Abstract
A continuous flow procedure for the gem-dichlorocyclopropanation of alkenes has been developed. The method is based on the generation of dichlorocarbene utilizing the classical biphasic aqueous sodium hydroxide/chloroform system. This reaction typically requires vigorous stirring for several hours in batch for completion. Tarry materials precipitate due to partial polymerization of dichlorocarbene and the process is difficult to scale. To overcome these problems and achieve very efficient mixing during the flow process a column reactor packed with PTFE beads as inert filling material has been used. PTFE beads have been found to be the optimal material to obtain fine dispersions of the aqueous phase in the organic solution. By heating the packed-bed reactor at 80 °C excellent conversions have been achieved after a residence time of only 4 min. The process has been applied for the synthesis of Ciprofibrate, a dichlorocyclopropane-containing drug used as treatment for several diseases associated with high lipid content in blood.
Avoid common mistakes on your manuscript.
Introduction
Biphasic liquid-liquid systems are very important in process chemistry and the pharmaceutical industry. Apart from multiphasic reactions, such as nitrations, saponifications and emulsion polymerizations, liquid-liquid systems are also involved in other processes like extractions [1,2,3,4]. The progress of these reactions is often limited by mass transfer, which in turn depends on the surface contact area between the two phases [5, 6]. In batch reactors highly dispersed biphasic liquid-liquid mixtures can only be obtained by vigorous stirring and, accordingly, stirring speed is an important parameter contributing to the reaction performance [7, 8]. Scale-up of liquid-liquid systems in batch is therefore problematic, since high shear forces are required and a larger scale results in lower interfacial surface area to volume ratio. To enhance the mass transfer in biphasic systems phase transfer catalysts (PTC) are often used. A PTC is an additive that facilitates the migration of a reactant from one phase into another. To shuttle anionic reactants quaternary ammonium salts such as benzyltriethylammonium chloride or tetrabutylammonium bromide are often employed [9]. PCTs in general are considered to be advantageous towards the goal of sustainable chemical processing and green chemistry, since they enable the use of water as a reaction medium, reducing the consumption of organic solvents [10, 11].
Continuous flow and microreactor technology has been shown as a powerful tool to overcome the problems associated with poor mixing in multiphasic systems. A very high surface-area-to-volume ratio is typically achieved within the microchannels of a flow reactor, dramatically increasing the mass transfer that can be achieved [12, 13]. Liquid-liquid biphasic mixtures can produce several flow patterns in continuous flow reactors (Fig. 1) [4, 14,15,16,17,18]. To make the interfacial area as large as possible a turbulent flow regime is desired, which leads to an emulsion with highly dispersed droplets (Fig. 1a). Adequate turbulent flow even at low flow rates can be achieved by carefully designed mixing structures. Such microstructured devices have proven very useful for the generation of emulsions for very fast reactions involving biphasic systems [19, 20]. Slow reactions, in contrast, are highly problematic. Despite the very efficient mixing achieved in a micromixer, the immiscible phases tend to separate within the residence time unit for longer reactions. Formation of larger segments within minutes can stop the reaction progress. Thus, for longer reactions the mixing structure should ideally be extended throughout the whole reactor channel.
A classic example of a challenging biphasic liquid-liquid reaction is the generation of dichlorocarbene (DCC) utilizing the chloroform/aqueous hydroxide system. DCC is a versatile reagent utilized for the generation of gem-dichlorocyclopropanes from olefins. It has also been utilized for the Hofmann isonitrile synthesis and for the Reimer-Tiemann formylation (Scheme 1) [21, 22]. Dichlorocarbene is a highly reactive, short-lived intermediate [23]. It is usually generated in situ in the presence of the substrate. In a typical batch gem-dichlorocyclopropanation reaction, a solution of concentrated NaOH is added over a vigorously stirred solution of the alkene and a PTC in chloroform under reflux conditions for several hours [24]. Carbene molecules that are not rapidly trapped by the substrate tend to polymerize resulting in tarry materials, or to decompose in the presence of hydroxyl sources producing carbon monoxide, NaCl and water [25]. In addition to the NaCl produced as by-product during the DCC generation, the precipitation of tar further complicates appropriate mixing of the reaction.
