Abstract
Semiconductors and the associated methodologies applied to electrochemistry have recently grown as an emerging field in energy materials and technologies. For example, semiconductor membranes and heterostructure fuel cells are new technological trend, which differ from the traditional fuel cell electrochemistry principle employing three basic functional components: anode, electrolyte, and cathode. The electrolyte is key to the device performance by providing an ionic charge flow pathway between the anode and cathode while preventing electron passage. In contrast, semiconductors and derived heterostructures with electron (hole) conducting materials have demonstrated to be much better ionic conductors than the conventional ionic electrolytes. The energy band structure and alignment, band bending and built-in electric field are all important elements in this context to realize the necessary fuel cell functionalities. This review further extends to semiconductor-based electrochemical energy conversion and storage, describing their fundamentals and working principles, with the intention of advancing the understanding of the roles of semiconductors and energy bands in electrochemical devices for energy conversion and storage, as well as applications to meet emerging demands widely involved in energy applications, such as photocatalysis/water splitting devices, batteries and solar cells. This review provides new ideas and new solutions to problems beyond the conventional electrochemistry and presents new interdisciplinary approaches to develop clean energy conversion and storage technologies.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Electrochemical devices, including fuel cells, batteries and electrolyzers have shown great potential for large-scale clean energy conversion and storage applications. In clean energy conversion, fuel cells directly convert the chemical energy from fuels into electricity with high efficiency and low emissions, while in clean energy storage, a battery is a typical storage device with high energy density and good reversibility and durability. We selected these two systems for the present study, because they represent the current and near-future energy conversion and storage technologies with a high potential to be combined with renewable and sustainable energy sources. We use them as examples to give a comprehensive review of current research frontiers with emphases on semiconductor and band theory applications in both materials and devices/technologies as well as fundamental sciences, followed by extensions to related fields, e.g., photoelectrolysis or photocatalysts, solar cells, metal-air batteries, and electrolyzers.
The knowledge and operating principles of fuel cells have largely remained the same over the last century [1, 2]. The yttrium-stabilized zirconia (YSZ) electrolyte, a typical oxide material, has been the main focus of research and development for solid oxide fuel cells (SOFCs). However, the full and successful commercialization of SOFCs is hampered by the requirement for high temperature materials associated with high costs despite the inherent benefits of the technology. The high operating temperature, which is directly related to the ionic conductivity of solid oxide electrolytes, requires special attention from the aspects of the material durability and manufacturing. It also adversely affects the costs, impeding wide application of SOFC technology. For instance, many works have focused on developing ultrathin film electrolyte layers of YSZ and alternative doped ceria (e.g., Sm0.2Ce0.8O2-δ (SDC)) to minimize device ohmic loss and maintain desirable power output at reduced temperatures.
The oxygen transport properties in the electrode and electrolyte materials are not only of technological interest, as noted above, but also of fundamental importance. The energetics of oxygen transport in these oxides are affected by the material structure, composition, oxygen stoichiometry, doping and elastic strain, which comprise a wide range of parameters in optimizing the transport properties. In addition, lowering the temperature can cause slower electrode and electrolyte dynamic processes. To speed up the development of SOFCs, new solutions to these key challenges for the electrolytes and electrodes need to be found, i.e., to achieve sufficient ionic conductivity electrolytes and highly electrocatalytically active electrodes at lower operating temperatures. In addition, more radical approaches alternative to the traditional approach of using the three-layer anode-electrolyte-cathode electrochemical device structure and material systems are greatly sought after. In 2013, Singh et al. [3] noted that the anode, cathode and electrolyte in SOFCs, in terms of semiconductor principles, can be associated with an n-type regime, a p-type regime and an ion conducting electrolyte, respectively, as shown in Fig. 1a. In 2018, Asghar et al. [4] further proposed that wide bandgap semiconductors can be used for replicating the electrolyte functionality. This means that an SOFC could be conceptually handled as a semiconductor fuel cell system. If the middle electrolyte layer of the SOFC is removed or replaced with a wide bandgap semiconductor, then the fuel cell effectively resembles a p–n junction device (as Fig. 1b); i.e., a p–n junction is formed in situ under a fuel cell atmosphere by the same material that can block electrons, thus preventing short circuiting of the fuel cell in much the same way as in solar cells. Based on this principle, two types of novel fuel cell devices may be constructed based on either O2− or H+ conduction/transfer, as illustrated in Fig. 1c, d. We designate these devices as electrolyte-layer-free fuel cells (EFFCs) to distinguish them from the traditional SOFC structure. Naturally, the EFFC has the same functionalities as the traditional SOFC, but these can be realized through a different methodology employing a vastly simplified configuration. This difference delivers several advantages over a conventional three-layer fuel cell such as the following: (1) the two anode/electrolyte and cathode/electrolyte interfaces that cause major power loss due to the ionic/electronic interfacial polarizations are eliminated [1, 5]; (2) redox reactions, such as the hydrogen oxidation reaction (HOR) and the oxygen reduction reaction (ORR), can be readily performed to generate electricity on the spot, and ionic transport occurs in a more efficient way to complete the fuel cell reactions (Fig. 1a); and (3) a built-in electric field is formed at the anode (n) and cathode (p) junction, which can increase the H+ (Fig. 1c) and O2− (Fig. 1d) transfer mechanics to enhance the overall efficiency of the cell. EFFCs with simpler configurations and harmonious cell components have led to significant progress in the SOFC field.
Transition of the fuel cell from an ionic electrolyte device (a) to an electrolyte-layer-free device, e.g., an n–p junction assembly (b), along with the further H+ conducting fuel cell (c) and the O2− conducting fuel (d), in which a built-in electric field is formed between the anode (n) and cathode (p) junction to promote H+ and O2− transfer to complete the redox reactions and electricity generation on-site without the interfacial polarization and losses caused by the ionic electrolyte separator in conventional fuel cells (a) [3]. Partially redrawn with permission from Ref. [3].
The transition from the conventional ionic electrochemistry to advanced semiconductor electrochemistry is widely evidenced as reported for many other energy conversion and storage devices [6, 7], which makes the application of semiconductors and associated methodologies to the electrochemistry in energy materials and relevant electrochemical devices an emerging field. For instance, the successful development of photoelectrolysis from photoelectrochemical devices using semiconductors has made photocatalysts and water splitting hot topics [8,9,10]. Lithium-ion batteries (LIBs) have also experienced new progress based on the semiconductor properties of materials used in devices, e.g., energy band/heterojunction/built-in electric field [11, 12]. Semiconductors and their methodologies complement the conventional electrochemistry, introducing the new topic “semiconductor electrochemistry” and a new frontier in ion conducting semiconductors and novel fuel cell devices [1, 13, 14]. The name of this topic refers to the similarities found between the development of photocatalysts and LIBs and that of fuel cells.
Fuel cells and photoelectrochemical electrolysis have much the same origin as electrolyte-based electrochemical devices with anodes and cathodes, i.e., photoelectrochemical cells (PECs). Today, PECs are well known to be transferrable to photo-water splitting based on semiconductors and band structures [15]. A similar situation occurs for batteries, where semiconductors and energy bands, as well as built-in-field effects, play a significant role in designing material functions and the device performance. Solar cells have taken a reverse route from typical physics to electrochemistry, where ions play an important role in state-of-the-art perovskite solar cells (PSCs). The electrochemistry of the semiconductor–electrolyte interface or semiconductor–semiconductor interface provides great promise to handle these challenges through a deeper understanding. A logical deduction can be made that by employing semiconductor electrochemistry, because a semiconductor provides two energy levels (the conduction band (CB) and the valence band (VB)), the charge transfer occurring at these two energy levels can be easily controlled. Therefore, our idea about the variation in the electron density that is helpful for ionic transport at these two energy levels and the interfaces for clean energy conversion and storage devices need to be recapitulated. This work provides a new view of these novel aspects of state-of-the-art research and development in the energy sector, where the two typical fields of fuel cells and batteries are focused on realizing a comprehensive understanding of the roles of semiconductors and bands in energy conversion and storage, with extension to other energy applications in a broader view.
2 Electrochemistry of the Semiconductor–Electrolyte Interface and Fuel Cells
Electrochemistry is the core of developing multilayer devices and is most commonly used in assembling two-electrode cells and for referring the voltage to a reference electrode (RE). The science of electrochemistry is central to the manufacturing, performance, and reliability of multilayer devices. Semiconductor electrochemistry is a particularly relevant and fundamental area for fuel cell devices, including ion transport in fuel cells [14]. Semiconductor heterostructure-based fuel cells are convenient to scale using the energy levels relative to the vacuum level (VL) compared to those with an intrinsic ionic conductor. This allows us to track the local variations in energy, electrostatic potential, and Fermi energy (EF) inside the solids by a standard representation. Control of the flowing charge carriers across the junction is the most important point for SOFCs in that electrons must be prevented from crossing over the electrolyte membrane. Before we discuss the properties of the semiconductor-electrolyte interface, examining some of the general aspects of electron and ion transfer reactions at the semiconductor-electrolyte interface from the perspective of the energy band positions is beneficial.
An electrochemical cell (e.g., an SOFC) contains two electrodes (anode and cathode) separated by an electrolyte for completion of the redox reactions. However, to realize the redox reactions and prevent electrons from crossing over the electrolyte membrane, many different methods can be employed, e.g., (1) creating a metal–electrolyte interface (the Schottky-type junction, see Fig. 2a, or the Mott transition from a metal conductor to an electronic insulator (Fig. 2b) related to the band description of the Mott transition induced by H2 or H+ incorporation) where electrons are localized while ions conduct (a typical example is the strongly correlated perovskite SmNiO3, Fig. 2c); (2) constructing a semiconductor (n-type)-semiconductor (p-type) heterostructure to suppress electron conduction (Fig. 2d); (3) using electronically insulating and ionically conductive electrolytes in conventional fuel cells with the anode/electrolyte/cathode configuration (Fig. 2e); and (4) replacing this configuration by the “three-in-one” single-layer device in which macro-redox reactions occur at the anode and cathode separated by the electrolyte while nano-redox reactions can occur at nano-redox units, where each anode, electrolyte and cathode component can be compacted into n-type, ionic and p-type particles, respectively, as shown in Fig. 2f. Since a metal can provide or accept electrons easily at the surface when it is used as either an anode or a cathode, control of the semiconductor-electrolyte interface (SEI) structure and properties to allow stable ionic conductivity is a critical and highly challenging task. That is why the semiconductor–semiconductor interface (Fig. 2f, fuel cell reaction at the nanoscale) can provide more advantages than metals or SEIs because they have distinct energy levels, the CB and the VB. A spontaneous charge transfer reaction can occur depending on the nature of the doping and charge carrier density. In the case of the SEI, a spontaneous charge transfer reaction can occur depending on the nature of the energy levels or their overlap with those of the redox electrolytes. There are three distinct possibilities for such an overlap: (1) overlap with the CB, (2) overlap with the VB, and (3) overlap with both bands. These processes lead to energy band alignment or energy band reorientation.

