncey EM, Morcillo SP, Douglas JJ, Sheikh NS, Leonori D (2018). Angew Chem Int Ed 57:744–748
Short MA, Shehata MF, Sanders MA, Roizen JL (2020). Chem Sci. 11:217-223
Short MA, Blackburn JM, Roizen JL (2018). Angew Chem Int Ed 57:296–299
Hu X, Zhang G, Bu F, Nie L, Lei A (2018). ACS Catal 8:9370–9375
Wang F, Stahl SS (2019). Angew Chem Int Ed 58:6385–6390
Del Castillo E, Muñiz K (2019). Org Lett 21:705–708
Herold S, Bafaluy D, Muniz K (2018). Green Chem 20:3191–3196
Zhang H, Muñiz K (2017). ACS Catal 7:4122–4125
Martínez C, Muniz K (2015). Angew Chem Int Ed 54:8287–8291
Nikolaienko P, Jentsch M, Kale AP, Rueping M (2019). Chem Eur J 25:7177–7184
Sugimoto A, Fukuyama T, Sumino Y, Takagi M, Ryu I (2009). Tetrahedron 65:1593–1598
Mühldorf B, Wolf R (2016). Angew Chem Int Ed 55:427–430
Kumar GS, Pieber B, Reddy KR, Kappe CO (2012). Chem Eur J 18:6124–6128
Kockmann N, Thenée P, Fleischer-Trebes C, Laudadio G, Noël T (2017). React Chem Eng 2:258–280
Gutmann B, Cantillo D, Kappe CO (2015). Angew Chem Int Ed 54:6688–6728
Partenheimer W (2005). Adv Synth Catal 347:580–590
Partenheimer W (2004). Adv Synth Catal 346:297–306
Gutmann B, Elsner P, Roberge D, Kappe CO (2013). ACS Catal 3:2669–2676
White MC (2012). Science 335:807–809
Newhouse T, Baran PS (2011). Angew Chem Int Ed 50:3362–3374
Halperin SD, Kwon D, Holmes M, Regalado EL, Campeau LC, DiRocco DA, Britton R (2015). Org Lett 17:5200–5203
Schultz DM, Lévesque F, DiRocco DA, Reibarkh M, Ji Y, Joyce LA, Dropinski JF, Sheng H, Sherry BD, Davies IW (2017). Angew Chem Int Ed 56:15275–15278
Laudadio G, Govaerts S, Wang Y, Ravelli D, Koolman HF, Fagnoni M, Djuric SW, Noel T (2018). Angew Chem Int Ed 57:4078–4082
Yamada K, Fukuyama T, Fujii S, Ravelli D, Fagnoni M, Ryu I (2017). Chem Eur J 23:8615–8618
Ravelli D, Fagnoni M, Fukuyama T, Nishikawa T, Ryu I (2017). ACS Catal 8:701–713
Wu WF, Fu ZH, Tang SB, Zou S, Wen X, Meng Y, Sun SB, Deng J, Liu YC, Yin DL (2015). Appl Catal B-Environ 164:113–119
Guo ML (2004). Green Chem 6:271–273
Tanielian C (1998). Coord Chem Rev 178:1165–1181
Lesieur M, Battilocchio C, Labes R, Jacq J, Genicot C, Ley SV, Pasau P (2019). Chem Eur J 25:1203–1207
Yuan T, Permentier H, Bischoff R (2015). Trac-Trends Anal Chem 70:50–57
Lohmann W, Karst U (2008). Anal Bioanal Chem 391:79–96
Yan M, Kawamata Y, Baran PS (2017). Chem Rev 117:13230–13319
Noel T, Cao Y, Laudadio G (2019). Acc Chem Res 52:2858–2869
Atobe M, Tateno H, Matsumura Y (2018). Chem Rev 118:4541–4572
Pletcher D, Green RA, Brown RCD (2018). Chem Rev 118:4573–4591
Mitsudo K, Kurimoto Y, Yoshioka K, Suga S (2018). Chem Rev 118:5985–5999
Folgueiras-Amador AA, Wirth T (2017). J Flow Chem 7:94–95
Laudadio G, Straathof NJ, Lanting MD, Knoops B, Hessel V, Noël T (2017). Green Chem 19:4061–4066
Kawamata Y, Yan M, Liu ZQ, Bao DH, Chen JS, Starr JT, Baran PS (2017). J Am Chem Soc 139:7448–7451
Roth GP, Stalder R, Long TR, Sauer DR, Djuric SW (2013). J Flow Chem 3:34–40
Permentier HP, Bruins AP, Bischoff R (2008). Mini-Rev Med Chem 8:46–56
Karst U (2004). Angew Chem Int Ed 43:2476–2478
Stalder R, Roth GP (2013). ACS Med Chem Lett 4:1119–1123
Scott KA, Njardarson JT (2018). Top Curr Chem 376:5
Inamoto K, Hasegawa C, Kawasaki J, Hiroya K, Doi T (2010). Adv Synth Catal 352:2643–2655