We were interested in the scale-up of the gem-dichlorocyclopropanation of alkenes with dichlorocarbene using the chloroform/aqueous NaOH system using continuous flow conditions. Challenged by the long reaction times required by the reaction, formation of solids, and the need for very efficient mixing during the whole process to avoid separation of the phases within the channel, we have carried out a comparative study of several mixer/reactor combinations. It was found that packed bed reactors using PTFE beads as inert filling material provided the best performance. Herein, the results of our study, including optimization of the reaction setup and conditions, and their application to the synthesis of the gem-dichlorocyclopropane containing drug Ciprofibrate [26] are presented.
Results and discussion
Our investigation began with a series of preliminary batch experiments using sealed vessels with the goal of obtaining an initial set of reaction conditions for our flow experiments. The gem-dichlorocyclopropanation of cyclohexene 1, providing 7,7-dichlorobicyclo[4.1.0]heptane 2 (Scheme 2), was chosen as a model reaction. Thus, in a 3 mL vial cyclohexene and 3 mol% benzyltriethylammonium chloride (BTEA-Cl) were dissolved in chloroform. An aqueous solution of sodium hydroxide 40 wt% was added and the mixture was vigorously stirred. Notably, the silicone septa ruptured (pressure ca. 6 bar) in most cases due to the formation of CO gas. The presence of this gas could be confirmed by approaching a CO detector to the reaction mixture. Under batch conditions, 97% conversion (GC-FID) to the desired product 2 was achieved after 1 h.
We then directly moved to continuous flow conditions. A simple continuous flow setup consisting of a Y-mixer (0.5 mm i.d.) and PFA tubing was initially tested. The setup consisted of three streams (Table 1). Aqueous NaOH (40 wt%) and the organic phase consisting of cyclohexene (1.95 M) and the PTC (3 mol%) in chloroform were pumped using a peristaltic and a syringe pump, respectively (see Supporting Information), and mixed using the Y-mixer. After the residence time coil (PFA tubing, 0.8 mm i.d.), the reaction was quenched with an aqueous stream, using a syringe pump. The system was pressurized at 5 bar. Utilizing this simple setup and mixer a segmented flow pattern was obtained. A series of phase transfer catalysts was examined at room temperature using a residence time of 10 min. Apart from the usual ammonium salts, secondary and tertiary amines were also evaluated as catalysts (Table 1). The use of amines as catalysts for dichlorocarbene reactions using the chloroform/aqueous hydroxide system, first reported by Isagawa et al [27], follows a different mechanism involving formation of ylides between the carbene and the amines in the aqueous phase [24, 28, 29]. Once the ylide is extracted to the organic phase it releases the carbene. Notably, conversions obtained using several inexpensive tertiary amines (Table 1, entries 2–8) were analogous to those for BTEA-Cl (entry 1). Other more bulky tertiary amines and secondary amines (entries 3, 6, and 9–11) gave poor results, in analogy to the reactivity described by Isagawa [27]. Best results were obtained using dimethylethylamine and diethylmethylamine (entries 4 and 5). Et2MeN was selected as catalyst for subsequent experiments due to its low boiling point (63–65 °C) [30] which simplifies the reaction workup as it can be removed by simple evaporation.