Reproduced with permission from Ref. [16]. Copyright 2016 Springer Nature. d Using a p–n heterostructure material to tune the semiconductor into an ionic conductor with no net electron conduction. Reproduced with permission from Ref. [17]. Copyright 2014 RSC. e Realizing the fuel cell redox reactions at the electrolyte-separated macroscale and f nonelectrolyte or EFFC nanoscale
Various methods to block electron transfer: a Creating a metal–electrolyte interface (the Schottky-type junction). b Using the Mott transition to block electrons transferring to the electrolyte; c the strongly correlated perovskite SmNiO3 is an example of such a fuel cell.
The electrode/electrolyte interfaces in SOFCs are of significant importance, which, on the one hand, provide the active sites for electrode electrochemical reactions and, on the other hand, contribute to a major loss of the power output due to the different ionic and electronic natures [3, 4]. Gleaning an understanding from the fundamental aspects is therefore necessary to develop sustainable SOFCs in the future. Normally, electron–hole (e−/h+) pairs are separated on the anode and cathode sides by the band structure, i.e., CB and VB, and built-in electric field (BIEF) depending on the nature of materials, so the energy band provides an important pathway for the electrochemical reactions to occur.
At the interface of two distinct materials, the free electrons and holes in the CB (Ec) and the VB (Ev) of the anode and cathode exchange their charges with the electrolyte by diffusion-based processes, and charge neutrality accumulates at the interface. The Fermi levels (EF) attempt to reach an equilibrium position due to charge diffusion processes, and the resultant EF can be associated with the difference in the positions of their respective EF(s). These processes force the energy band structures to be rearranged and hence induce band bending. In semiconductors, the position of EF normally depends on the dopants (n-type or p-type) and their contents; in addition, there are no other reference energy states to assess the energy levels. Therefore, the position of EF is assessable only when the distance between EF and Ec or Ev is known. The position of EF is composed of two parts, the chemical potential (μe) and electrostatic potential (ex), and can be expressed as the following Eq. (1), providing that there is no additional charge:
where \(\mu_{\rm e}\) is the chemical potential of the bulk, while ex arises from the surface dipole; in the case of heterostructure semiconductors, dipoles are formed by the surface structure due to a particular ionic charge distribution. These effects depend on the crystal plane and the reconstruction of the surface atoms. This dipole also influences the electron affinity. In agreement with the band of a semiconductor with an electrolyte, the electrolyte can be visualized as possessing two distinct energy levels: an empty level as the oxidized level (represented by the most probable energy level \(E_{{{\text{ox}}}}^{{\text{O}}}\)) and a filled level as the reduced level (represented by the most probable energy level \(E_{{\rm re}}^{\rm O}\)).
Generally, band bending due to charge transfer at the interface can be of two types: (1) upward band bending and (2) downward band bending, depending on the type of material. In the case of an n-type semiconductor (the anode), the Fermi level is usually at higher energy (less negative) than the Fermi level of the electrolyte, and electrons try to move from the semiconductor (the anode) to the electrolyte to equilibrate the Fermi level. In this case, the Fermi level of the semiconductor decreases in energy until both Fermi levels overlap; hence, EF shifts to lower energy, which results in upward band bending, as shown in Fig. 3a, and creates a depletion layer close to the surface of the anode/electrolyte interface. In the case of p-type (normally on the cathode side) semiconductors, the Fermi level normally exists at lower energy (near the VB and more negative) than that of the electrolyte, and its EF shifts to higher energy (downward band bending) at the interface. A positive space charge region (h+) is therefore created near the surface of the anode interface due to electronic transformation in the electrolyte to achieve electronic equilibrium. A positive space charge means that fewer electrons are in this region. Hence, a charge accumulation/depletion region is formed at the cathode/electrolyte interface. Additionally, on the electrolyte side, when it is fully charged, the electrolyte interface will be left with non-absorbed ions with opposite charges, and they will form a Helmholtz double layer (Fig. 3a).
A space charge region (created due to a change in EF position) can be expected on the semiconductor side due to the difference in electrostatic potential in the process, and its charge will be transferred to the electrolyte, leading to energy loss and band structure rearrangement (see Fig. 3a). The concentration of the charges in the space charge region depends on the potential difference between the semiconductor and the electrolyte. The potential drop in the space charge region is much more than that in the Helmholtz layer because the former spreads over a large area and exists due to ionization of the acceptor solid, while the latter is built up at the single-atom level away from the electrolyte surface. The inhomogeneous distribution of charges in the interfacial region establishes a BIEF that crosses over the electrode–electrolyte interface (EEI), which can also aid in ion or charge carrier crossing of the interface barrier. In other words, the created electric field produces a “built-in potential difference”. Another significant aspect of such a built-in potential at the interface is to oppose the flow of electrons and holes across the junction, so it is denoted as the potential barrier to establish the device voltage. In practical devices, instead of using two interfaces (anode/electrolyte and cathode/electrolyte), a one-interface device (as shown in Fig. 3b), such as by joining an n-type semiconductor and a p-type semiconductor, can work efficiently (low space charge region) by creating a BIEF to block electron penetration while enabling high ionic transport. This advanced configuration should be employed to construct a device with one electrode by ohmic contact to reduce the polarization loss.
3 Semiconductor Electrochemistry in Fuel Cells
Based on the fundamentals of semiconductor electrochemistry briefly discussed in the above section, we further discuss both conventional ion-based electrolytes and semiconductor-based membranes for fuel cells, with an emphasis on how semiconductor-ionic conductors replace conventional ionic electrolytes, resulting in different scientific principles.
Generally, SOFC reactions can be expressed in Kröger-Vink notation as follows: in an O2- conducting electrolyte, the cathode reaction is \(0.5{\text{O}}_{2} + {\text{~}}2{\text{e}}^{ - } + {\text{~}} \to {\rm O}_{{\text{O}}}^{\rm x}\), and the anode reaction is \({\text{H}}_{2} + {\rm O}_{{\text{O}}}^{\rm x} \to {\text{H}}_{2} {\text{O}} + 2{\text{e}}^{ - } + V_{{\text{O}}}^{{ \cdot \cdot }}\), where \(V_{{\text{O}}}^{{ \cdot \cdot }}\) is the oxygen ion vacancy and \({\rm O}_{{\text{O}}}^{\rm x}\) is the lattice oxygen ion in the electrolyte.
In H+ conducting fuel cells, the reaction at the fuel side (anode) is \({\text{H}}_{2} {\text{~}} \to 2{\text{H}}^{ + } + 2{\text{e}}^{ - }\), and that at the air side (cathode) is \(2{\text{H}}^{ + } + \frac{1}{2}{\text{O}}_{2} + 2{\text{e}}^{ - } \to {\text{H}}_{2} {\text{O}}\). In both cases, the overall cell reaction is \({\text{~H}}_{2} + \frac{1}{2}{\text{O}}_{2} \to {\text{H}}_{2} {\text{O}}\). Both anode and cathode materials can now easily be conceptualized as semiconductors in a strongly reducing or oxidizing atmosphere. More importantly, electronic exchange with chemicals or electron/hole transport is crucial to realize electrochemical reactions and current collection. However, semiconducting properties were not explicitly noted until they were addressed by K. Singh in 2013 (see also Fig. 1a) [3]. The electrical conduction behavior of the electrode material of SOFCs changes at different oxygen partial pressures, leading to electron or hole conduction in reducing or oxidizing atmospheres, respectively. A particular example is the perovskite oxide series that has been extensively developed and investigated as electrode materials for SOFCs [18,19,20,21,22,23,24,25]. They have been used as electrode materials to yield reduced material and cell fabrication costs and higher resistance to carbon deposition and sulfur poisoning due to amphiprotic electron and hole conduction under different oxygen partial pressures [26, 27].
3.1 Electrolyte-based SOFCs
Regarding the electrolyte materials in SOFCs, significant effort has been put in developing materials with higher ionic conductivity but limited electronic conduction in a fuel cell atmosphere. Indeed, a large number of new oxide ionic conductor materials have been developed and reported in the literature, such as doped ceria [28], La0.9Sr0.1Ga0.8Mg0.2O3 [29], BaZr0.1Ce0.7Y0.2−xYbxO3−δ [30], Ln10(SiO4)6O3 [31], calcium-doped LaNbO4 [32], Na0.5Bi0.5TiO3 [33], and Sr1–xNaxSiO3–0.5x [34]. Most of them show higher ionic conductivity than that of the state-of-the-art electrolyte YSZ, particularly for low-temperature operation. In addition, proton conductors for low-temperature (LT) SOFCs have recently stimulated worldwide interest and research input [23, 25, 35,36,37,38]. However, none of these materials can replace YSZ for industrial deployment yet due to the intrinsic issues with these materials, such as the high reactive activity with cell components and instability under fuel cell conditions. In addition, neither YSZ nor the newer generation of O2− or H+ conducting materials can meet the high ionic conductivity demand of 0.1 S cm−1 below 600 °C, the so-called LT range. The electrolyte material and resultant system challenges remain in SOFCs.
In parallel, another stream of LT SOFC research involves developing two-phase composite electrolytes that have better ionic conductivity than single-phase electrolytes, as discussed above for single O2− or H+ ion conducting electrolytes, which highly rely on the oxygen vacancy concentration of the oxide matrix. These composite electrolytes originate from ceria-salt composites [39], with a focus on ceria-carbonate composite systems [5, 40,41,42,43,44,45,46,47,48]. In addition, ceria-carbonate electrolytes have a unique characteristic of hybrid or dual H+ and O2− conduction [5, 48,49,50,51,52,53,54,55,56,57,58,59,60], which was even stable over 6000 h [61]. Figure 4 illustrates these three types of ion conducting electrolyte-based SOFC devices. In all these electrolyte-based electrode devices, semiconductors, commonly perovskite oxides, for example, (La,Sr)(Co,Fe)O3, (La,Sr)MnO3, (La,Sr)CoO3, and (Ba,Sr)(Co,Fe)O3 [62,63,64, 64, 66], and transition metal oxides, e.g., LiNiO2 and LiNiCoAlO2 [67, 68], are used as electrode materials, and they can be described in the semiconductor electrochemical way as discussed in Sect. 2. Another interesting report given by Kim et al. [69] presented the development of a hybrid solid oxide cell-based mixed H+ and O2− conductive BaZr0.1Ce0.7Y0.1Yb0.1O3−δ electrolyte for the simultaneous electrolysis of water at the anode and cathode; significantly improved the electrolysis cell performance and stable operation during 60 h of nonstop testing were achieved.
Different from classic heteroelement structural doping, the oxygen ionic conduction in doped ceria occurs through bulk diffusion via oxygen vacancies, while Zhu et al. [68, 70] recently reported that nondoped CeO2 itself acts as a super proton conductor under fuel cell conditions. The precipitated CeO2−x materials treated at different temperatures (as-synthesized, 600 and 1000 °C) all showed defective particle surfaces with many oxygen defects on the surface according to careful high-angle annular dark-field scanning transmission electron microscopy and electron energy loss spectroscopy analyses. The lower the treatment temperature, the higher the oxygen vacancy concentration is. In addition, a charge depletion layer is formed between the interface of core ceria (intrinsic-type semiconductor, Ce4+) and surface ceria (n-type semiconductor, Ce3+), which favors superionic conduction. Moreover, the ionic transport through ceria was determined to be a proton conduction contribution instead of an oxygen ion contribution by intentionally adding a pure proton conductor layer between the electrolyte and cathode. An isotopic effect study also supported the claims. Consequently, the constrained surface region of CeO2−δ is believed to build long-term proton shuttles, suggesting a new methodology for and understanding of the proton transport in general oxides and a promising electrolyte material for new-generation proton ceramic fuel cells.
3.2 Semiconductor Membrane Fuel Cells
According to traditional knowledge, electrolytes with partial electronic conductivity will significantly reduce the cell voltage and system energy efficiency [71]. In 1992, Riess proposed the use of mixed ionic and electronic conductors (MIECs) to replace solid-state electrolytes to run SOFCs when the FC is operated close to its maximum power output [72]. More recently, a new research emphasis has been put on using semiconductor oxides, more specifically electronic insulating oxides under an applied fuel cell atmosphere, to substitute conventional ion conducting electrolytes for SOFCs. This research was initiated by the revolutionary inventions called a single-layer fuel cell (SLFC) and an EFFC. In these cases, one-layer semiconductor-ionic conductor composite components can replace the conventional three-layer components of the anode, electrolyte and cathode while providing functions that can make the fuel cell operate in the same way [73,74,75,76,77]. Such a single-layer material is made of a semiconductor-ionic mixed or heterostructure composite, which leads to the same fuel cell functionality and holds the potential for lower temperature and higher performance applications. Later, a series of semiconductor materials were shown as SLFCs in the same way, e.g., La0.9Sr0.1InO3−δ [78, 79], LixAl0.5Co0.5O2 [80], SmNiO3 [16], La0.2Sr0.25Ca0.45TiO3 [81], TiO2 [82], LiZnO [83], and Na0.5Bi0.5TiO3 [33]. From a classic SOFC perspective, electronic conduction through the electrolyte/membrane will cause significant losses in both cell voltage (open-circuit voltage, OCV) and energy efficiency, while for the semiconductor fuel cell, the fuel cell behavior is not influenced. Generally, the electrical conduction behavior of semiconducting materials is well recognized to be a function of the applied oxygen partial pressure, as seen from the aforementioned symmetric perovskite oxide electrode materials in SOFCs. Moreover, some materials show interesting electronic, pure ionic and hole conduction under low, intermediate and high oxygen partial pressure conditions at a certain temperature [78]. For example, the electrical conductivity of La0.9Sr0.1InO3−δ changes only slightly with oxygen partial pressures from 10–5 to 10–20 between 600 and 800 °C, a typical ionic conduction behavior, while it exhibits typical p-type and n-type semiconducting behavior under higher and lower oxygen partial pressure conditions [78, 79]. The constructed SOFCs based on only the La0.9Sr0.1InO3−δ material, called SLFCs, gave an OCV of higher than 1.0 V and a maximum power output of 3 mW • cm−2 at 800 °C. However, in such a single layer fuel cell, a functional layer of La0.9Sr0.1InO3−δ performing as an electrolyte layer remains, which is similar to that in the classic SOFC, its p- and n-type conduction behavior is utilized for electrode functionality.
Therefore, in semiconductor-based fuel cell devices, the working principles built on semiconductor and heterostructure membranes should differ from those of ion electrolyte membrane-based fuel cells. A technical illustration of the difference between classic and newly developed semiconductor-based SOFCs is shown in Fig. 5, which shows the SOFC based on the O2− conducting electrolyte without electron passage. However, in Fig. 5b, the coexistence of both ions (e.g., the O2− case) and electrons, which is commonly the case in semiconductors and their heterostructures, takes over the electrolyte. Surprisingly, the latter (Fig. 5b) can display even better performance than the electrolyte-based SOFC. This is truly a new phenomenon, and therefore, the semiconductor electrochemistry of fuel cells shows high potential for further deployment. It is naturally introduced in term of semiconductors and physical principles in the following.
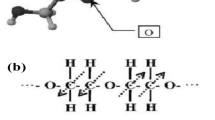
Different from the above case, recently, mixed ionic and electronic conducting single-phase oxides have also been transformed into ionic conductors with ionic transfer number close to unity under fuel cell conditions, more accurately by proton incorporation, according to the reports by Lan [80] and Zhou et al. [16]. As a typical LIB electrode, the layered structure LixAl0.5Co0.5O2 (LCAO) can accommodate protons in the layer between two transition metal oxide layers or by replacing lithium ions (Fig. 6a). Therefore, the obtained proton-intercalated LixHyCoO2 at the cathode leads to a change in the oxidation states of transition metal ions, which consequently relieves the electronic conduction of traditional semiconducting LiCoO2; the substitution of Co with Al also leads to suppressed electronic conduction in the materials, changing the semiconductor to a pure ionic conductor with an almost unity ionic transfer number.

