Abstract
Evaluation of a team-based intervention in primary care that includes cognitive behavioural therapy elements and case management and was supported by eHealth components in patients with panic disorder with or without agoraphobia (PD ± AG) and/or depression. In a two-armed cluster-randomised controlled trial (cRCT), mental health conditions were assessed by the Mental Health Index (MHI-5), Patient Health Questionnaire (PHQ-9), Overall Anxiety Severity and Impairment Scale (OASIS), Panic and Agoraphobia Scale (PAS), Mobility Inventory for Agoraphobia (MIA), and Patient Assessment of Chronic Illness Care (PACIC) at baseline (T0), after 6 months (T1), and after 12 months (T2). Scores were analysed as differences from baseline using a mixed linear model with general practitioner (GP) as a random intercept and treatment, time point, and respective baseline value as fixed factors. The majority of participants (mean age 54 years, SD 12.8 years) were women (n = 40, 67.8%). We found consistent mean effects in favour of the intervention group (MHI-5 index, 6.66 [−7.38; 20.70]; PACIC, 15.92 [4.58; 27.26]; PHQ-9, −3.43 [−5.71; −1.14]; OASIS, −2.89 [−5.41; −0.37]). A cautious interpretation indicates promising effects of the intervention. Obstacles to recruitment included the workload for GPs and medical assistants (MAs), potential reservations regarding eHealth, and the onset of the COVID-19 pandemic.
Trial registration:
The study was registered at the German Clinical Trials Register (DRKS00016622) on February 22nd, 2019. https://drks.de/search/de/trial/DRKS00016622.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Panic disorder (PD) is an anxiety disorder characterised by reoccurring unexpected episodes of intense fear (panic attacks) accompanied by physical symptoms that may include sweating, chest pain, heart palpitations, shortness of breath, dizziness, or abdominal distress (Nay et al., 2013). The maximum degree of symptoms occurs within minutes. There may be ongoing worries about having further attacks, especially in public, and avoidance of places where attacks have occurred in the past (Roy-Byrne et al., 2006). PD can be complicated by agoraphobia, a type of anxiety disorder that occurs when one is in a public or crowded place, from which a potential escape is difficult or help may not be readily available. It involves the fear that a panic attack or panic-like symptoms may occur in these situations, accompanied with feelings of being trapped, helpless, or embarrassed. Individuals with agoraphobia, therefore, strive to avoid such situations or locations, e.g. using public transportation, being in open or enclosed spaces, standing in line, or being in a crowd. Panic disorder with or without agoraphobia (PD± AG) is classified within the International Statistical Classification of Diseases and Related Health Problems (ICD)-10 (F41.0, F40.01). PD affects about 1.7–5% of people at some point in their life (De Jonge et al., 2016; Roy-Byrne et al., 2006). Women are more often affected than men (Roy-Byrne et al., 2006). Depression (ICD-10 F32-F34) is a mental disorder characterised by sadness, loss of interest or pleasure, feelings of guilt, low self-worth or hopelessness, disturbed sleep or appetite, feelings of tiredness, and poor concentration. Some people experience physical aches or pains, cramps, or digestive problems that do not have a clear physical cause and do not go away with treatment. Thoughts of death can occur which can lead to suicide attempts and suicide. To be diagnosed with depression, people must experience some of these signs and symptoms for at least 2 weeks most of the day or nearly every day. The average lifetime prevalence of depression in the general population is 10.8% (Lim et al., 2018), with a higher prevalence in women (Kuehner, 2017).
In primary care settings, PD and depression are common mental disorders, with prevalences of 4–7% (Kroenke et al., 2007; Roy-Byrne et al., 2005) for PD and 13.1% for depression (Jackson et al., 2022). Clinical and epidemiological studies have found a high comorbidity rate between PD and depression (Lamers et al., 2011; Rudden et al., 2003). Nevertheless, there exists conflicting data regarding the precise co-occurrence rate of these two conditions: Bystritsky et al. (2010) estimated that 32 to 70% of individuals may exhibit both disorders concurrently (Bystritsky et al., 2010). Another study reported a lifetime comorbidity rate of 63% between PD and major depressive disorder (Tilli et al., 2012), while Hirschfeld (2001) found that 12.6% of people with panic attacks went on to develop major depression (Barrio-Martínez et al., 2021; Hirschfeld, 2001).