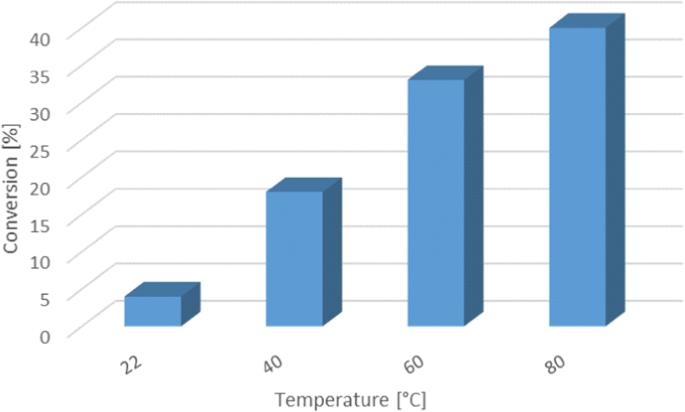
Monitoring of the reaction conversion after a series of flow experiments with increasing residence time (Fig. 2) at 40 °C revealed that, while the reaction is relatively fast during the initial 2–3 min, it then becomes exceedingly slow. Nearly 50% conversion was observed after 5 min residence time. Increase of the residence time to 10 min only produced an increase of conversion of 6%. This effect was ascribed to the above mentioned aggregation of the phases in the residence time PFA tube, producing longer segments of liquid and significantly decreasing the surface contact area between the immiscible liquids (Fig. 2). Temperature had a positive effect in the reaction outcome. Keeping the residence time constant at 1 min, the reaction temperature was gradually increased from room temperature to 80 °C. The conversion increased from <10% to 40% (Fig. 3). No side products were observed by GC or NMR analysis. However, a significant amount of tar was produced at higher temperatures, presumably due to the polymerization of the excess of carbene (the same tarry material was observed when running the reaction in the absence of cyclohexene). Tar precipitation eventually caused clogging of the reactor and irreproducible results [31]. Clogging could not be avoided by applying ultrasound.
Effect of the residence time on the formation of 2 at 40 °C (left). The continuous flow setup depicted in Table 1 was used. Conversion was determined by GC-FID analysis. Image of the reaction mixture at the end of the residence time coil. Long liquid segments can be observed (right)
Effect of the reaction temperature on the gem-dichlorocyclopropanation of cyclohexene. The reactor depicted in Table 1 was used. Conversion was determined by GC-FID analysis
To ensure active mixing during the complete residence time and avoid aggregation of the liquid phases, a glass reactor plate with an active mixing geometry was also evaluated. Unfortunately, appropriate mixing was only achieved at relatively high flow rates (Fig. 4). Gradual increase of the flow rates of a biphasic water/chloroform mixture revealed that the desired dispersion is only achieved at a total flow rate of 1.5 mL/min. This resulted in a residence time for a 4 mL plate below 3 min and poor conversions were observed for the model reaction. Analogous problems were observed with a 3 mm inner diameter static Kenics type mixer.
We next turned our attention towards a packed bed reactor filled with inert material (beads). This strategy has previously been utilized to provide biphasic systems with excellent mixing even at low flow rates [16, 32,33,34,35,36]. Thus, an Omnifit glass column (12 mL) was utilized as reactor, and heated in a commercially available column heater (Syrris). Several inert materials, namely glass, stainless steel and PFTE beads, and particle sizes were tested as stationary phase for comparison. Pressure drop, reaction conversion and reactor dead volume were examined in all cases (Table 2). As expected, highest conversions were achieved when the smallest particles where utilized (entries 4–6), under otherwise analogous conditions. These results clearly demonstrate the importance of effective mixing for this reaction. The flow regime within the packed-bed reactor could not be visually inspected. This type of static mixers are known to produce droplet/dispersion regimes for biphasic liquid-liquid system [37,38,39]. Indeed, an excellent dispersion of the aqueous phase in the organic phase could be visually observed in the tubing at the mixer output by adding a dye to the water solution (Fig. 5c). PTFE particles provoked the lowest pressure drop in the system (entry 6) and, more importantly, a constant pressure profile over time even for long run reactions (> 4 h). In contrast, the system pressure gradually increased when using stainless steel and sand particles, probably due to the accumulation of polymeric material within the packed-bed. These results could be ascribed to the very low van-der-Waals forces characteristic of PTFE, which prevent materials to easily stick to its surface [40]. Moreover, this material is fully inert under most reaction conditions. PTFE was therefore selected as packing material for subsequent optimization studies.