Reproduced with permission from Ref. [80]. Copyright 2014 Wiley–VCH. b For the SNO case. Reproduced with permission from Ref. [16]. Copyright 2016 Springer Nature
Working principle of SLFCs operated based on existing SOFC science, i.e., ionic (in the case of H+ conduction) electrolyte devices formed during in situ fuel cell operation, regardless of the construction of the semiconductor layer configuration. a For the LCAO case.
Similarly, a distinguished work by Zhou et al. [16] reported that the highly electronically conducting perovskite oxide SmNiO3 (SNO) was transformed into a unity-transference ionic conductor under fuel cell conditions (H2, Pt/SNO/Pt, air, Fig. 6b). An in-depth investigation showed that when SNO is exposed to a hydrogen anode, hydrogen dissociates into protons and electrons accompanied by the reduction of Ni3+ cations in SNO to Ni2+. The proton incorporation into the SNO lattice forms H-SNO because the concentration and potential gradient lead to filling-controlled Mott transitions such that electrons are no longer able to transport through the \(E_{{\text{g}}}^{2}\) manifold because of the large electronic bandgap. Such a transition enables hydrogenated SNO to provide the electrolyte functionality for SOFCs. Moreover, according to experimental measurements, the proton conductivity in H-SNO is higher than that in well-known high proton conductivity Ba(Ce,Zr)O3-based perovskite oxides at reduced temperatures. A micro-SOFC with optimal electrolyte thickness and an active Pt metal electrode gave a maximum power density of 225 mW• cm−2 at 525 °C, which is comparable to the best value in the applied temperature range. Therefore, both LCAO and SNO materials eventually transform from high electron (hole) conductors to common proton conducting electrolyte-based fuel cells, i.e., the proton ceramic fuel cell (PCFC) process, even though the SOFC devices are initially constructed as a semiconductor device. Hydrogenation of oxides leads to wide electronic bandgap materials, which is an interesting approach to design and obtain new-generation electrolytes for SOFCs working at low temperatures [4].
Another work by Dong et al. [82] reported that thin film TiO2, a typical semiconductor, could be used as an electrolyte for LT SOFCs. SOFCs based on a TiO2 thin film were fabricated by a spin coating method on an electrode supporter, giving an OCV of 1.1 V and a maximum power output of 364 mW• cm−2 at 550 °C. This certificates that typical semiconductors can be used as electrolytes for SOFCs. In this work, Dong proposed the use of the universal energy band principle instead of electronic conduction theory to evaluate the possible application of novel materials for fuel cell and photocatalysis applications. They proposed that the electronic conduction in electrolyte materials should not be blamed if the energy band diagram can be well aligned in a real fuel cell. Under a fuel cell atmosphere, TiO2 possesses a higher CB above the redox potential of H2/H+ and a lower VB than the redox potential of O2/O2−, which can help effectively block electron transport through the electrolyte. When TiO2 is combined with the electrode material LiNi0.8Co0.15Al0.05O2, a LT high-activity symmetric electrode, the electrons produced at the anode cannot move to the CB of TiO2; therefore, they can only follow the external circuit of the cell and transfer to the cathode, realizing the fuel cell function. The nearly Nernst theoretical voltage and excellent LT power output verified the feasibility and correctness of the adopted approach. More recently, SOFCs using the pure SrTiO3 “electrolyte” delivered a peak power output of 620 mW • cm−2 at 550 °C [81, 84, 85], while it is an intrinsic semiconductor and presents a high n-type electronic conductivity. Moreover, the ionic conductivity reached 0.24 S • cm−1 at 550 °C, which is higher than that of most current single-phase pure solid-state ionic conductive electrolyte materials. A deep examination of the material texture and morphology confirmed that a superhigh ionic conductivity layer with an amorphous structure is formed on the surface of SrTiO3 [85]. Regardless, the superior electrochemical activity suggests a new approach for LT SOFC applications.
3.3 Semiconductor Heterostructure Materials
Commonly, compared to the single-phase electrolyte drawback that it cannot meet all the strict requirements for reliable, robust and durable SOFCs, semiconductor heterostructures with one ionic material or another semiconductor phase incorporated can provide better performance, such as higher ionic conductivities, than conventional electrolytes and increase the triple-phase boundary for electrode reactions [1, 86]. For example, bulk p-n heterostructure materials have been proposed to tune semiconductors to superionic conduction because the p and n materials can balance free electrons/holes, thus suppressing electron leakage and resulting in no net electrons [17, 67, 87,88,89,90,91,92,93,94,95]. Compositing p-type LiZnO with the ionic conductor doped ceria (n-type under fuel electrode conditions) for LT SOFCs has been reported. Interestingly, this bulk hybrid electrolyte exhibits superionic conductivity (> 0.1 S • cm−1 over 300 °C) and excellent electrolytic performance (400–630 mW • cm−2) between 480 and 550 °C. Unexpectedly, the hybrid semiconductor shows net-electron-free transport in the electrolyte, as reflected by the sufficient OCV under H2/air fuel cell conditions. Later, as demonstrated by Zhu et al. [77] and Wang et al. [89], fuel cells based on the planar/bulk n-type/p-type semiconducting heterostructure gave impressive fuel cell performance under H2/air conditions. Wu et al. [96] synthesized a ZnO/SrTiO3 semiconductor by a hydrothermal method and used it as a photocatalytic hydrogen production catalyst and a fuel cell “electrolyte layer”. The photocatalytic cell based on this n/n-type semiconducting heterostructure gave a H2 production rate of 1317.44 μmol • g−1 within 5 h under solar-light irradiation and an OCV of 1.14 V, and SOFCs presented a maximum power density of 564 mW • cm−2 at 550 °C. In the interesting work by Cai et al. [97], they composited all the materials in a typical SOFC, such as a Ni(O)-YSZ anode, a YSZ electrolyte and an LSM-YSZ cathode, to identify each component that could function as a semiconductor-ionic membrane for electrolyte functioning. They also made an ‘all-components-in-one’ fuel cell, and the complex hybrid heterostructure layer with the NCAL electrode displayed a three times higher device performance than the conventional YSZ electrolyte-based fuel cell under the same operating conditions (temperature and fuel/oxidant partial pressure). The “all-in-one” heterostructures appear to have much better ionic transport properties than existing ionic materials by creating a motorway for ionic transport along the semiconductor and ionic interfaces, which normally contain a space charge layer and present band bending and an induced BIEF, facilitating ionic transport/transfer, which cannot be manipulated by single-phase materials.
Moreover, an EFFC can be superior to traditional electrochemical fuel cells due to the physical removal of the conventional ionic electrolyte layer and two physical interfaces between the electrolyte and the electrodes (the anode/the cathode). In particular, in semiconductors and heterostructures, coupling interactions between charge carriers, electrons (holes) and ions impact ionic transport and redox reactions from the conventional macro-redox in three-layer devices to the nano-redox in single-layer configuration devices (as shown in Fig. 2f) as new-generation energy devices. These results suggest that semiconductor electrochemistry could play an important role in improving the fuel cell performance and developing new science and technology to continuously increase fuel cell R&D [1, 2]. By integrating the band structure and alignment fundamentals into fuel cell design, many new energy devices/technologies jointly harnessing semiconductors and electrochemistry have been demonstrated with plentiful examples. In addition, band alignment can also make significant contributions to speed up the HOR and ORR by adjusting the reactant and intermediate phase absorption energies, thus lowering the required activation energy, which has been commonly demonstrated in the photochemical water splitting process and other related fields [15, 98]. From a reaction product aspect, facilitating fuel cell functions for a reversible process via photochemical water splitting to H2 and O2 is desirable to generate H2O and simultaneously produce electricity. These new emerging trends of semiconductors and the associated methodologies have changed the fuel cell electrochemistry and brought new scenarios for fuel cell R&D toward semiconductor-fuel cell science, which may pave the commercialization roadmap for SOFCs and impact other areas in chemistry, such as photocatalysis/electrolysis and batteries.
Without the ion conducting/electron insulating layer, a key question arises: how does such a device work? In fact, various semiconductors and heterostructures have been widely applied without by using the traditional anode/electrolyte/cathode structure based on multiple built-in junctions. These devices have been developed by introducing new functional semiconductor materials or a semiconductor with an ionic conductor to form heterostructures. Figure 7 presents an overview of these novel fuel cells based on three-layer, two-layer, and single-layer structures. A three-layer fuel cell device (Fig. 7a) employs a semiconductor (S) or a semiconductor heterostructure (S+) membrane instead of the sole ionically conductive electrolyte, but it can be tuned to an ion conducting electrolyte (function) via a semiconductor-ionic conductor transition in the fuel cell environment. This device has the three-layer configuration of the anode (n-semiconductor or metal)/S or S+/cathode (p-semiconductor). The abovementioned LCAO and SNO fuel cells belong to this type. This configuration may also include an electrolyte layer because the ionic conductor or wide bandgap semiconductor can be treated/tuned as an electrolyte [2]. Fig. 7b illustrates the fuel cell device constructed by using only two layers, an anode and a cathode; refer to Fig. 7b for a planar p–n junction device. Conventional SOFC technology commonly uses a three-layer structure, two electrodes using a mixture of semiconductor oxides, e.g., NiO for the anode and perovskite oxide for the cathode, and an ionic electrolyte, e.g., doped ceria or zirconia, to enhance the ionic conductivity in the electrodes and effectively minimize the electrode polarization while enhancing the power output. The double-layer device was previously reported [77] and has been verified by other interesting research work. These devices were constructed by using typical SOFC components/layers in contact without an electrolyte between them. By removing the middle electrolyte layer, the fuel cell can still function due to the p–n junction principle.

Reproduced with permission from Ref. [77]. Copyright 2011 RSC. (d) Fuel cell constructed by a Schottky junction device, in which the Schottky junction barrier is caused by band bending at the metal/semiconductor (electrolyte) interface
Schematic illustration of (a) a three-layer fuel cell device consisting of an n-semiconductor anode/semiconductor (S) or semiconductor heterostructure (S+) membrane/p-semiconductor cathode; (b) a two-layer fuel cell: A, anode; C, cathode; and (c) a 3-in-1 SLFC device. CC stands for current collection. All cases are considered with both H+ and O2− conduction; in the case of only one type of ions, either H+ or O2−, the corresponding H2O formation is only on one side, the former in the cathode (O2, which can be air) and the latter in the anode.
In the new semiconductor approach, as illustrated in Fig. 7c, the configuration looks very similar to that of the SOFC electrode component of mixed electrolyte and electrode materials: all three layer materials of anode, electrolyte, and cathode (where the anode and the cathode could be the same material, leading to symmetrical fuel cells) can function within a single component, i.e., the “three-in-one” type device [99], outperforming the traditional three-layer fuel cell functions, thus stimulating significant industrial and fundamental research interest with remarkable and additional contributions to fuel cell science and technology. From a technical aspect, the bulk heterojunction (BHJ) requires delicate nanoengineering to tailor the n and p conducting properties at the nanolevel using heterostructure composite technology.
In later developments, metal–semiconductor-based Schottky junctions were discovered to have a significant impact on fuel cell performance [100]. The Schottky junction fuel cell device, as shown in Fig. 7d, is constructed using only one type of semiconductor, either n- or p-type (more commonly) or a semiconductor-ionic heterostructure composite, as reported [100, 101]. In this case, a Schottky barrier is formed between the anodic reduced metal/alloy surface and a single layer of the p-type (or n-type) semiconductor (Fig. 7d), thus preventing electrons from passing through the device internally. Moreover, by applying energy band design/alignment to design fuel cells as a new fuel-to-electricity conversion technology, the performance can arguably surpass that of fuel cells [102]. The designed energy band and alignment based on PSC science can isolate the device (Fig. 8a, b), avoiding the electronic short-circuiting problem and simultaneously generating electricity with high power outputs > 1000 mW • cm−2 at 550 °C. There is a unique charge separation mechanism, discovered by Zhu et al. [103], as shown in Fig. 8c.

Reproduced with permission from Ref. [103]. Copyright 2017 Elsevier
a Nanoparticle contacts between the LSCF and Sm and Ca co-doped Ceria (SCDC), b energy diagram of the p–n BHJ for charge separation on the H2 contact side at the particle level, and c energy band diagram at the device level.
This can be a general methodology for new conversion devices based on semiconductors and principles. In the latest research, Dong et al. [82] also used the same strategy to construct a fuel cell with TiO2 as the electrolyte. They claimed that electronic conduction in electrolyte materials should not be blamed if the energy band diagram can be well aligned in a real fuel cell. In the fuel cell, the CB of the electrolyte, either a pure ionic conductor or a semiconductor, should be higher than the redox potential of H2/H+; therefore, the electrons cannot jump to the CB of the electrolyte, or are blocked by the electrolyte layer. TiO2 is a typical semiconductor with great application potential in photovoltaics and photoelectrochemistry [104,105,106,107,108]. At first glance, the use of TiO2 will cause the short-circuiting issue in fuel cell conditions. This does not happen in this fuel cell case since TiO2 possesses a higher CB above the redox potential of H2/H+ and a lower VB than the redox potential of O2/O2− [98, 105, 109, 110], which can help effectively block electron transport through the electrolyte. When combined with the advanced NCAL symmetric electrode for demo-SOFCs, the reduced electrode materials in the anode have a lower CB than the redox potential of H2/H+ and a higher VB than the redox potential of O2/O2−. The electrons and holes can easily move to and be collected in the electrode. Such a fuel cell device has been successful demonstrated, delivering 1.10 V (OCV) and 364 mW cm−2 at 550 °C under hydrogen/air conditions. The nearly Nernst theoretical voltage and excellent power output have verified the proposed methodology. Electronic conduction in electrolytes could be well managed by properly choosing the electrode material based on the energy band diagram method [111, 112]. More details will be further discussed in Sect. 5.
To this end, a summary of the developed semiconductor and/or heterostructure material-based fuel cells and their corresponding electrical properties, including ionic conductivity and fuel cell electrochemical performance, is presented in Table 1. The ionic conductivities of semiconductor or heterostructure materials are much higher than those of single-phase oxide ion conducting electrolytes, which enables superior fuel cell electrochemical performance at low temperatures. Semiconductor-based fuel cells may suggest an alternative promising approach to develop next-generation SOFCs or PCFCs.
4 Semiconductors and Semiconductor Electrochemistry in Batteries
4.1 LIB as a Typical System
Extensive examples of employing semiconductor bands and physics in electrochemistry can be widely found. One of the featured fields is the rechargeable batteries or secondary batteries, such as LIBs, Na-ion batteries, and Zn- and Mg-ion batteries, which reversibly convert electrical and chemical energy via redox reactions, thus storing electrical energy as a chemical potential in their electrodes [122, 123]. Metal ions are reversibly transported between the anode and cathode and through the electrolyte, releasing or storing energy. Generally, the specific capacity of electrodes and the working voltage of the battery determine the energy density of these devices, i.e., the differential chemical potentials between the cathode and the anode. In these typical electrochemical devices, semiconductor properties and band theories provide a new comprehensive understanding of the scientific principles for improving the electrochemical reaction kinetics to increase the rate performance. Taking the LIB as a typical system, Fig. 9 shows an example of understanding the lithium battery electrochemistry and cathode (positive electrode) materials from the semiconductor band structure and physics (Fig. 9a). The LiCoO2 positive electrode (cathode) energy as a function of the density of states, being relevant to the Fermi level of Co4+/3+ redox couples, is presented in the device energy level diagram with respect to the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of the liquid electrolyte, as shown in Fig. 9b [124]. The battery voltage, i.e., the OCV VOC can be expressed as Voc = (μc − μa)/e, where e is the magnitude of the electronic charge and the difference in chemical potential between the anode (a) and the cathode (c).

Reproduced with permission from Ref. [126]
a Schematic energy level diagram for a lithium cell; b with LiCoO2 as the cathode and lithium as the anode, illustrating the origin of Voc and relative energy positions with respect to the HOMO and the LUMO of the liquid electrolyte. The Fermi level of the lithium anode is drawn above the LUMO of the electrolyte, indicating that reduction of the electrolyte is usually observed. Note that the Fermi level of the cathode shifts downward during deintercalation. c Electronic band structure of LiCoO2 (Li = 1, left), Li0.5CoO2 (Li = 0.5, middle) and CoO2 (Li = 0, right), as deduced from DFT calculations and experimental measurements.
Normally, this working voltage is also determined by the electrochemical window of the electrolyte (see Fig. 9b), which is controlled by the energy gap between the HOMO and the LUMO. To work properly, the anode and cathode materials must follow the rule that μa is below the LUMO and μc is above the HOMO; otherwise, the electrolyte will be reduced on the anode surface or oxidized in the cathode zone, forming a passivating solid electrolyte interphase (SEI) film [125]. From a physical point of view, one may relate the cell voltage to the difference in the (electro)chemical potential of the electrons between the cathode and anode, as given by the position of their Fermi levels (see Fig. 9a). However, we should keep in mind that the Fermi level (electron chemical potential) of (single) electrode materials in the discussion of electrode potentials is not exact but quite often provides reasonable accuracy and results.
In Ensling’s work, the authors highlighted the significance of the (partially) covalent character of the device capacity and stability as well as electrode kinetics after investigating the electronic structure of the LiCoO2 cathode materials. Figure 9c presents three typical structures of LiCoO2 (Li = 1, left), Li0.5CoO2 (Li = 0.5, middle) and CoO2 (Li = 0, right) after Li+ removal from the LiCoO2 structure with different electronic bands, along with the differences in the chemical instability with respect to oxygen loss due to the overlapping of the Co4+/3+:t2g band with the O 2p orbitals. The changes within the structure and occupation of the density of states (DOS) of LixCoO2 during deintercalation are schematically summarized. The Fermi level (EF) reaches a critical value when the Co 3d states (Co4+/3+ couple) cross the top of the O 2p bands, which is accompanied by hole transfer to the O 2p states. A significant number of electrons can be removed from the O 2p band, leading to oxidation of Co ions and/or formation of oxygen vacancies, which is not favored for cathodic durability. Therefore, the proximity in energy of the t2g and O 2p bands determines the intrinsic voltage limit for the LiCoO2 cathode material [126]. The loss of oxygen from the lattice in the Li1 − xCoO2 system may explain the limited capacity (140 mAh • g−1), which is significantly lower than the theoretical value. Therefore, investigating the metastable character of partially deintercalated semiconductors of LixCoO2 is very important for the device process kinetics and durability, where the band structure plays an important role.
In the LIB field, further improvements in electrodes and cells are generally limited by an incomplete understanding of the interfaces, involved charge transfer, charge compensation, and chemical reaction processes. Much attention has been paid in the past to the electric potential gradient at the interface, although double (dipole) layers can have a significant impact on the energy level (mis)alignment [127]. Additionally, for ionic phases with low charge carrier concentrations, diffuse charge layers (space charge layers) are encountered at interfaces [128], which induce band bending and related charge carrier concentration changes that have a significant impact on the charge transfer rate and interface stability. Figure 10 shows an energy level diagram for the LiCoO2/diethyl carbonate (DEC) (alkyl carbonate solvent) electrolyte interface with a focus on the formation of the electrochemical double layer. Li ions are generally aligned with the ionic electrochemical potential of Li+ in the two phases (the chemical potential of Li ions is initially very low in the DEC adsorbate phase). According to theoretical considerations, the Li-ion energy level is bent in the opposite direction to the electron energy levels due to the opposite charge of the ion. With such an energy level diagram, the interface and its kinetic properties can be understood, as it contains energy level offsets as well as any charge carrier concentration changes, as evidenced by band bending [129].
Electron and ion energy level diagram of the LiCoO2/DEC interface established from LT adsorption of DEC (alkyl carbonate solvent) electrolyte. DEC: diethyl carbonate. Note that the Li-ion levels are deduced from theoretical considerations. Reprinted with permission from Ref. [130].
4.1.1 Band Theory for Tuning from Semiconducting to Ion Conducting Electrolytes
Currently, efforts are mainly devoted to the development of new solid-state electrolytes, such as oxide and sulfide, to increase the energy density and safety of lithium batteries. However, the ionic conductivity in solid-state ionic electrolytes is much lower than that in liquid electrolytes. The traditional design of ionic conductors is primarily based on substitutional doping of dissimilar valence cations into the unit lattice of the structure, resulting in inherent limits on the dopant concentration and thereby ionic conductivity. Recently, a very interesting example of applying semiconductors and electronic states to batteries is the strongly correlated perovskite SmNiO3 (SNO), which shows applicable lithium ion conductivity at room temperature. SNO has a metal conduction nature and can be electrochemically transformed by Li+ injection from an electronic conductor to an electronic insulator and a superior Li+ conductor as the electrolyte for a LIB [131]. Based on extensive experimental and theoretical calculations [131], Li+ ion shuttling and simultaneous suppression of electronic transport via the Mott transition are induced in such a system, which provides an alternative approach to develop novel LIBs or other energy devices.
Figure 11a–c shows that a large amount of mobile Li+ can be found in an electrochemically lithiated SmNiO3 (Li-SNO) system, which is located in interstitial sites of the perovskite, approaching one dopant ion per unit cell, accompanied by significant lattice expansion. Such interstitially inserted Li+ ions can facilitate fast Li+ conduction associated with a lower activation energy in comparison with the structural case. Figure 11b presents a design principle for solid-state Li+ conductors based on SNO. Pristine SNO is a narrow gap semiconductor after lithiation, i.e., the lithium-doped/inserted thin film SNO (Li-SNO), then transforms into an insulator, with a gap of approximately 3 eV (Fig. 11c). First-principles density functional theory (DFT) calculations showed that the pristine SNO exhibits a monoclinic cell without distortion, while with lithium entering SNO, Ni3+ is converted to Ni2+, resulting in a much different total DOS (Fig. 11d).