A high utilisation of services is particularly notable among patients with PD comorbid with other psychiatric conditions (Simon & Fischmann, 2005). Despite the help-seeking behaviour of patients, correctly identifying and treating depression and PD can be difficult for the general practitioner (GP) due to the often unspecific symptoms (Hanel et al., 2009; Mitchell et al., 2009; Mitchell et al., 2011; Olariu et al., 2015), and thus, these disorders remain underrecognized (Tylee & Walters, 2007). GPs offer guidance based on their intuitive understanding of patients’ issues, which may draw from personal experiences rather than formal mental health training (Dahli et al., 2022). For the GP, the care of patients with mental disorders such as depression and PD ± AG is a time- and resource-intensive challenge; referral to a specialist might be an option, but waiting lists are long and patients are often reluctant to accessing specialised care for mental health problems due to the stigma that still surrounds mental health (Archer et al., 2021). Thus, the GP is often the patient’s first and only contact.
The German national guidelines for the treatment of unipolar depression ("Nationale VersorgungsLeitlinie Unipolare Depression – Leitlinienreport, Version 3.0. 2022", 2022) and anxiety disorder (Bandelow et al., 2021) mention cognitive behavioural therapy (CBT) elements for the use in primary care. In short, CBT aims to correct problem behaviour in patients through an individual problem analysis and psychoeducation, as well as to develop an improved problem-solving repertoire including activity planning, daily structure, and pleasure goal setting. It also includes relapse prevention (Margraf et al., 1993). CBT shows effectiveness for a variety of mental illnesses, including affective and anxiety-related disorders (Hofmann et al., 2012; Van Dis et al., 2020). Several randomised controlled trials have shown that CBT works well in the treatment of PD ± AG or depression (Gensichen et al., 2019; Lepping et al., 2017; López-López et al., 2019; Pompoli et al., 2016; Van Dis et al., 2020), even when provided by a non-specialist (Parker et al., 2021).
Case management programs (CMP) in primary care have shown potential for improving the quality, efficiency, and effectiveness of healthcare delivery, patient outcomes, and provider work experience while reducing costs for frequent users with complex needs (Hudon et al., 2019). In this collaborative approach, a case manager—often a nurse or medical assistant (MA)—evaluates and plans assessments and coordinates services, prioritising individual needs while providing self-management support (Hudon et al., 2022). Case management consists of five essential components: (1) identification of patients in need of services, (2) assessment of individual patient needs, (3) developing a treatment plan, (4) coordination of care, and (5) monitoring outcomes and altering care when favourable outcomes are not achieved (Norris et al., 2002). A structured care programme including a MA as case manager for depression and PD is effective in routine GP care (Gensichen et al., 2009; Gensichen et al., 2019). In addition, eHealth services such as internet-based interventions for anxiety disorders and depression were effective in reducing symptomatology for both depression and anxiety, especially when guided by a trained expert (Saddichha et al., 2014). Despite an ongoing debate, there is still no consensus about the definition of eHealth (services) (van der Kleij et al., 2019). The widely cited definition by Eysenbach describes eHealth as health services and information delivered or enhanced through the Internet and related technologies (Eysenbach, 2001). Shaw et al. offer a more detailed view of eHealth, outlining three key functions: (a) inform, monitor, and track, which involves using eHealth technologies to observe and study health parameters; (b) interaction, focused on facilitating communication among healthcare stakeholders; and (c) data utilisation, which involves collecting and managing health and medical data for medical decision-making and intervention development (Shaw et al., 2017). In essence, eHealth includes not only mobile apps for tracking patient behaviour but also communication technology for information exchange and ‘big data’ research for risk assessment tool development. Monitoring, informing, and improving communication are particularly relevant to primary care providers. However, implementation of eHealth tools in primary care is challenging. Barriers include cost, privacy, security problems, and a lack of recognised standards for eHealth tools. Additionally, the attitude of GPs toward technology might be an issue, especially in Germany, where the average age of practicing physicians increased from 49.8 to 54.6 years between 2001 and 2021 (https://www.zi.de/detailansicht/mai-2023, accessed October 15th, 2023). eHealth technologies such as electronic health records (EHRs) and patient assessment tools are facilitators of case management (Teper et al., 2020). However, healthcare professionals, especially physicians, often require specific training in these technologies.