Further optimization of the reaction conditions using the packed bed reactor was carried out by varying the catalyst loading, NaOH concentration, temperature and residence time for the reaction (Table 3). Initially, a solution of 0.5 M cyclohexene in chloroform containing 3 mol% of catalyst was used. Increasing the amount of the catalyst up to 10 mol% improved the conversion significantly (Table 3, entries 1–3). Higher catalyst loading only showed minor improvement (entry 4). Temperature had an important influence in the reaction outcome. While decreasing the temperature resulted in much lower conversions, increase to 90 °C provoked clogging of the reactor (entries 5 and 6). The conversion increased when higher concentrations of sodium hydroxide were applied (entry 7). This may be attributed to the fact that hydroxide anions are less solvated by water and therefore the reactivity increases [41]. Increasing the concentration of the sodium hydroxide solution to 40 wt% led to clogging of the reactor (entry 8). The optimal concentration was 35 wt% of sodium hydroxide in water. To extend the residence time, an additional column was added to enlarge the reactor volume by 50%, providing a total volume of 6.9 mL. Excellent conversion could finally be achieved by gradually increasing the NaOH/CHCl3 ratio (entries 10–11). Under optimal conditions 97% conversion of the substrate and > 99% selectivity for the desired product 2 was obtained (entry 11).
The continuous flow procedure was then applied for the synthesis of Ciprofibrate, a gem-dichlorocyclopropane-containing drug used as lipid-lowering agent (Fig. 6) [42,43,44,45]. Precursor 3 was prepared according to the literature [46]. Using the optimized conditions (Table 3, entry 11) full conversion of 3 and complete selectivity for the desired Ciprofibrate 4 was observed by GC-FID analysis. Notably, the high purity with which 4 was obtained permitted a very simple workup consisting in extraction of the product with DCM/water and evaporation of the organic phase. Using this protocol, the reaction was run for 4 h. 18.8 g of Ciprofibrate 4 (98% yield) was isolated, corresponding to a productivity of ca. 5 g/h.
Conclusions
In summary, a continuous flow method for the gem-dichlorocyclopropanation of alkenes with in situ generated dichlorocarbene has been developed. The classical biphasic aqueous NaOH/CHCl3 system has been utilized. The challenges associated with the very efficient mixing required by this process have been overcome on lab scale by using a packed-bed reactor filled with PTFE beads as inert material. This mixer enabled the generation of very fine dispersions of the aqueous solution in the organic phase even at relatively low flow rates, which was not achievable using other mixing elements. Moreover, using this strategy intense mixing is applied during all the residence time, avoiding aggregation of the liquid droplets. N,N-diethylmethylamine, was found as the best catalyst for the transformation. Notably, utilization of amines instead of quaternary ammonium salts significantly simplified the reaction work-up. Under optimal conditions at 80 °C and a 2:1 aqueous (NaOH 35 wt%)/CHCl3 ratio, excellent conversion and selectivity for the desired gem-dichlorocyclopropane was obtained, in a readily scalable protocol. The process was stable for at least 4 h, producing 19 g of the target compound.
Experimental section
General remarks
1H NMR spectra were recorded on a Bruker 300 MHz instrument. 13C NMR spectra were recorded on the same instrument at 75 MHz. Chemical shifts (δ) are expressed in ppm downfield from TMS as internal standard. The letters s, d, dd, t, q, and m are used to indicate singlet, doublet, doublet of doublets, triplet, quadruplet, and multiplet. GC-FID analysis was performed on a ThermoFisher Focus GC with a flame ionization detector, using a TR-5MS column (30 m × 0.25 mm ID × 0.25 μm) and helium as carrier gas (1 mL min−1 constant flow). The injector temperature was set to 280 °C. After 1 min at 50 °C, the temperature was increased by 25 °C min−1 to 300 °C and kept constant at 300 °C for 4 min. The detector gases for flame ionization were hydrogen and synthetic air (5.0 quality). GC-MS spectra were recorded using a ThermoFisher Focus GC coupled with a DSQ II (EI, 70 eV). A TR-5MS column (30 m × 0.25 mm × 0.25 μm) was used, with helium as carrier gas (1 mL min−1 constant flow). The injector temperature was set to 280 °C. After 1 min at 50 °C, the temperature was increased by 25 °C min−1 to 300 °C and kept at 300 °C for 3 min. All chemicals were purchased from standard commercial vendors and utilized without further purification.