Reproduced with permission from Ref. [131] Copyright 2018 National Academy of Sciences
Strongly correlated nickelate as the ionic conductor in a LIB. a Electronic configuration of pristine SmNiO3 (SNO), a narrow gap semiconductor, at room temperature. b Self-designed electrochemical cell for lithiation of SNO. c Electronic configuration of lithiated SNO (Li-SNO). Electron filling leads to a large on-site Coulomb interaction, suppressing the electronic conduction pathway. Li+ occupies multiple interstitial sites of the unit cell. Electron localization-induced perovskite lattice expansion facilitates Li+ interstitial transport, which enables Li-SNO to be a potential lithium-ion conductor. d First-principles simulation of the total DOS for Li-SNO with 0–1 intercalated Li/SNO (gray). The projected DOS (PDOS) of the unoccupied Ni Eg states are shown in color. The lighter hues indicate Eg states of Ni3+, and the darker hues indicate those of Ni2+ including the newly occupied Eg states due to the localization of the added electron from the intercalated lithium below the Fermi energy.
The Li+-inserted Li-SNO has an expanded structure that allows for a 3D interconnected Li+ diffusion channel, and the interstitial Li+ is weakly bonded with oxygen ions, therefore exhibiting fast Li+ conduction behavior. Accompanying this transition, the pristine SNO shows insulation due to suppression of electron transport in SNO. The Li+ conductivity was determined to be 3.1 × 10–3 S·cm−1 at room temperature with an activation barrier of ∼0.24 eV. The corresponding Li-SNO battery demonstrated LIB capacity and good lithiation and delithiation cycle life with average coulombic efficiency of 96.8%. This work provides evidence for ion shuttling in the lattice and atomistic pathways [132,133,134]. Moreover, this strategy can be extended to other ions, such as Na+, demonstrating the generality of the approach.
A theoretical design of the Li+ conductor could be based on wide bandgap semiconductors. Wide bandgap semiconductors, especially dielectric materials, can potentially be used as electrolytes because of their large bandgap, which precludes the possibility of electrons being excited from the VB to the CB by thermal means. In addition, under electric field conditions, the electric dipole component in the dielectric materials can be adjusted, which may further affect the ionic transport properties [135,136,137,138]. Li+ and Na+ ions are large enough to form bent O-A-O symmetric bonds in some condensates of larger anion clusters with electric dipole moments, as illustrated in Fig. 12 for Na glass. The high-frequency dielectric constant is important because it represents the intrinsic property of the material and is directly related to the DC conductivity. Accompanying this electric dipole component, the electric dipole component of the electrolyte interface charge is increased by dipole coalescence. Using dielectric materials in the electrolyte may produce a very large energy gap window that makes it stable upon contact with both an alkali-metal anode and a high-voltage cathode without the formation of an SEI. The glass also contains electric dipoles that have a large dielectric constant. The Goodenough group applied dielectric materials as electrolytes for Li+ and Na+ batteries. For example, the Li+ and Na+ glass electrolytes A2.99Ba0.005O1+xCl1−2x with A = Li or Na were reported [128,129,130,−131]. Bragg and Goodenough et al. [128] reported that the dipoles are oriented under an applied electric field and aligned parallel to the field axis, which enables solid glass electrolytes A2.99Ba0.005O1+xCl1−2x with A = Li or Na to yield an ionic conductivity σi > 10–2 S • cm−1 and a very large dielectric constant, which is extremely important to develop safe rechargeable batteries of high energy density and long cycle life.

Reproduced with permission from Ref. [139]. Copyright 2016 ECS
Ab initio molecular dynamics calculations showing electric dipoles with a ratio of elementary dipoles to several unaligned clusters of 7:1 at 100 °C. a Box containing 81 Na+, 7 Cl− and 37 O2− ions. b Elementary dipoles and clusters. c Chain segments calculated before and proposed after alignment.
4.1.2 Role of the BIEF in Batteries
One of the most exciting characteristics of semiconductor and energy band theories in the battery field is that the band alignment of different battery components or compositions that induces a BIEF or alters the local electric field (LEF) can significantly improve the electrochemical performance of the device, including promotion of ion transport, increase of the cycle life and high-speed discharge–charge cycle, and enlargement of the battery capacity.
To speed up electrode Li intercalation at high charge–discharge rates and promote the Li+ ion diffusion process, a BIEF at the semiconductor particle (material) level has been reported. A typical example is reported by Xia et al. [11] for a TiO2-based electrode, as shown in Fig. 13. In comparison to bulk TiO2 (Fig. 13a) and nanocrystal TiO2 (Fig. 13b), Fig. 13c shows a structure consisting of a nanocrystalline stoichiometric core of an intrinsic-type TiO2 semiconductor and an amorphized nonstoichiometric outer layer TiO2−d n-type shell. Such a structure creates an i-n heterostructure [11] with the BIEF direction from the outer layer to the core, which is expected to promote Li+ diffusion during the lithium discharge process, i.e., help Li+ insertion (Fig. 13d). Once Li+ has been fully inserted or discharged into the TiO2 nanocrystal, the core will become LiyTiO2, turning into an intrinsic semiconductor. In contrast, the disordered layer takes up much less Li+ due to the existence of oxygen vacancies and becomes Liy−2x−rTiO2 − d, corresponding to a relative p-type semiconductor compared with LiyTiO2. Therefore, a new i-p heterostructure and a BIEF will be formed at the core/shell interface; thus, the new BIEF directed from the disordered layer to the crystalline core facilitates Li+ transport from the core to the shell, i.e., helps Li+ extraction in the charging process (Fig. 13e). In the end, with the assistance of the in situ formed i-n and i-p heterostructure and BIEFs, a higher ionic diffusion capability is obtained during the Li+ ion insertion/extraction process, increasing the rate performance.

Reproduced with permission from Ref. [11]. Copyright 2013 ACS
Comparison of charge diffusion in electrode materials made of a bulk crystals, b nanocrystals, and c surface-amorphized nanocrystals, and illustration of the facilitation of charge transport under a BIEF during discharge (d) and charge (e) processes. The length and width of the arrows illustrate the relative penetration depth and the charge transfer/transport coefficients of the lithium ion in the material, respectively.
Another interesting case is reported by Yan et al. [140] based on carbon-doped Co3O4 nanocrystals for superior Li-ion storage. The imbalanced charge distribution emerging from the carbon-dopant can induce an LEF to greatly facilitate charge transfer within unique sub-10 nm nanocrystal-assembled Co3O4 hollow nanotubes (HNTs). The resulting carbon-doped Co3O4 HNT-based electrodes demonstrated an excellent reversible capacity of 950 mAh • g−1 at 0.5 A • g−1 after 300 cycles and superior rate performance of the full battery with 853 mAh • g−1 at 10 A • g−1. Zheng et al. [12] also reported that the LEF effect can be built at atomic interfaces on ultrathin Bi2MoO6 (BMO) nanosheets (Fig. 14a–c). The unbalanced charge distribution between the [Bi2O2]2+ and [MoO4]2− layers induces an interfacial electric field at the interfaces, while the lopsided charge distribution around oxygen vacancies results in a local in-plane electric field, thus promoting ion diffusion/electron transport. Additionally, the 2D ultrathin construction can provide interconnected charge migration paths formed on the open surfaces, which could facilitate the charge transfer process in the BMO sheets. Therefore, both the in-plane and interfacial electric fields, as illustrated in Fig. 14d, induced by atomic-level engineering (Fig. 14e) can cause an unbalanced charge distribution within the crystal and induce a BIEF, which can significantly boost the lithium-ion transfer dynamics. Impressively, the lithium battery using the BMO nanosheet electrode shows a long-term cycling performance at 2000 mA • g−1. A possible mechanism for the improvement in lithium storage in atomically thin BMO nanosheets is proposed as shown in Fig. 14f.

Reproduced with permission from Ref. [12]. Copyright 2017 Wiley–VCH
Morphology of obtained BMO sheets. a TEM image, b enlarged high-resolution TEM image, and c atomic force microscopy (AFM) image with corresponding height. d Schematic diagram of the induced in-panel electric field and the interface electric field within the BMO sheets viewed in different directions. e Electric field distribution and corresponding crystal structure of the BMO sheets in (d). f Relationship between the electric field, migration paths, and Li transfer kinetics in BMO sheets and bulk BMO. d–f Summary of the enhanced high rate capacity mechanism of the BMO sheets for Li-ion storage.
In addition to LIBs, the construction of a BIEF/LEF has also been demonstrated to be very effective in developing high-performance sodium-ion batteries [141, 142]. Ni et al. [141] found that a BIEF can reduce the activation energy and accelerate charge transport significantly based on the synergy of the ordered architecture of iron oxide-based nanotube arrays and a BIEF. Figure 15a schematically illustrates such a material system and the BIEF-related mechanism. A BIEF spontaneously develops at the heterogeneous interface of n-Fe2O3 and p-FeS2, as verified by Kelvin probe force microscopy measurements across the heterointerface. Upon discharge (sodiation), the BIEF that has an n-to-p direction will push Na+ ions from Fe2O3 to neighboring FeS2, leading to enhanced transport kinetics. Then, for recharging, desodiation grows relatively Na+-rich FeS2 domains and Na+-deficient Fe2O3 domains, yielding a new BIEF with an electric field direction from FeS2 to Fe2O3 due to the potential difference. Therefore, the Na+ diffusion kinetics can be boosted in such an S-Fe2O3 system in both sodiation and desodiation states. More specifically, S-Fe2O3 exhibits a high initial current efficiency of 83%. The assembled S-Fe2O3//Na0.67(Mn0.67Ni0.23Mg0.1)O2 battery shown in Fig. 15b afforded a reversible capacity of approximately 500 mAh • g−1 (based on anode mass) and an average voltage of 2.5 V (Fig. 15c), leading to a specific energy of 160 Wh • kg−1 (based on both active materials) at a power density of 60 W • kg−1. The energy remained at 142 Wh • kg−1 under a specific power of 330 W • kg−1, which is comparable to that of commercial LiFePO4 batteries. Similar promotion effects were also observed in Bi2S3-Bi2O3 [142] for Na-ion batteries and in C@N–C@N P–C graded heterostructures for aluminum-ion batteries (AIBs) [143]. Such a design also serves as a perfect platform for exploring the underlying correlation of the BIEF and the electrochemical performance of secondary batteries.

Reproduced with permission from ref. [141]. Copyright 2019 Wiley–VCH
a Schematic of the formation of a BIEF and Na+ storage mechanism upon discharge and recharge, and electrochemical performance of the full cell of Fe2O3//Na0.67(Mn0.67Ni0.23Mg0.1)O2. b Schematic of the coin-type full cell. c Initial galvanostatic profiles of the S-Fe2O3//Na0.67(Mn0.67Ni0.23Mg0.1)O2 full cell at a rate of 0.2 A g−1 (based on the anode mass).
4.2 Coupled Energy Conversion and Storage Technologies with Semiconductors
The application of semiconductors to new energy conversion and storage has been widely reported. Coupling devices through the joining principle is an emergent frontier. Here, two typical examples of designing fuel cells by combining the approaches of solar cell coupling and using photoelectrolysis principles to design semiconductor-ionic fuel cell (SIFC) devices are reviewed. Figure 16a–c illustrates the band design of a fuel cell based on the PSC principle with the band structure and alignment as a new fuel-to-electricity conversion approach [102]. Similar to the PSC, we used electron and hole transport layers (ETL and HTL) and a semiconductor-ionic LSCF-SCDC material layer sandwiched between the ETL and HTL (Fig. 16b, c). Such a novel system consists of an LSCF-SCDC functional layer (Fig. 16d). The electrochemical performance was compared for a conventional 3-layer fuel cell, the type-I cell with an ETL and an HTL similar to a solar cell, and a type-II symmetrical heterojunction device (Fig. 16e). The latter two cells showed much-improved fuel cell performance over the conventional 3-layer fuel cell. In particular, the type-II cell provided a peak power density of 1080 mW • cm−2 at 550 °C with hydrogen fuel/air oxidant. The outstanding electrochemical performance, we believe, is ascribed to the much-improved ionic conductivity of the LSCF-SCDC layer, i.e., 0.188 S • cm-1 (4 times higher than that of the individual SCDC electrolyte at 600 °C) because of the enriched oxygen at the interface of LSCF and SCDC [121]. This work highlighted exploring wide bandgap semiconductors and energy band design for new-generation fuel-to-electricity conversion. Well-aligned energy bands can be designed and realized by unique semiconductor-ionic materials from anode n-type conducting to cathode p-type conducting materials with junction formation incorporated with ionic functions to maintain fuel cell electrochemical reactions, all were integrated into one device [113, 122, 123]. The key issue in such a semiconductor-ionic device is that there must be a charge separation mechanism to prevent the electrons and holes from passing through the device internally and forming an electron short-circuiting problem [103].