eHealth might be one of the solutions to improve the efficiency of care and might facilitate the shift toward personalised medicine, self-care, and collaborative decision-making in primary healthcare (Meier et al., 2013). To achieve this goal, specific requirements must be met to ensure the safety, evidence-based nature, and high quality of eHealth tools. Thus, in the present study, a practice team consisting of the GP and a MA as case manager was supported by eHealth components that supported them in the screening, diagnosis, and treatment of patients with depression or PD ± AG. This blended care approach, i.e. combining face-to-face care with remote options, personalised to the individual patient, is considered to have the potential to improve the quality and efficiency of care, while maintaining—or even improving—patient and provider satisfaction with care (van der Kleij et al., 2019). Security and data protection were ensured by our partner who developed the online content and provided the platform. Participating primary care teams were trained and also received manuals.
The aim of the study was to investigate a new intervention combining case management, CBT, and eHealth components in a primary care setting. An intervention group was compared with a control group regarding clinical parameters such as depression, panic disorder, and quality of chronic care.
Methods
Study Design and Subjects
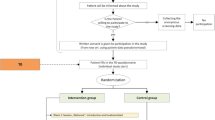
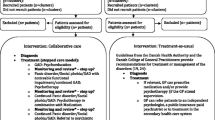
PREMA was a two-armed cluster randomised controlled trial (cRCT). The study was conducted in GP practices in Hesse, Germany. Randomisation was performed at cluster level with the practice as cluster; practices (and thus all recruited patients) were randomly assigned to either the intervention group (IG) or the control group (CG). In IG, patients were scheduled for four appointments (psychoeducation; interoceptive and situational exposure exercises; success monitoring; and relapse prophylaxis) with the GP and received case management and training over a period of 12 months, supported by the practice team and eHealth components. The case management by the MA consisted of 17 monitoring telephone calls (each about 10 min), in which the MA followed checklists (Gensichen et al., 2006; Hiller et al., 2018) and motivated the patients if necessary. In CG, patients received a standardised TAU: treatment based on the current German recommendations for the diagnosis and treatment of depression ("Nationale VersorgungsLeitlinie Unipolare Depression – Leitlinienreport, Version 3.0. 2022 ", 2022) and anxiety disorders (Bandelow et al., 2021).
A detailed study protocol was published previously (Lukaschek et al., 2019). In short, patients were eligible for the study if they had a primary diagnosis of PD ± AG (ICD-10 F41.0, F40.01) and/or depression (ICD-10 F32-34), were ≥ 18 years of age and able to give written informed consent, had sufficient knowledge of German, and had Internet and telephone connection at home. The study has been approved by the ethics committee of the Goethe-University, Frankfurt/Main, Germany. The study was registered at the German Clinical Trials Register (DRKS00016622).
Training of Practice Teams
Basic Training
All participating practice teams received general information on diagnosis, treatment, and management of PD ± AG and depression. The material provided was based on the national guidelines for depression ("Nationale VersorgungsLeitlinie Unipolare Depression – Leitlinienreport, Version 3.0. 2022", 2022) and for anxiety disorders (Bandelow et al., 2021). Participating practice teams were offered a training regarding study procedures, recruitments of patients, and the use of the eHealth components.