Zhang GT, Liu C, Yi H, Meng QY, Bian CL, Chen H, Jian JX, Wu LZ, Lei AW (2015). J Am Chem Soc 137:9273–9280
Cheng YN, Yang J, Qu Y, Li PX (2012). Org Lett 14:98–101
Tabakovic I, Trkovnik M, Batusic M, Tabakovic K (1979). Synthesis 8:590–592
Wang P, Tang S, Lei AW (2017). Green Chem 19:2092–2095
Qian X-Y, Li S-Q, Song J, Xu HC (2017). ACS Catal 7:2730–2734
Folgueiras-Amador AA, Qian XY, Xu HC, Wirth T (2018). Chem Eur J 24:487–491
Huang C, Qian XY, Xu HC (2019). Angew Chem Int Ed 58:6650–6653
Cantillo D, Kappe CO (2017). React Chem Eng 2:7–19
Gustafsson T, Gilmour R, Seeberger PH (2008). Chem Commun:3022–3024
Meanwell M, Lehmann J, Eichenberger M, Martin RE, Britton R (2018). Chem Commun 54:9985–9988
Britton R, Yuan Z, Nodwell M, Yang H, Malik N, Merkens H, Benard F, Martin R, Schaffer P (2018). Angew Chem Int Ed 57:12733–12736
Nodwell MB, Yang H, Čolović M, Yuan Z, Merkens H, Martin RE, Bénard F, Schaffer P, Britton R (2017). J Am Chem Soc 139:3595–3598
Halperin SD, Fan H, Chang S, Martin RE, Britton R (2014). Angew Chem Int Ed 53:4690–4693
Nodwell MB, Bagai A, Halperin SD, Martin RE, Knust H, Britton R (2015). Chem Commun 51:11783–11786
Brixen K, Chapurlat R, Cheung AM, Keaveny TM, Fuerst T, Engelke K, Recker R, Dardzinski B, Verbruggen N, Ather S, Rosenberg E, de Papp AE (2013). J Clin Endocrinol Metab 98:571–580
Wang J-t, Pan Y, Zhang L-m, Yu S-q (2007). Chin J Chem Phys 20:395
Garner A, Wilkinson F (1976). J Chem Soc Faraday Trans 2(72):1010–1020
Cantillo D, de Frutos O, Rincón JA, Mateos C, Kappe CO (2014). J Organomet Chem 79:8486–8490
Ehrich H, Linke D, Morgenschweis K, Baerns M, Jähnisch K (2002). Chimia 56:647–653
Fukuyama T, Tokizane M, Matsui A, Ryu I (2016). React Chem Eng 1:613–615
Strauss FJ, Cantillo D, Guerra J, Kappe CO (2016). React Chem Eng 1:472–476
Li ZH, Fan KN, Wong MW, Phys J (2001). Chem A 105:10890–10898
Manabe Y, Kitawaki Y, Nagasaki M, Fukase K, Matsubara H, Hino Y, Fukuyama T, Ryu I (2014). Chem Eur J 20:12750–12753
Cambié D, Noël T (2018). Top Curr Chem 376:45
Schultz DM, Yoon TP (2014). Science 343:1239176
Protti S, Ravelli D, Fagnoni M, Albini A (2009). Chem Commun:7351–7353
Kim YJ, Jeong MJ, Kim JE, In I, Park CP (2015). Aust J Chem 68:1653–1656
Henderson RK, Jimenez-Gonzalez C, Constable DJC, Alston SR, Inglis GGA, Fisher G, Sherwood J, Binks SP, Curzons AD (2011). Green Chem 13:854–862
Cantillo D, de Frutos O, Rincon JA, Mateos C, Kappe CO (2014). J Organomet Chem 79:223–229
Šterk D, Jukič M, Časar Z (2013). Org Process Res Dev 17:145–151
O’Brien M, Cooper D (2016). Synlett 27:164–168
Sawama Y, Monguchi Y, Sajiki H (2012). Synlett 23:959–972
Lockley WJS, McEwen A, Cooke R, Labelled Compd J (2012). Radiopharm. 55:235–257
Habraken E, Haspeslagh P, Vliegen M, Noël T (2014). J Flow Chem 5:2–5
Acknowledgements
We acknowledge financial support from the Dutch Science Foundation (NWO) for a VIDI grant for T.N. (SensPhotoFlow, No. 14150). S.G. is grateful to the European Union for receiving Erasmus+ grant. A.N. and T.N. acknowledge financial support from AbbVie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Govaerts, S., Nyuchev, A. & Noel, T. Pushing the boundaries of C–H bond functionalization chemistry using flow technology. J Flow Chem 10, 13–71 (2020). https://doi.org/10.1007/s41981-020-00077-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-020-00077-7