Reproduced with permission from ref. [102]. Copyright 2016 Elsevier
Energy level diagram of a a perovskite-type solar cell, b a fuel-to-electricity conversion device (fuel cell) inspired by the PSC structure (type I) and c a symmetrical fuel-to-electricity conversion device (type II) with an in situ formed Schottky junction. d Cross-sectional SEM image of the type-II device and close-up view of the LSCF-SCDC functional layer. e Device electrochemical performance comparison of the conventional 3-layer fuel cell, the type-I fuel-to-electricity device with an ETL and an HTL, and the type-II symmetrical heterojunction fuel-to-electricity device.
In another case, fuel cell devices based on energy band alignment were experimentally demonstrated to show no e−/h+ flow causing short circuiting based on a TiO2 functional layer as the electrolyte (Fig. 17) [82], in which a TiO2 thin film was deposited on the p-conducting semiconductor NCAL (LiNi0.8Co0.15Al0.05O2) ceramic substrate, with another NCAL thin electrode on top completing the fuel cell construction (Fig. 17a, b). As a wide bandgap semiconductor, TiO2 is suggested to hold the capability to suppress electron and hole conduction through the bulk (Fig. 17c), as reflected by an OCV close to the theoretical value based on the Nernst equation (Fig. 17d), and the cell gave a peak power output larger than 300 mW • cm−2 at 550 °C and an impressive electrochemical impedance value (Fig. 17e). This fundamental understanding and scientific design are important and naturally related to the photoelectrolysis and photocatalysis fields. The TiO2 fuel cell provides for the first time, from the detailed band parameters relevant to the photo-water splitting process and the products, the view that the photo-water electrolysis process, H2O = H2 + O2, is the reverse process to that in a fuel cell: H2 + O2 = H2O. Compared to photocatalyst TiO2 water splitting, there are some similarities in the TiO2 electrolyte fuel cell band structure and alignment. Therefore, a new and universal design for TiO2 electrolyte or other semiconductor-based fuel cells from the band structure and alignment in comparison with the TiO2 photo-water splitting process is shown in Fig. 18, in which the electrolyte layer is extended from a typical electronic conductor to a wide bandgap semiconductor, providing the specific requirement mentioned in the following paragraph. According to energy band theory, to adjust the carrier transport behavior in a device, materials with different energy band structures should be carefully designed. For example, in a PEC water splitting device [15, 144], the Fermi level of the metal counter electrode must be higher than the redox potential of H+/H2, while the VB level of the semiconductor photoanode must be lower than the redox potential of H2O/O2 (Fig. 18a). The fuel cell reaction product can be regarded as a reverse product of water splitting. In a typical fuel cell, electrons are generated at the anode/electrolyte interface and are then supposed to flow through the anode to the outer circuit when the circuit is connected. Meanwhile, no electrons are expected to flow through the electrolyte; otherwise, short circuiting occurs (Fig. 18b). Therefore, the electrons in the anode should be blocked by the electrolyte from the perspective of energy band theory. In a typical Ni-YSZ (Y-stabilized ZrO2) fuel cell, the redox potential of H2/H+ is approximately 4.5 eV relative to the vacuum energy (RVE), and the work function for a typical anode material Ni is approximately 5.1 eV RVE, which is lower than the potential of H2/H+; hence, electrons stemming from the oxidation of H2 can flow to the anode. Meanwhile, the CB of the YSZ electrolyte is higher than the redox potential of H2/H+; electrons cannot jump to the CB of the electrolyte; namely, the electrons are blocked by the electrolyte. However, if an electrolyte material has a lower CB level, then short-circuiting problems might occur (Fig. 18c). For example, the CB level of SDC, which is measured to be at approximately 5.65 eV, then the electron may flow to electrolyte, and the short-circuiting problem is often observed in SDC electrolyte fuel cells. When SDC is reduced by H2 in fuel cell operation, its electronic conductivity rapidly increases, and electrons can flow through it [145]. This is why the short-circuiting problem was observed in an SDC electrolyte fuel cell after a period of operation. In short, the energy band structure of a good SOFC should obey the following rules: (1) the CB level of the electrolyte should be higher than the redox potential of H2/H+, (2) the Fermi level of the metal anode (or the CB level of the semiconductor anode) should be lower than the redox potential of H2/H+, (3) the VB level of the electrolyte should be lower than the redox potential of O2/O2−, and (4) the CB level of the cathode should be higher than the redox potential of O2/O2−.

Reproduced with permission from Ref. [82]. Copyright 2019 RSC
a Construction of the fuel cell device using a TiO2 electrolyte functional layer; b SEM images of the cell configuration of the TiO2 electrolyte-based fuel cell device, c energy band diagram of the resulting thin film TiO2 electrolyte fuel cell with NCAL electrodes; d, e electrochemical performance of a proof-of-concept of the fuel cell at 550 °C in H2/air condition.

Reproduced with permission from Ref. [82]. Copyright 2019 RSC
Energy band diagrams of a PEC water splitting (the Honda–Fujishima effect), b a fuel cell, and c the fuel cell with the occurrence of short circuiting. If the anode of the fuel cell is not a metal but a semiconductor, then the CB level of this semiconductor should be lower than the energy level of H+/H2, as the electrons generated by H2 oxidation are expected to flow to the CB level of this semiconductor.
In addition to wide applications of semiconductors in fuel cells, coupling or integrating semiconductors/bands and fuel cell electrochemistry has recently emerged, involving metal-air batteries, photocatalytic fuel cells, photoenhanced fuel cells and many others [110, 146,147,148,149,150,151,152]. Rechargeable Li-O2 batteries are frequently reported to be intensified by the photoassisted process. Generally, rechargeable Li-air batteries show high energy density above that of Li-ion batteries. However, their practical application is limited by the high voltage loss related to the cathode processes, oxygen reduction and evolution reactions, and coupling with the low conductivity lithium oxide that forms [153,154,155]. Reduction of the energy loss to accelerate the formation and decomposition of Li2O2 and O2 evolution is the key. C3N4-carbon is the key to realizing such a coupling process of lithium-air batteries to realize high energy efficiency. Such a system consists of a Li anode, a nonaqueous electrolyte combined with an I− ion redox mediator and g-C3N4 grown on carbon paper simultaneously as an oxygen electrode and a photoelectrode, as shown in Fig. 19a. Upon charging under illumination, the I− ions are oxidized to I3− ions by photoexcited holes from the photocatalyst, and subsequently, I3− ions diffuse to the oxygen electrode surface, oxidize Li2O2 to O2 and are reduced back to complete a full redox cycle. Meanwhile, the photoexcited electrons transfer to the anode through the external circuit and reduce Li+ to Li metal. Accordingly, the charging voltage of a Li–O2 battery is compensated by the photovoltage generated on the photoelectrode. The photoassisted charging voltage equals the energy difference between the redox potential of the Li+/Li couple and the CB of the photocatalyst, as shown in Fig. 19b. A suitable photocatalyst for photoassisted rechargeable batteries must fulfill two essential conditions: the CB potential should be lower than the O2/Li2O2 couple potential (that is, 2.96 V), and the VB potential should be higher than the I−/I3− couple potential to realize oxidization of I− ions to I3− ions. The battery can deliver discharge and charge voltages of 2.76 and 3.56 V, respectively, proving that g-C3N4 can act as an ORR catalyst for Li-O2 batteries. Solar cells and solid battery/fuel cells both convert energy of different forms into electricity. A combination of these two battery systems has demonstrated clean and energy-efficient functional integrity [7, 156,157,158,159]. Li-O2 batteries have been the most focused on system to be successfully loaded with a photoresponsive electrode, where the in situ generated holes possessing positive charges directly or indirectly catalyze the oxygen evolution reaction (OER) [157, 160, 161]. Therefore, the notoriously large overpotential of the OER has been efficiently suppressed via this synergistic process, where solar energy is nominally stored in the rechargeable battery system and further consumed to compensate for the energy needed for battery charge. Additionally, recently, the utilization of solar energy to improve the OER kinetics has also been demonstrated in zinc-air batteries, which consist of a Zn electrode and a BiVO4/α-Fe2O3 heterostructure semiconductor photoelectrode as an air electrode assembled in an alkaline electrolyte (Fig. 19c). The charging process is associated with the OER on the air electrode, which has intrinsically sluggish reaction kinetics, resulting in a large overpotential and low energy efficiency. As illustrated in the proposed sunlight-promoted charging mechanism (Fig. 19d), a photovoltage is generated when the photoelectrode undergoes photoexcitation, which compensates for the high charge potential of the zinc-air battery; therefore, a higher electrical efficiency is achieved, as expected [157, 161]. These results can benefit from a more comprehensive understanding of fuel cell semiconductor electrochemistry and motivate new interesting frontier research and breakthroughs in scientific fundamentals, technologies, and applications.

Reproduced with permission from Ref. [161] and [157]. Copyright 2015 RSC and Copyright 2019 Springer Nature, respectively
Schematic illustration of the photoassisted a rechargeable Li-O2 battery and b zinc-air battery and c, d their corresponding energy band as well as the proposed mechanism of the sunlight-promoted process.
Another interesting topic is the solar photocatalytic fuel cell (PFC), which can realize wastewater treatment and electricity generation simultaneously [163]. Figure 20a schematically shows the configuration of such a device, which consists of an effective photoanode and an ORR cathode. The photoanode is made of a CdS quantum dot-sensitized TiO2 nanorod array deposited on FTO glass, and a gas diffusion electrode was employed to improve the ORR at the cathode. The ORR reaction utilizes protons produced from wastewater treatment to complete the fuel cell cathode reaction for power generation. Another interesting PFC constructed by using a photoanode made of heterostructured ZnFe2O4/TiO2-NTs and an air-breathing cathode was also proposed for simultaneous pollutant removal and power generation (Fig. 20b) [162]. Similar to the fuel cell electrochemical process, the electrolyte conductivity can effectively improve the PFC performance. The photoanode is based on band alignment to perform effective pollutant removal, and the cathode reaction can be realized again by taking protons produced from the photo-pollutant treatment process, thus simultaneously producing electricity. The same concept should be extended to future energy technology, i.e., an integrated highly efficient photo-water-splitting fuel cell, as illustrated in Fig. 20c, which not only treats wastewater but also acts as a standard water-powered electricity generator or self-generation device. In this device, H+ can be produced by photo-water splitting and then used on-site in combination with the fuel cell air electrode to complete the ORR reaction for power generation. This may be an ideal solution to realize water as both “fuel” and “power”, which is an advanced environmentally friendly and sustainable power generation system.

Reproduced with permission from Ref. [157]. Copyright 2019 Springer Nature
Schematic diagram of a photocatalytic fuel cell (PFC) a for treating wastewater and simultaneously converting chemically stored energy from wastewater into electricity [162]. b Schematic diagram of the PFC system with the ZnFe2O4/TiO2-NT photoanode and air-breathing cathode for simultaneous pollutant removal and power production [163]. c Schematic diagram of the PFC system with a photo-water splitting anode and an air diffusion catalyst cathode for simultaneous water oxidation and power production.
Furthermore, joint fuel cell and hydrogen production may be designed based on the same band design and alignment. For example, semiconductor SrTiO3/ZnO heterostructure materials have been assembled, as illustrated in Fig. 21. They showed a joint photocatalyst functionality for H2 production (Fig. 21a, b) and fuel cell electricity generation (Fig. 21c, d) [96]. Wu et al. reported excellent results for both applications: a 1 317 µmol • g−1 H2 production rate was generated at room temperature (Fig. 21a, b), reaching an advanced level, and an excellent fuel cell power output of 564 mW • cm−2 was achieved at 550 °C (Fig. 21c, d). This joint production of H2 and electricity presents a good example of a comprehensive understanding of semiconductor-based fuel cell science. We expect that based on new understanding and guidance provided by semiconductor electrochemistry, many new advanced energy applications will be explored and developed for practical uses, which should not be limited to the listed examples, e.g., photovoltachromic supercapacitors [7] and others.

Reproduced with permission from Ref. [96]. Copyright 2018 Elsevier
Schematic illustration of Energy band alignment for photocatalytic hydrogen evolution (a), with the corresponding H2 production performance (b), and for fuel cell applications (c), with the full cell performance of the ZnO/SrTiO3 heterostructure (d). The performances of control samples are also given in (d).
In photoelectrolysis, semiconductor heterostructure materials are often employed, in which the energy band and semiconducting physics play important roles, as shown in Fig. 22. The basic mechanism and process of photocatalysis are focused on two strategies: (1) energy band modification to broaden the light absorption and (2) surface modification (surface sensitization, semiconductor combination, and noble metal deposition) to increase the lifetime of the carrier, as well as the reaction kinetics. Overall, a suitable band structure is key for visible-light harvesting and effective separation of the carriers of semiconductor photocatalysts. Following a similar working principle for novel semiconductor-based fuel cells, what is required is rather the same, that is, in terms of charge creation, separation, transport and reaction (‘closing the circuit’), with h+/e− being the key charges on electrodes and H+/O2− being the charge carriers, to realize the charge transfer and redox reactions based on the band design.
Even in the photovoltaic field, typically, a perovskite-type solar cell is a very emerging concept [164], which greatly involves a deep fundamental understanding and technology scaling up and applications. Ion transport is responsible for unusual phenomena such as current–voltage hysteresis in photovoltaic devices, band structure changes, durability and anomalous above-bandgap photovoltages. Ion transport is governed in PSCs by intrinsic (ions, e.g., MA+, Pb2+, I−, and O2−; and point and extended defects) and extrinsic (light, heat, electrical fields, and chemical gradients) factors [165, 166]. These factors affect the photonic conductivity and offer valuable directions for both limiting ion transport, where required, and harnessing it to enable new functionalities, as shown in Fig. 23 [167, 168]. The transport of these ion defects exerts strong effects on the PSC performance, suggesting that electrons and ions are coupled and correlated in energy conversion and storage systems, although the focuses and applications are different because the device performances are dominated by either ions or electrons/holes. In our recent development, a semiconductor-ionic oxide was used as the PV functional layer, with successful demonstration of a PV cell with significant enhancement of both the device voltage and photocurrent output (see Fig. 24) [169]. Co3O4 is a p-type semiconductor that is incorporated with lithium to form an ionic-type LixCo3−xO4 semiconductor oxide. The use of both Co3O4 and LixCo3−xO4 as the PV functional layer resulted in a very different device performance; an OCV enhanced by more than double and a current output enhanced nine times were obtained, suggesting the feasibility of the project.

Reproduced with permission from Ref. [168]. Copyright 2018 ACS
Effect of factors on mobile defects and the consequences for physical and chemical properties.