Representative procedure for the continuous flow synthesis of gem-dichlorocyclopropanes
Flow experiments were performed using the continuous flow setup described in the Fig. S1 in the Supporting Information. The organic feed contained 0.5 M of the olefin and 3 mol% of diethylmethylamine in chloroform. The aqueous solution feed consisted of 35 wt% NaOH in water. The organic solution was pumped by using a Syrris® Asia syringe pump and the alkaline aqueous solution was pumped using a Vapourtec® SF-10 peristaltic pump. Both streams were combined in a Y-mixer before entering an Omnifit® packed-bed reactor filled with PTFE particles (>40 μm). To extend the reactor volume, a second packed-bed was connected in series to give a total reaction volume of 6.9 mL. Both columns were arranged in a vertical orientation and the reaction mixture was passed through them from the bottom upwards. The columns were heated to 80 °C using a Syrris® column heater. After the column reactors the reaction mixture was diluted inline with water. A 5 bar back-pressure-regulator was installed in the reaction output. The crude reaction mixture collected from the reactor outlet was extracted with DCM. The organic layer was dried over MgSO4 and concentrated under reduced pressure yielding the desired gem-dichlorocyclopropane.
Ciprofibrate methylester (4)
18.8 g (98% yield), 1H-NMR (300 MHz, CDCl3): δ = 7.13 (2H, d, 3J = 8.3 Hz), 6.82 (1H, d, 3J = 8.3 Hz), 3.78 (3H, s), 2.90–2.77 (1H, m), 1.94 (1H, dt, 2J = 15.8 Hz, 3J = 7.9 Hz), 1.87–1.74 (1H, m), 1.61 (6H, s). 13C-NMR (75 MHz, CDCl3): δ = 174.7, 154.8, 129.7, 128.3, 79.2, 60.9, 52.5, 34.8, 25.8, 25.4.
References
Kulkarni AA (2014) Beilstein. J Org Chem 10:405–424
Alenezi R, Baig M, Wang J, Santos R, Leeke GA (2010). 32:460–468
Bataille P, Van BT, Pham QB (1982). J Polym Sci Polym Chem Ed. 20:795–810
Mielke E, Plouffe P, Mongeon SS, Aellig C, Filliger S, Macchi A, Roberge DM (2018). Chem Eng J 352:682–694
Plouffe P, Roberge DM, Macchi A (2016). Chem Eng J 300:9–19
Wang K, Li L, Xie P, Luo G (2017). React Chem Eng 2:611–627
Starks CM (1999). Tetrahedron 55:6261–6274
Piradashvili K, Alexandrino EM, Wurm FR, Landfester K (2016). Chem Rev 116:2141–2169
Weber WP, Gokel GW (1977). Phase transfer catalysis in organic synthesis, Vol 4
Metzger JO (1998). Angew Chemie Int Ed 37:2975–2978
Makosza M (2000). Pure Appl Chem 72:1399–1403
Nieves-Remacha MJ, Kulkarni AA, Jensen KF (2012). Ind Eng Chem Res 51:16251–16262
Lobry E, Theron F, Gourdon C, Le Sauze N, Xuereb C, Lasuye T (2011). Chem Eng Sci 66:5762–5774
Nguyen NT, Wu Z (2005). J Micromech Microeng 15:R1–R16
Hessel V, Löwe H, Schönfeld F (2005). Chem Eng Sci 60:2479–2501
Naber JR, Buchwald SL (2010). Angew Chemie Int Ed 49:9469–9474
Dessimoz AL, Cavin L, Renken A, Kiwi-Minsker L (2008). Chem Eng Sci 63:4035–4044
Ahmed B, Barrow D, Wirth T (2006). Adv Synth Catal 348:1043–1048