Reproduced with permission from Ref. [169]. Copyright 2016 Elsevier
PV devices using Co3O4 and LixCo3−xO4 as the functional layer. a LixCo3−xO4 structure; b schematic diagram of TiO2/LixCo3−xO4 heterojunction solar cells; c energy alignment diagram for TiO2/LixCo3−xO4 heterojunction solar cells; d J–V characteristics of the device under 100 mW cm−2.
5 Summary and Remarks
This review intends to present new ideas and explore new solutions to clean electrochemical energy conversion and storage going beyond the existing knowledge raising a fundamental question: are there other ways to further develop fuel cells, e.g., by employing semiconductors and semiconductor electrochemistry? Other rapidly developing energy research could be taken as examples for further exploration. For example, the solar cell field is experiencing a highly interesting phase of development from physical p–n junction devices to electrochemical dye-sensitized and new perovskite-type solar cells. The latest developments show a trend of combining multiple types of energy devices (silicon solar cell (SSC), Cu(In,Ga)(Sn,Se)2 (CIGS), organic solar cell (OSC), dye-sensitized solar cell (DSSC), perovskite solar cell (PSC), lithium-ion battery (LIB), nanogenerator (NG), supercapacitor (SC), photoelectrosynthetic cell (PESC), biofuel cell (BFC), photodetector (PD), and electrolysis cell (ELC), etc.,) into a single device, two of them, at most cases, can be integrated into one unit, such as DSSC/LIB, DSSC/SC, DSSC/NG, DSSC/LIB/NG, PSC/OSC, PSC/CIGS, PSC/PSC, PSC/SSC, SSC/SC, PSC/SC, OSC/SC, DSSC/PESC, PSC/PESC, PSC/ELC, LIB/SC, LIB/NG, BFC/NG, PD/BFC, SC/PD, and NG/SC/DSSC, for various applications [15, 123]. This field is strongly affected by interdisciplinary approaches. It is often complementary to traditional semiconductor p–n junction devices progressing towards new types of practical uses as well as new science. We also recognized early on the SLFC with a joint solar cell and fuel cell as the working principle [87]. Photoelectrolysis or water splitting by photons was well developed around the PEC in the past, but today, photocatalysis can be realized by using semiconductors and band structures [8,9,10, 170]. Notably, there is a very high degree of similarity historically between the fuel cell and the PEC.
The traditional electrolyte in the fuel cell has served as both an ionic conductor and as an electronic insulator preventing short circuiting. In semiconductor and heterostructure membrane fuel cells, the short-circuiting problem can be solved through energy band alignment/junction formation and design, which have been successfully demonstrated in solar cells and photoelectrolysis, but now also adopted for fuel cells, e.g., SLFCs or semiconductor membrane fuel cells. Understanding of semiconductor bands and physics enabled conventional electrochemical photoelectrolysis and lithium battery to make use from the new aspects, which now may extend to fuel cell electrochemistry. A wide range of energy conversion and storage applications from the new scientific discoveries across the different disciplines of materials, technology, engineering, physics, and chemistry/electrochemistry could be envisioned as summarized in Fig. 25, where an overall understanding of semiconductors and semiconductor electrochemistry plays a vital role.
Availability of Data and Material
Please contact Professor Bin Zhu (Email: binzhu@kth.se).
Change history
13 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s41918-022-00130-0
References
Zhu, B., Yun, S.N., Lund, P.D.: Semiconductor-ionic materials could play an important role in advanced fuel-to-electricity conversion. Int. J. Energy Res. 42, 3413–3415 (2018). https://doi.org/10.1002/er.4105
Lund, P.D., Zhu, B., Li, Y.D., et al.: Standardized procedures important for improving single-component ceramic fuel cell technology. ACS Energy Lett. 2, 2752–2755 (2017). https://doi.org/10.1021/acsenergylett.7b00997
Singh, K., Nowotny, J., Thangadurai, V.: Amphoteric oxide semiconductors for energy conversion devices: a tutorial review. Chem. Soc. Rev. 42, 1961–1972 (2013). https://doi.org/10.1039/c2cs35393
Asghar, M.I., Jouttijärvi, S., Jokiranta, R., et al.: Wide bandgap oxides for low-temperature single-layered nanocomposite fuel cell. Nano Energy 53, 391–397 (2018). https://doi.org/10.1016/j.nanoen.2018.08.070
Fan, L.D., Zhu, B., Su, P.C., et al.: Nanomaterials and technologies for low temperature solid oxide fuel cells: recent advances, challenges and opportunities. Nano Energy 45, 148–176 (2018). https://doi.org/10.1016/j.nanoen.2017.12.044
Garcia-Barriocanal, J., Rivera-Calzada, A., Varela, M., et al.: Colossal ionic conductivity at interfaces of epitaxial ZrO2: Y2O3/SrTiO3 heterostructures. Science 321, 676–680 (2008). https://doi.org/10.1126/science.1156393
Zhou, F.C., Ren, Z.W., Zhao, Y.D., et al.: Perovskite photovoltachromic supercapacitor with all-transparent electrodes. ACS Nano 10, 5900–5908 (2016). https://doi.org/10.1021/acsnano.6b01202
Feng, X.J., LaTempa, T.J., Basham, J.I., et al.: Ta3N5 nanotube arrays for visible light water photoelectrolysis. Nano Lett. 10, 948–952 (2010). https://doi.org/10.1021/nl903886e
Osterloh, F.E.: Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 42, 2294–2320 (2013). https://doi.org/10.1039/c2cs35266d
Linsebigler, A.L., Lu, G.Q., Yates, J.T., Jr.: Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem. Rev. 95, 735–758 (1995). https://doi.org/10.1021/cr00035a013
Xia, T., Zhang, W., Murowchick, J., et al.: Built-in electric field-assisted surface-amorphized nanocrystals for high-rate lithium-ion battery. Nano Lett. 13, 5289–5296 (2013). https://doi.org/10.1021/nl402810d
Zheng, Y., Zhou, T.F., Zhao, X.D., et al.: Atomic interface engineering and electric-field effect in ultrathin Bi2MoO6 nanosheets for superior lithium ion storage. Adv. Mater. 29, 1700396 (2017). https://doi.org/10.1002/adma.201700396
Fong, D.D., Ramanathan, S.: Preface for special topic: ionotronics. APL Mater. 5, 042201 (2017). https://doi.org/10.1063/1.4982238
Gerischer, H.: The impact of semiconductors on the concepts of electrochemistry. Electrochim. Acta 35, 1677–1699 (1990). https://doi.org/10.1016/0013-4686(90)87067-C
Yun, S.N., Vlachopoulos, N., Qurashi, A., et al.: Dye sensitized photoelectrolysis cells. Chem. Soc. Rev. 48, 3705–3722 (2019). https://doi.org/10.1039/c8cs00987b
Zhou, Y., Guan, X.F., Zhou, H., et al.: Strongly correlated perovskite fuel cells. Nature 534, 231–234 (2016). https://doi.org/10.1038/nature17653
Fan, L.D., Ma, Y., Wang, X.D., et al.: Understanding the electrochemical mechanism of the core–shell ceria–LiZnO nanocomposite in a low temperature solid oxide fuel cell. J. Mater. Chem. A 2, 5399 (2014). https://doi.org/10.1039/c3ta14098
Boukamp, B.A.: Fuel cells: The amazing perovskite anode. Nat Mater 2, 294–296 (2003). https://doi.org/10.1038/nmat892
Shao, Z.P., Haile, S.M.: A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 431, 170–173 (2004). https://doi.org/10.1038/nature02863
Huang, Y.H., Dass, R.I., Xing, Z.L., et al.: Double perovskites as anode materials for solid-oxide fuel cells. Science 312, 254–257 (2006). https://doi.org/10.1126/science.1125877
Tao, S.W., Irvine, J.T.S.: A redox-stable efficient anode for solid-oxide fuel cells. Nat. Mater. 2, 320–323 (2003). https://doi.org/10.1038/nmat871
Neagu, D., Tsekouras, G., Miller, D.N., et al.: In situ growth of nanoparticles through control of non-stoichiometry. Nat. Chem. 5, 916–923 (2013). https://doi.org/10.1038/nchem.1773
Duan, C.C., Tong, J.H., Shang, M., et al.: Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 349, 1321–1326 (2015). https://doi.org/10.1126/science.aab3987
Sengodan, S., Choi, S., Jun, A., et al.: Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells. Nat. Mater. 14, 205–209 (2015). https://doi.org/10.1038/nmat4166
Vøllestad, E., Strandbakke, R., Tarach, M., et al.: Mixed proton and electron conducting double perovskite anodes for stable and efficient tubular proton ceramic electrolysers. Nat. Mater. 18, 752–759 (2019). https://doi.org/10.1038/s41563-019-0388-2
Liu, Q., Dong, X.H., Xiao, G.L., et al.: A novel electrode material for symmetrical SOFCs. Adv. Mater. 22, 5478–5482 (2010). https://doi.org/10.1002/adma.201001044
Su, C., Wang, W., Liu, M.L., et al.: Progress and prospects in symmetrical solid oxide fuel cells with two identical electrodes. Adv. Energy Mater. 5, 1500188 (2015). https://doi.org/10.1002/aenm.201500188
Steele, B.C.H.: Appraisal of Ce1−yGdyO2−y/2 electrolytes for IT-SOFC operation at 500 °C. Solid State Ion. 129, 95–110 (2000). https://doi.org/10.1016/S0167-2738(99)00319-7
Ishihara, T., Matsuda, H., Takita, Y.: Doped LaGaO3 perovskite type oxide as a new oxide ionic conductor. J. Am. Chem. Soc. 116, 3801–3803 (1994). https://doi.org/10.1021/ja00088a016
Yang, L., Wang, S.Z., Blinn, K., et al.: Enhanced sulfur and coking tolerance of a mixed ion conductor for SOFCs: BaZr0.1Ce0.7Y0.2−xYbxO3−δ. Science 326, 126–129 (2009). https://doi.org/10.1126/science.1174811
Nakayama, S., Aono, H., Sadaoka, Y.: Ionic conductivity of Ln10(SiO4)6O3 (Ln = La, Nd, Sm, Gd and Dy). Chem. Lett. 24, 431–432 (1995). https://doi.org/10.1246/cl.1995.431
Haugsrud, R., Norby, T.: Proton conduction in rare-earth ortho-niobates and ortho-tantalates. Nat. Mater. 5, 193–196 (2006). https://doi.org/10.1038/nmat1591
Li, M., Pietrowski, M.J., de Souza, R.A., et al.: A family of oxide ion conductors based on the ferroelectric perovskite Na0.5Bi0.5TiO3. Nat. Mater. 13, 31–35 (2014). https://doi.org/10.1038/nmat3782
Singh, P., Goodenough, J.B.: Monoclinic Sr1−xNaxSiO3–0.5x: new superior oxide ion electrolytes. J. Am. Chem. Soc. 135, 10149–10154 (2013). https://doi.org/10.1021/ja4042737
Norby, T.: The promise of protonics. Nature 410, 877–878 (2001). https://doi.org/10.1038/35073718
Duan, C.C., Kee, R.J., Zhu, H.Y., et al.: Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 557, 217–222 (2018). https://doi.org/10.1038/s41586-018-0082-6
An, H., Lee, H.W., Kim, B.K., et al.: A 5 × 5 cm2 protonic ceramic fuel cell with a power density of 1.3 W cm–2 at 600 ℃. Nat. Energy 3, 870–875 (2018). https://doi.org/10.1038/s41560-018-0230-0
Duan, C.C., Kee, R., Zhu, H.Y., et al.: Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 4, 230–240 (2019). https://doi.org/10.1038/s41560-019-0333-2
Zhu, B.: Advantages of intermediate temperature solid oxide fuel cells for tractionary applications. J. Power Sources 93, 82–86 (2001). https://doi.org/10.1016/S0378-7753(00)00564-4
Zhu, B.: Functional ceria-salt-composite materials for advanced ITSOFC applications. J. Power Sources 114, 1–9 (2003). https://doi.org/10.1016/S0378-7753(02)00592-X
Zhu, B., Yang, X.T., Xu, J., et al.: Innovative low temperature SOFCs and advanced materials. J. Power Sources 118, 47–53 (2003). https://doi.org/10.1016/S0378-7753(03)00060-0
Zhu, B.: Next generation fuel cell R&D. Int. J. Energy Res. 30, 895–903 (2006). https://doi.org/10.1002/er.1195
Wang, X.D., Ma, Y., Raza, R., et al.: Novel core-shell SDC/amorphous Na2CO3 nanocomposite electrolyte for low-temperature SOFCs. Electrochem. Commun. 10, 1617–1620 (2008). https://doi.org/10.1016/j.elecom.2008.08.023
Zhu, B., Li, S., Mellander, B.E.: Theoretical approach on ceria-based two-phase electrolytes for low temperature (300–600 ℃) solid oxide fuel cells. Electrochem. Commun. 10, 302–305 (2008). https://doi.org/10.1016/j.elecom.2007.11.037
Zhu, B.: Solid oxide fuel cell (SOFC) technical challenges and solutions from nano-aspects. Int. J. Energy Res. 33, 1126–1137 (2009). https://doi.org/10.1002/er.1600
Ma, Y., Wang, X.D., Li, S.H., et al.: Samarium-doped ceria nanowires: novel synthesis and application in low-temperature solid oxide fuel cells. Adv. Mater. 22, 1640–1644 (2010). https://doi.org/10.1002/adma.200903402
Raza, R., Wang, X.D., Ma, Y., et al.: Improved ceria-carbonate composite electrolytes. Int. J. Hydrog. Energy 35, 2684–2688 (2010). https://doi.org/10.1016/j.ijhydene.2009.04.038
Fan, L.D., Wang, C.Y., Chen, M.M., et al.: Recent development of ceria-based (nano)composite materials for low temperature ceramic fuel cells and electrolyte-free fuel cells. J. Power Sources 234, 154–174 (2013). https://doi.org/10.1016/j.jpowsour.2013.01.138
Fan, L.D., Zhang, G.Q., Chen, M.M., et al.: Proton and oxygen ionic conductivity of doped ceria-carbonate composite by modified Wagner polarization. Int. J. Electrochem. Sci. 7, 8420–8435 (2012). https://doi.org/10.1002/cvde.201206981
Zhu, B., Mat, M.D.: Studies on dual phase ceria-based composites in electrochemistry. Int. J. Electrochem. Sci. 1, 383–402 (2006).
Zhu, B., Albinsson, I., Andersson, C., et al.: Electrolysis studies based on ceria-based composites. Electrochem. Commun. 8, 495–498 (2006). https://doi.org/10.1016/j.elecom.2006.01.011
Zhu, B., Liu, X.R., Schober, T.: Novel hybrid conductors based on doped ceria and BCY20 for ITSOFC applications. Electrochem. Commun. 6, 378–383 (2004). https://doi.org/10.1016/j.elecom.2004.01.015
Huang, J.B., Mao, Z.Q., Liu, Z.X., et al.: Development of novel low-temperature SOFCs with co-ionic conducting SDC-carbonate composite electrolytes. Electrochem. Commun. 9, 2601–2605 (2007). https://doi.org/10.1016/j.elecom.2007.07.036
Zhao, Y.C., Xia, C., Wang, Y.J., et al.: Quantifying multi-ionic conduction through doped ceria-carbonate composite electrolyte by a current-interruption technique and product analysis. Int. J. Hydrog. Energy 37, 8556–8561 (2012). https://doi.org/10.1016/j.ijhydene.2012.02.053
Zhao, Y.C., Xia, C., Xu, Z.R., et al.: Validation of H+/O2− conduction in doped ceria-carbonate composite material using an electrochemical pumping method. Int. J. Hydrog. Energy 37, 11378–11382 (2012). https://doi.org/10.1016/j.ijhydene.2012.05.015
Zhao, Y.C., Xu, Z.R., Xia, C., et al.: Oxide ion and proton conduction in doped ceria-carbonate composite materials. Int. J. Hydrog. Energy 38, 1553–1559 (2013). https://doi.org/10.1016/j.ijhydene.2012.11.004
Xia, C., Li, Y., Tian, Y., et al.: Intermediate temperature fuel cell with a doped ceria-carbonate composite electrolyte. J. Power Sources 195, 3149–3154 (2010). https://doi.org/10.1016/j.jpowsour.2009.11.104
Xia, C., Li, Y., Tian, Y., et al.: A high performance composite ionic conducting electrolyte for intermediate temperature fuel cell and evidence for ternary ionic conduction. J. Power Sources 188, 156–162 (2009). https://doi.org/10.1016/j.jpowsour.2008.11.068
Zhang, G.H., Li, W.J., Huang, W., et al.: Strongly coupled Sm0.2Ce0.8O\(_{2^-}\)Na2CO3 nanocomposite for low temperature solid oxide fuel cells: one-step synthesis and super interfacial proton conduction. J. Power Sources 386, 56–65 (2018). https://doi.org/10.1016/j.jpowsour.2018.03.035
Wang, X.D., Ma, Y., Zhu, B.: State of the art ceria-carbonate composites (3C) electrolyte for advanced low temperature ceramic fuel cells (LTCFCs). Int. J. Hydrog. Energy 37, 19417–19425 (2012). https://doi.org/10.1016/j.ijhydene.2011.09.096
Benamira, M., Ringuedé, A., Hildebrandt, L., et al.: Gadolinia-doped ceria mixed with alkali carbonates for SOFC applications: II—an electrochemical insight. Int. J. Hydrog. Energy 37, 19371–19379 (2012). https://doi.org/10.1016/j.ijhydene.2011.10.062
Afzal, M., Raza, R., Du, S.F., et al.: Synthesis of Ba0.3Ca0.7Co0.8Fe0.2O3-δ composite material as novel catalytic cathode for ceria-carbonate electrolyte fuel cells. Electrochimica Acta 178, 385–391 (2015). https://doi.org/10.1016/j.electacta.2015.07.183
Sun, X.L., Li, S., Sun, J.C., et al.: Electrochemical performances of BSCF cathode materials for ceria-composite electrolyte low temperature solid oxide fuel cells. Int. J. Electrochem. Sci. 2, 462–468 (2007)
Asghar, M.I., Heikkilä, M., Lund, P.D.: Advanced low-temperature ceramic nanocomposite fuel cells using ultra high ionic conductivity electrolytes synthesized through freeze-dried method and solid-route. Mater. Today Energy 5, 338–346 (2017). https://doi.org/10.1016/j.mtener.2017.07.017
Rajesh, S., Pereira, J.R.S., Figueiredo, F.M.L., et al.: Performance of carbonate - LaCoO3 and La0.8Sr0.2Co0.2Fe0.8O3-δ composite cathodes under carbon dioxide. Electrochimica Acta 125, 435–442 (2014). https://doi.org/10.1016/j.electacta.2014.01.157
Huang, J.B., Gao, R.F., Mao, Z.Q., et al.: Investigation of La2NiO4+δ-based cathodes for SDC-carbonate composite electrolyte intermediate temperature fuel cells. Int. J. Hydrog. Energy 35, 2657–2662 (2010). https://doi.org/10.1016/j.ijhydene.2009.04.022
Xia, C., Mi, Y.Q., Wang, B.Y., et al.: Shaping triple-conducting semiconductor BaCo0.4Fe0.4Zr0.1Y0.1O3-δ into an electrolyte for low-temperature solid oxide fuel cells. Nat. Commun. 10, 1707 (2019). https://doi.org/10.1038/s41467-019-09532-z
Xing, Y.M., Wu, Y., Li, L.Y., et al.: Proton shuttles in CeO2/CeO2–δ core–shell structure. ACS Energy Lett. 4, 2601–2607 (2019). https://doi.org/10.1021/acsenergylett.9b01829
Kim, J., Jun, A., Gwon, O., et al.: Hybrid-solid oxide electrolysis cell: a new strategy for efficient hydrogen production. Nano Energy 44, 121–126 (2018). https://doi.org/10.1016/j.nanoen.2017.11.074
Wang, B.Y., Zhu, B., Yun, S.N., et al.: Fast ionic conduction in semiconductor CeO2-δ electrolyte fuel cells. NPG Asia Mater. 11, 51 (2019). https://doi.org/10.1038/s41427-019-0152-8
Duncan, K.L., Lee, K.T., Wachsman, E.D.: Dependence of open-circuit potential and power density on electrolyte thickness in solid oxide fuel cells with mixed conducting electrolytes. J. Power Sources 196, 2445–2451 (2011). https://doi.org/10.1016/j.jpowsour.2010.10.034
Riess, I.: The possible use of mixed ionic electronic conductors instead of electrolytes in fuel cells. Solid State Ion. 52, 127–134 (1992). https://doi.org/10.1016/0167-2738(92)90098-A
Zhu, B., Ma, Y., Wang, X.D., et al.: A fuel cell with a single component functioning simultaneously as the electrodes and electrolyte. Electrochem. Commun. 13, 225–227 (2011). https://doi.org/10.1016/j.elecom.2010.12.019
Zhu, B., Qin, H.Y., Raza, R., et al.: A single-component fuel cell reactor. Int. J. Hydrog. Energy 36, 8536–8541 (2011). https://doi.org/10.1016/j.ijhydene.2011.04.082
Zhu, B., Raza, R., Abbas, G., et al.: An electrolyte-free fuel cell constructed from one homogenous layer with mixed conductivity. Adv. Funct. Mater. 21, 2465–2469 (2011). https://doi.org/10.1002/adfm.201002471
Zhu, B., Raza, R., Qin, H.Y., et al.: Single-component and three-component fuel cells. J. Power Sources 196, 6362–6365 (2011). https://doi.org/10.1016/j.jpowsour.2011.03.078
Zhu, B., Raza, R., Qin, H.Y., et al.: Fuel cells based on electrolyte and non-electrolyte separators. Energy Environ. Sci. 4, 2986 (2011). https://doi.org/10.1039/c1ee01202a
He, H.P., Huang, X.J., Chen, L.Q.: A practice of single layer solid oxide fuel cell. Ionics 6, 64–69 (2000). https://doi.org/10.1007/BF02375548
He, H.P., Huang, X.J., Chen, L.Q.: Sr-doped LaInO3 and its possible application in a single layer SOFC. Solid State Ion. 130, 183–193 (2000). https://doi.org/10.1016/S0167-2738(00)00666-4
Lan, R., Tao, S.W.: Novel proton conductors in the layered oxide material LixAl0.5Co0.5O2. Adv. Energy Mater. 4: 1301683. (2014). https://doi.org/10.1002/aenm.201401461
Dong, W.J., Yaqub, A., Janjua, N.K., et al.: All in one multifunctional perovskite material for next generation SOFC. Electrochim. Acta 193, 225–230 (2016). https://doi.org/10.1016/j.electacta.2016.02.061
Dong, W.J., Tong, Y.Z., Zhu, B., et al.: Semiconductor TiO2 thin film as an electrolyte for fuel cells. J. Mater. Chem. A 7, 16728–16734 (2019). https://doi.org/10.1039/c9ta01941c
Xia, C., Qiao, Z., Shen, L.P., et al.: Semiconductor electrolyte for low-operating-temperature solid oxide fuel cell: Li-doped ZnO. Int. J. Hydrog. Energy 43, 12825–12834 (2018). https://doi.org/10.1016/j.ijhydene.2018.04.121
Chen, G., Zhu, B., Deng, H., et al.: Advanced fuel cell based on perovskite La–SrTiO3 semiconductor as the electrolyte with superoxide-ion conduction. ACS Appl. Mater. Interfaces 10, 33179–33186 (2018). https://doi.org/10.1021/acsami.8b10087
Chen, G., Liu, H.L., He, Y., et al.: Electrochemical mechanisms of an advanced low-temperature fuel cell with a SrTiO3 electrolyte. J. Mater. Chem. A 7, 9638–9645 (2019). https://doi.org/10.1039/c9ta00499h
Shi, Y.N., Wang, L.Y., Wang, Z.Y., et al.: Defect engineering for tuning the photoresponse of ceria-based solid oxide photoelectrochemical cells. ACS Appl. Mater. Interfaces 13, 541–551 (2021). https://doi.org/10.1021/acsami.0c17921
Zhu, B., Raza, R., Liu, Q.H., et al.: A new energy conversion technology joining electrochemical and physical principles. RSC Adv. 2, 5066 (2012). https://doi.org/10.1039/c2ra01234k
Zhu, B., Lund, P., Raza, R., et al.: A new energy conversion technology based on nano-redox and nano-device processes. Nano Energy 2, 1179–1185 (2013). https://doi.org/10.1016/j.nanoen.2013.05.001
Wang, G.J., Wu, X.Y., Cai, Y.X., et al.: Design, fabrication and characterization of a double layer solid oxide fuel cell (DLFC). J. Power Sources 332, 8–15 (2016). https://doi.org/10.1016/j.jpowsour.2016.09.011
Shao, K., Li, F.J., Zhang, G.H., et al.: Approaching durable single-layer fuel cells: promotion of electroactivity and charge separation via nanoalloy redox exsolution. ACS Appl. Mater. Interfaces 11, 27924–27933 (2019). https://doi.org/10.1021/acsami.9b08448
Fan, L.D., Wang, C.Y., Osamudiamen, O., et al.: Mixed ion and electron conductive composites for single component fuel cells: I. Effects of composition and pellet thickness. J. Power Sources 217, 164–169 (2012). https://doi.org/10.1016/j.jpowsour.2012.05.045
Wu, J.S., Zhu, B., Mi, Y.Q., et al.: A novel core-shell nanocomposite electrolyte for low temperature fuel cells. J. Power Sources 201, 164–168 (2012). https://doi.org/10.1016/j.jpowsour.2011.10.084
Cai, Y.X., Chen, Y., Akbar, M., et al.: A bulk-heterostructure nanocomposite electrolyte of Ce0.8Sm0.2O2-δ-SrTiO3 for low-temperature solid oxide fuel cells. Nano Micro Lett. 13, 1–14 (2021). https://doi.org/10.1007/s40820-020-00574-3
Rauf, S., Zhu, B., Yousaf Shah, M.A.K., et al.: Application of a triple-conducting heterostructure electrolyte of Ba0.5Sr0.5Co0.1Fe0.7Zr0.1Y0.1O3–δ and Ca0.04Ce0.80Sm0.16O2–δ in a high-performance low-temperature solid oxide fuel cell. ACS Appl. Mater. Interfaces 12, 35071–35080 (2020). https://doi.org/10.1021/acsami.0c10061
Lu, Y.Z., Akbar, M., Xia, C., et al.: Catalytic membrane with high ion-electron conduction made of strongly correlated perovskite LaNiO3 and Ce0.8Sm0.2O2-δ for fuel cells. J. Catal. 386, 117–125 (2020). https://doi.org/10.1016/j.jcat.2020.04.004
Wu, Y., Dong, B., Zhang, J., et al.: The synthesis of ZnO/SrTiO3 composite for high-efficiency photocatalytic hydrogen and electricity conversion. Int. J. Hydrog. Energy 43, 12627–12636 (2018). https://doi.org/10.1016/j.ijhydene.2018.03.206
Cai, Y.X., Wang, B.Y., Wang, Y., et al.: Validating the technological feasibility of yttria-stabilized zirconia-based semiconducting-ionic composite in intermediate-temperature solid oxide fuel cells. J. Power Sources 384, 318–327 (2018). https://doi.org/10.1016/j.jpowsour.2018.03.012
Han, F., Yun, S.N., Shi, J., et al.: Efficient dual-function catalysts for triiodide reduction reaction and hydrogen evolution reaction using unique 3D network aloe waste-derived carbon-supported molybdenum-based bimetallic oxide nanohybrids. Appl. Catal. B: Environ. 273, 119004 (2020). https://doi.org/10.1016/j.apcatb.2020.119004
Fuel cells: Three in one. Nat. Nanotechnol. 6, 330 (2011). https://doi.org/10.1038/nnano.2011.93.
Zhu, B., Lund, P.D., Raza, R., et al.: Schottky junction effect on high performance fuel cells based on nanocomposite materials. Adv. Energy Mater. 5, 1401895 (2015). https://doi.org/10.1002/aenm.201401895
Afzal, M., Saleemi, M., Wang, B.Y., et al.: Fabrication of novel electrolyte-layer free fuel cell with semi-ionic conductor (Ba0.5Sr0.5Co0.8Fe0.2O3−δ- Sm0.2Ce0.8O1.9) and Schottky barrier. J. Power Sources 328, 136–142 (2016). https://doi.org/10.1016/j.jpowsour.2016.07.093
Zhu, B., Huang, Y.Z., Fan, L.D., et al.: Novel fuel cell with nanocomposite functional layer designed by perovskite solar cell principle. Nano Energy 19, 156–164 (2016). https://doi.org/10.1016/j.nanoen.2015.11.015
Zhu, B., Wang, B.Y., Wang, Y., et al.: Charge separation and transport in La0.6Sr0.4Co0.2Fe0.8O3-δ and ion-doping ceria heterostructure material for new generation fuel cell. Nano Energy 37, 195–202 (2017). https://doi.org/10.1016/j.nanoen.2017.05.003
Yun, S.N., Zhou, H.W., Wang, L., et al.: Economical hafnium oxygen nitride binary/ternary nanocomposite counter electrode catalysts for high-efficiency dye-sensitized solar cells. J. Mater. Chem. A 1, 1341–1348 (2013). https://doi.org/10.1039/c2ta00680d
Yun, S.N., Zhang, H., Pu, H.H., et al.: Metal oxide/carbide/carbon nanocomposites: in situ synthesis, characterization, calculation, and their application as an efficient counter electrode catalyst for dye-sensitized solar cells. Adv. Energy Mater. 3, 1407–1412 (2013). https://doi.org/10.1002/aenm.201300242
Yun, S.N., Wu, M.X., Wang, Y.D., et al.: Pt-like behavior of high-performance counter electrodes prepared from binary tantalum compounds showing high electrocatalytic activity for dye-sensitized solar cells. Chemsuschem 6, 411–416 (2013). https://doi.org/10.1002/cssc.201200845
Yun, S.N., Wang, L., Zhao, C.Y., et al.: A new type of low-cost counter electrode catalyst based on platinum nanoparticles loaded onto silicon carbide (Pt/SiC) for dye-sensitized solar cells. Phys. Chem. Chem. Phys. 15, 4286 (2013). https://doi.org/10.1039/c3cp44048f
Yun, S.N., Wang, L., Guo, W., et al.: Non-Pt counter electrode catalysts using tantalum oxide for low-cost dye-sensitized solar cells. Electrochem. Commun. 24, 69–73 (2012). https://doi.org/10.1016/j.elecom.2012.08.008
Vedhanarayanan, B., Shi, J., Lin, J.Y., et al.: Enhanced activity and stability of MoS2 through enriching 1T-phase by covalent functionalization for energy conversion applications. Chem. Eng. J. 403, 126318 (2021). https://doi.org/10.1016/j.cej.2020.126318
Yun, S.N., Si, Y.M., Shi, J., et al.: Electronic structures and catalytic activities of niobium oxides as electrocatalysts in liquid-junction photovoltaic devices. Sol. RRL 4, 1900430 (2020). https://doi.org/10.1002/solr.201900430
Filip, M.R., Hillman, S., Haghighirad, A.A., et al.: Band gaps of the lead-free halide double perovskites Cs2BiAgCl6 and Cs2BiAgBr6 from theory and experiment. J. Phys. Chem. Lett. 7, 2579–2585 (2016). https://doi.org/10.1021/acs.jpclett.6b01041
Pessoa, R.S., Fraga, M.A., Santos, L.V., et al.: Nanostructured thin films based on TiO2 and/or SiC for use in photoelectrochemical cells: a review of the material characteristics, synthesis and recent applications. Mater. Sci. Semicond. Process. 29, 56–68 (2015). https://doi.org/10.1016/j.mssp.2014.05.053
Xing, Y.M., Hu, E.Y., Wang, F.Z., et al.: Cubic silicon carbide/zinc oxide heterostructure fuel cells. Appl. Phys. Lett. 117, 162105 (2020). https://doi.org/10.1063/5.0021460
Mushtaq, N., Xia, C., Dong, W.J., et al.: Tuning the energy band structure at interfaces of the SrFe0.75Ti0.25O3−δ-Sm0.25Ce0.75O2−δ heterostructure for fast ionic transport. ACS Appl. Mater. Interfaces 11, 38737–38745 (2019). https://doi.org/10.1021/acsami.9b13044
Meng, Y.J., Wang, X.Y., Zhang, W., et al.: Novel high ionic conductivity electrolyte membrane based on semiconductor La0.65Sr0.3Ce0.05Cr0.5Fe0.5O3-δ for low-temperature solid oxide fuel cells. J. Power Sources 421, 33–40 (2019). https://doi.org/10.1016/j.jpowsour.2019.02.100