Dummann G, Quittmann U, Gröschel L, Agar DW, Wörz O, Morgenschweis K (2003). Catal Today 79–80:433–439
Kulkarni AA, Kalyani VS, Joshi RA, Joshi RR (2009). Org Process Res Dev 13:999–1002
Fedoryński M (2003). Chem Rev 103:1099–1132
Mąkosza M, Fedoryński M (2011). Russ Chem Bull 60:2141–2146
Bertrand, G. Carbene chemistry: from fleeting intermediates to powerful reagents (2003)
Mąkosza M, Wawrzyniewicz M (1969). Tetrahedron Lett 10:4659–4662
Dehmlow E, Wilkenloh J, Dehmlow EV, Wilkenloh JJ (1984). J Chem Res Synop:396
Aguilar-Salinas CA, Assis-Luores-Vale A, Stockins B, Rengifo HM, Filho JD, Neto AA (2004). Cardiovasc Diabetol 3:1–6
Isagawa K, Kimura Y, Kwon SJ (1974). Org Chem 39:3171–3172
Reeves WP, Hilbrich RG (1976). Tetrahedron: 2235–2237
Makosza M, Kacprowicz A, Fedorynaki M (1975). Tetrahedron Lett 16:2119–2122
Stephenson RM (1993). J Chem Eng Data 38:625–629
Dehmlow EV, Lissel M (1978). Chem Ber 111:3873–3878
Yang JC, Niu D, Karsten BP, Lima F, Buchwald SL (2016). Angew Chemie Int Ed 55:2531–2535
Noël T, Musacchio AJ (2011). Org Lett 2011, 13:5180–5183
Reichart B, Kappe CO, Glasnov T (2013). Synlett 24:2393–2396
Bogdan A, McQuade DTA (2009). Beilstein J Org Chem 5:34–36
Noël T, Kuhn S, Musacchio AJ, Jensen KF, Buchwald SL (2011). Angew Chemie Int Ed 50:5943–5946
Su Y, Zhao Y, Jiao F, Chen G, Yuan Q (2011). AICHE J 57:1409–1418
Su Y, Zhao Y, Chen G, Yuan Q (2010). Chem Eng Sci 65:3947–3956
Shang M, Noel T, Wang Q, Hessel V (2013). Chem Eng Technol 36:1001–1009
Dhanumalayan E, Joshi GM (2018). Adv Compos Hybrid Mater 1:247–268
Landini D, Maia A, Podda G (1982). J Org Chem 47:2264–2268
Hunninghake DB, Peters JR (1987). Am J Med 83:44–49
Zimetbaum P, Frishman WH, Kahn S (1991). J Clin Pharmacol 31:25–37
Brown WV (1987). Am J Med 83:85–89
Knipscheer HC, De Valois JC, Van Den Ende B, Wouter J, Kastelein JJ (1996). Atherosclerosis 124:75–81
Jia C, Chen W, Huang F, Wang T (2013). CN105237389 a synthetic method of Ciprofibrate
Acknowledgments
Open access funding provided by University of Graz. The CC Flow Project (Austrian Research Promotion Agency FFG No. 862766) is funded through the Austrian COMET Program by the Austrian Federal Ministry of Transport, Innovation and Technology (BMVIT), the Austrian Federal Ministry of Science, Research and Economy (BMWFW), and by the State of Styria (Styrian Funding Agency SFG).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
The online version of this article (https://doi.org/10.1007/xxxxxxx) contains supplementary material, which is available to authorized users. (PDF 263 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
von Keutz, T., Cantillo, D. & Kappe, C.O. Enhanced mixing of biphasic liquid-liquid systems for the synthesis of gem-dihalocyclopropanes using packed bed reactors. J Flow Chem 9, 27–34 (2019). https://doi.org/10.1007/s41981-018-0026-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-018-0026-1