Chen, G., Sun, W.K., Luo, Y.D., et al.: Advanced fuel cell based on new nanocrystalline structure Gd0.1Ce0.9O2 electrolyte. ACS Appl. Mater. Interfaces 11, 10642–10650 (2019). https://doi.org/10.1021/acsami.8b20454
Wu, Y., Liu, L., Yu, X.X., et al.: Natural hematite ore composited with ZnO nanoneedles for energy applications. Compos. B: Eng. 137, 178–183 (2018). https://doi.org/10.1016/j.compositesb.2017.11.020
Qiao, Z., Xia, C., Cai, Y.X., et al.: Electrochemical and electrical properties of doped CeO2-ZnO composite for low-temperature solid oxide fuel cell applications. J. Power Sources 392, 33–40 (2018). https://doi.org/10.1016/j.jpowsour.2018.04.096
Xia, C., Cai, Y.X., Wang, B.Y., et al.: Strategy towards cost-effective low-temperature solid oxide fuel cells: a mixed-conductive membrane comprised of natural minerals and perovskite oxide. J. Power Sources 342, 779–786 (2017). https://doi.org/10.1016/j.jpowsour.2016.12.120
Wu, Y., Xia, C., Zhang, W., et al.: Natural hematite for next-generation solid oxide fuel cells. Adv. Funct. Mater. 26, 938–942 (2016). https://doi.org/10.1002/adfm.201503756
Wang, B.Y., Wang, Y., Fan, L.D., et al.: Preparation and characterization of Sm and Ca co-doped ceria–La0.6Sr0.4Co0.2Fe0.8O3–δsemiconductor–ionic composites for electrolyte-layer-free fuel cells. J. Mater. Chem. A 4, 15426–15436 (2016). https://doi.org/10.1039/c6ta05763b
Yun, S.N., Zhang, Y.W., Xu, Q., et al.: Recent advance in new-generation integrated devices for energy harvesting and storage. Nano Energy 60, 600–619 (2019). https://doi.org/10.1016/j.nanoen.2019.03.074
Yun, S.N., Qin, Y., Uhl, A.R., et al.: New-generation integrated devices based on dye-sensitized and perovskite solar cells. Energy Environ. Sci. 11, 476–526 (2018). https://doi.org/10.1039/c7ee03165c
Goodenough, J.B., Park, K.-S.: The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135(4), 1167–1176 (2013). https://doi.org/10.1021/ja3091438
Goodenough, J.B., Kim, Y.: Challenges for rechargeable Li batteries. Chem. Mater. 22, 587–603 (2010). https://doi.org/10.1021/cm901452z
Ensling, D., Cherkashinin, G., Schmid, S., et al.: Nonrigid band behavior of the electronic structure of LiCoO2 thin film during electrochemical Li deintercalation. Chem. Mater. 26, 3948–3956 (2014). https://doi.org/10.1021/cm501480b
Jaegermann, W., Klein, A., Mayer, T.: Interface engineering of inorganic thin-film solar cells - materials-science challenges for advanced physical concepts. Adv. Mater. 21, 4196–4206 (2009). https://doi.org/10.1002/adma.200802457
Maier, J.: Physical chemistry of ionic materials. Chichester, UK: John Wiley & Sons, Ltd, 2004. https://doi.org/10.1002/0470020229
Sato, N.: Mixed electrodes. Electrochemistry at Metal and Semiconductor Electrodes. Amsterdam: Elsevier, 1998: 373–389. https://doi.org/10.1016/b978-044482806-4/50011-8
Becker, D., Cherkashinin, G., Hausbrand, R., et al.: Adsorption of diethyl carbonate on LiCoO2 thin films: formation of the electrochemical interface. J. Phys. Chem. C 118, 962–967 (2014). https://doi.org/10.1021/jp405714x
Sun, Y.F., Kotiuga, M., Lim, D., et al.: Strongly correlated perovskite lithium ion shuttles. PNAS 115, 9672–9677 (2018). https://doi.org/10.1073/pnas.1805029115
Wang, C., Yun, S.N., Fan, Q.Y., et al.: A hybrid niobium-based oxide with bio-based porous carbon as an efficient electrocatalyst in photovoltaics: a general strategy for understanding the catalytic mechanism. J. Mater. Chem. A 7, 14864–14875 (2019). https://doi.org/10.1039/c9ta03540k
Yun, S.N., Zhou, X., Even, J., et al.: Theoretical treatment of CH3NH3PbI3 perovskite solar cells. Angewandte Chemie Int. Ed. 56, 15806–15817 (2017). https://doi.org/10.1002/anie.201702660
Yun, S.N., Hagfeldt, A., Ma, T.L.: Pt-free counter electrode for dye-sensitized solar cells with high efficiency. Adv. Mater. 26, 6210–6237 (2014). https://doi.org/10.1002/adma.201402056
Yun, S.N., Wang, X.L., Li, J.F., et al.: Investigation of dielectric relaxation mechanism in bismuth doped barium calcium titanate ceramics by dielectric and Raman spectroscopy. Mater. Chem. Phys. 116, 339–343 (2009). https://doi.org/10.1016/j.matchemphys.2009.02.057
Yun, S.N., Wang, X.L., Li, J.F., et al.: Linear dielectric response and dielectric relaxation behavior of bismuth-doped (Ba1–xCax/2Srx/2)TiO3 ceramics. Phys. Stat. Sol. (a) 206, 303–310 (2009). https://doi.org/10.1002/pssa.200824272
Yun, S.N., Wang, X.L.: Dielectric properties of (Ba1–2x Srx Cax)TiO3 ferroelectric ceramics. J. Electroceramics 21, 585–588 (2008). https://doi.org/10.1007/s10832-007-9252-x
Yun, S.N., Wang, X.L.: Dielectric properties of bismuth doped Ba1−xCaxTiO3 ceramics. Mater. Lett. 60, 2211–2213 (2006). https://doi.org/10.1016/j.matlet.2005.12.122
Braga, M.H., Ferreira, J.A., Murchison, A.J., et al.: Electric dipoles and ionic conductivity in a Na+ glass electrolyte. J. Electrochem. Soc. 164, A207–A213 (2016). https://doi.org/10.1149/2.0691702jes
Yan, C.S., Zhu, Y., Li, Y.T., et al.: Local built-in electric field enabled in carbon-doped Co3O4 nanocrystals for superior lithium-ion storage. Adv. Funct. Mater. 28, 1705951 (2018). https://doi.org/10.1002/adfm.201705951
Ni, J.F., Sun, M.L., Li, L.: Highly efficient sodium storage in iron oxide nanotube arrays enabled by built-in electric field. Adv. Mater. 31, 1902603 (2019). https://doi.org/10.1002/adma.201902603
Luo, W., Li, F., Li, Q., et al.: Heterostructured Bi2S3-Bi2O3 nanosheets with a built-in electric field for improved sodium storage. ACS Appl. Mater. Interfaces 10, 7201–7207 (2018). https://doi.org/10.1021/acsami.8b01613
Li, C., Dong, S., Tang, R., et al.: Heteroatomic interface engineering in MOF-derived carbon heterostructures with built-in electricfield effects for high performance Al-ion batteries. Energy Environ. Sci. 11, 3201–3211 (2018). https://doi.org/10.1039/c8ee01046c
Yun, S.N., Freitas, J.N., Nogueira, A.F., et al.: Dye-sensitized solar cells employing polymers. Prog. Polym. Sci. 59, 1–40 (2016). https://doi.org/10.1016/j.progpolymsci.2015.10.004
Shen, S.L., Yang, Y.P., Guo, L.J., et al.: A polarization model for a solid oxide fuel cell with a mixed ionic and electronic conductor as electrolyte. J. Power Sources 256, 43–51 (2014). https://doi.org/10.1016/j.jpowsour.2014.01.041
Zhang, H., Luo, Z.M., Liu, Y.L., et al.: Noble-metal-free Ni3C as co-catalyst on LaNiO3 with enhanced photocatalytic activity. Appl. Catal. B: Environ. 277, 119166 (2020). https://doi.org/10.1016/j.apcatb.2020.119166
Zeng, Q., Lai, Y.Q., Jiang, L.X., et al.: Integrated photorechargeable energy storage system: next-generation power source driving the future. Adv. Energy Mater. 10, 1903930 (2020). https://doi.org/10.1002/aenm.201903930
Ishii, Y., Kurimoto, K., Hosoe, K., et al.: Photo-rechargeable fuel cell using photo-hydrogenation reactions of quinone molecules. New J. Chem. 44, 2275–2280 (2020). https://doi.org/10.1039/c9nj04782d
Zhu, Z., Shi, X.M., Fan, G.L., et al.: Photo-energy conversion and storage in an aprotic Li-O2 battery. Angew. Chem. Int. Ed. 58, 19021–19026 (2019). https://doi.org/10.1002/anie.201911228
Zhu, D.D., Zhao, Q.C., Fan, G.L., et al.: Photoinduced oxygen reduction reaction boosts the output voltage of a zinc-air battery. Angew. Chem. Int. Ed. 58, 12460–12464 (2019). https://doi.org/10.1002/anie.201905954
Wang, K., Mo, Z.H., Tang, S.T., et al.: Photo-enhanced Zn–air batteries with simultaneous highly efficient in situ H2O2 generation for wastewater treatment. J. Mater. Chem. A 7, 14129–14135 (2019). https://doi.org/10.1039/c9ta04253a
Xu, X.Y., Zhao, H., Wang, R., et al.: Identification of few-layer ReS2 as photo-electro integrated catalyst for hydrogen evolution. Nano Energy 48, 337–344 (2018). https://doi.org/10.1016/j.nanoen.2018.03.078
Lv, J., Abbas, S.C., Huang, Y.Y., et al.: A photo-responsive bifunctional electrocatalyst for oxygen reduction and evolution reactions. Nano Energy 43, 130–137 (2018). https://doi.org/10.1016/j.nanoen.2017.11.020
Cai, C., Han, S.B., Caiyang, W.N., et al.: Tracing the origin of visible light enhanced oxygen evolution reaction. Adv. Mater. Interfaces 6, 1801543 (2019). https://doi.org/10.1002/admi.201801543
Liu, Z.R., Wang, L.W., Yu, X., et al.: Piezoelectric-effect-enhanced full-spectrum photoelectrocatalysis in p-n heterojunction. Adv. Funct. Mater. 29, 1807279 (2019). https://doi.org/10.1002/adfm.201807279
Chen, P., Li, G.R., Li, T.T., et al.: Solar-driven rechargeable lithium-sulfur battery. Adv. Sci. 6, 1900620 (2019). https://doi.org/10.1002/advs.201900620
Liu, X.R., Yuan, Y.F., Liu, J., et al.: Utilizing solar energy to improve the oxygen evolution reaction kinetics in zinc-air battery. Nat. Commun. 10, 1–10 (2019). https://doi.org/10.1038/s41467-019-12627-2
Liu, Y., Li, N., Liao, K.M., et al.: Lowering the charge voltage of Li–O2 batteries via an unmediated photoelectrochemical oxidation approach. J. Mater. Chem. A 4, 12411–12415 (2016). https://doi.org/10.1039/c6ta03583c
Zhang, B.Q., Wang, S.Y., Fan, W.J., et al.: Photoassisted oxygen reduction reaction in H2–O2 fuel cells. Angew. Chem. Int. Ed. 55, 14748–14751 (2016). https://doi.org/10.1002/anie.201607118
Paolella, A., Faure, C., Bertoni, G., et al.: Light-assisted delithiation of lithium iron phosphate nanocrystals towards photo-rechargeable lithium ion batteries. Nat. Commun. 8, 1–10 (2017). https://doi.org/10.1038/ncomms14643
Liu, Y., Li, N., Wu, S.C., et al.: Reducing the charging voltage of a Li–O2 battery to 1.9 V by incorporating a photocatalyst. Energy Environ. Sci. 8, 2664–2667 (2015). https://doi.org/10.1039/c5ee01958c
Wang, B., Zhang, H., Lu, X.Y., et al.: Solar photocatalytic fuel cell using CdS-TiO2 photoanode and air-breathing cathode for wastewater treatment and simultaneous electricity production. Chem. Eng. J. 253, 174–182 (2014). https://doi.org/10.1016/j.cej.2014.05.041
Shan, X., Ke, O.Y., Yan, S.: A solar responsive photocatalytic fuel cell with a heterostructured ZnFe2O4/TiO2-NTs photoanode and an air-breathing cathode. Int. J. Hydrog. Energy 42, 29201–29209 (2017). https://doi.org/10.1016/j.ijhydene.2017.10.059
Huang, J.S., Yuan, Y.B., Shao, Y.C., et al.: Understanding the physical properties of hybrid perovskites for photovoltaic applications. Nat. Rev. Mater. 2, 17042 (2017). https://doi.org/10.1038/natrevmats.2017.42
Aristidou, N., Eames, C., Sanchez-Molina, I., et al.: Fast oxygen diffusion and iodide defects mediate oxygen-induced degradation of perovskite solar cells. Nat. Commun. 8, 15218 (2017). https://doi.org/10.1038/ncomms15218
Li, C., Guerrero, A., Huettner, S., et al.: Unravelling the role of vacancies in lead halide perovskite through electrical switching of photoluminescence. Nat. Commun. 9, 5113 (2018). https://doi.org/10.1038/s41467-018-07571-6
Eames, C., Frost, J.M., Barnes, P.R.F., et al.: Ionic transport in hybrid lead iodide perovskite solar cells. Nat. Commun. 6, 7497 (2015). https://doi.org/10.1038/ncomms8497
Walsh, A., Stranks, S.D.: Taking control of ion transport in halide perovskite solar cells. ACS Energy Lett. 3, 1983–1990 (2018). https://doi.org/10.1021/acsenergylett.8b00764
Wang, B.Y., Cai, Y.X., Dong, W.J., et al.: Photovoltaic properties of LixCo3−xO4/TiO2 heterojunction solar cells with high open-circuit voltage. Sol. Energy Mater. Sol. Cells 157, 126–133 (2016). https://doi.org/10.1016/j.solmat.2016.05.036
Christoforidis, K.C., Fornasiero, P.: Photocatalytic hydrogen production: a rift into the future energy supply. ChemCatChem 9, 1523–1544 (2017). https://doi.org/10.1002/cctc.201601659
Funding
This work was supported by the National Natural Science Foundation of China (51772080, 51672208, 51774259, and 51402093), the Natural Science Foundation of Guangdong Province (2021A1515012356 and 2017A030313289) and the project foundation from the Ministry of Education of Guangdong Province (2019KTSCX151), Shenzhen Government Plan of Science and Technology (JCYJ20180305125247308), the National Laboratory of Solid State Microstructures, Nanjing University, EPSRC (EP/I013229/1), Royal Society and Newton Fund (NAF\R1\191294), and Key Program for International S&T Cooperation Projects of Shaanxi Province (2019JZ-20, 2019KWZ-03). The leading author Prof. Bin Zhu acknowledges the Hubei Provincial 100-Talent Distinguished Professor Grant at the China University of Geoscience and Hubei University. Authors thank PhD student Jingjing Yang for correcting. References by using Endnote and getting high-resolution Figures from the original literatures.
Author information
Authors and Affiliations
Contributions
BZ Conceptualization, Supervision, Original draft preparation, Writing-Review & editing, Project administration, Funding acquisition. LF Conceptualization, Literature survey & analysis, Original draft preparation, Writing—Review & editing, Funding acquisition. NM Original draft preparation and editing, Literature survey & analysis. RR Writing—Review & editing, Figure edition, Literature survey. MS Figure re-edition and beautification. YW Literature survey & analysis, Writing—Review & editing. WL Writing—Review & editing. J-SK Writing—Review & editing, Funding acquisition. PDL Writing—Review & editing. SY Literature survey, Original draft preparation, Writing—Review & editing, Funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
All authors claim no conflicts of interest.
Additional information
The original online version of this article was revised: affiliation 5 and 6 were not correct.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, B., Fan, L., Mushtaq, N. et al. Semiconductor Electrochemistry for Clean Energy Conversion and Storage. Electrochem. Energy Rev. 4, 757–792 (2021). https://doi.org/10.1007/s41918-021-00112-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41918-021-00112-8