Training for Practices in the Intervention Group
Practices allocated to the IG were offered additional training including (1) rationale of exposure techniques in relation to PD ± AG and depression, (2) treatment plan, (3) structure of study materials, (4) case management, (5) information for physicians on the treatment process, (6) information for MAs on conducting telephone contacts with patients using a monitoring checklist.
Study Outcomes
Primary Outcome
The Mental Health Index-5 (MHI-5) was used to assess mental health disorders (depression and panic disorder) of the study participants (Berwick et al., 1991). Each of its five questions is answered on a five-point Likert scale (from 1 not at all to 5 more than five times a week/strongly; total range 5–25). A higher score indicates better mental health. Using a standard linear transformation, the score is transformed into a range of 0–100. The internal consistency (Cronbach’s α) ranges from 0.67 to 0.95 (Means-Christensen et al., 2005). The MHI-5 was validated in a primary care setting (Means-Christensen et al., 2005); ROC analyses showed that a cut-off score of 23 on the MHI-5 gave a sensitivity of 91% and a specificity of 58% for predicting the preliminary diagnosis of major depression or panic disorder. The MHI-5 was assessed on-line at baseline (T0) and at follow-up 6 (T1) and 12 (T2) months after study entry.
Secondary Outcomes
Depression measured by the Patient Health Questionnaire (PHQ-9) (score range, 0–27. Higher scores indicate more severe depressive symptoms) (Lowe et al., 2003); anxiety measured by the Overall Anxiety Severity and Impairment Scale (OASIS) (score range, 0–25. Higher scores indicate higher anxiety severity and greater clinical impairment) (Norman et al., 2011); number and severity of panic attacks, measured by two items (A1, A2) of the Panic and Agoraphobia Scale (PAS) (Bandelow, 1997) (PAS 1: score range, 1–5. Higher scores for more frequent panic attacks. PAS 2: score range, 1–5. Higher scores for more intense panic attacks); agoraphobic avoidance behaviour measured with the Mobility Inventory for Agoraphobia (MIA) (Chambless et al., 1985) (score range, 1–5. Higher scores indicate more intense agoraphobic avoidance behaviour); medical care assessment measured with the Patient Assessment of Chronic Illness Care (PACIC) (higher scores indicate better assessment of outpatient care) (Gugiu et al., 2009); medication adherence measured with the Morisky score (Morisky et al., 1986). PHQ-9 and OASIS were assessed at screening and at follow-up after 6 months (T1) and 12 months (T2). All other outcomes were assessed at baseline (T0), 6-month follow-up (T1), and 12-month follow-up (T2).
Planned Sample Size
We assumed an effect size on the primary outcome (MHI-5) of 0.2 standard deviations (SD), which is considered clinically relevant in the GP setting (Means-Christensen et al., 2005). With a power of 0.9, a two-sided α of 0.05, an intra-class correlation coefficient of 0.05, a correlation of baseline values with the primary outcome of 0.5, and assuming a drop-out rate of about 20% for the practices and about 20% for the patients, the aim was to recruit 1844 patients in 148 GP practices with an average of 12.5 patients per practice. Due to difficulties and delays in patient recruitment, a recalculation was carried out, accepting a lower power of 0.8 and an average of ten patients per practice; thus, the recruitment of a total of 1280 patients from 128 practices was targeted under otherwise identical assumptions.
Descriptions of Study Sample
Patients were recruited from 18 GP practices (9 IG, 9 CG). A total of 85 patients were included. Socio-demographic data were available from 59 patients (see Table 1). The majority of participants were female (n = 40, 67.8%); the mean age was 54 years (SD 12.8 years; women, 53.6 SD 12.0 years; men, 54.9 SD 14.5 years).
The IG and CG were sufficiently balanced regarding the demographic factors. The larger proportion of pensioners in the intervention group (33.3% in the IG, 0% in the CG) and the higher number of individuals living alone in the CG (36.4% CG, 14.6% IG) is noticeable.
Description and Evaluation of the Primary Outcome and Secondary Outcome
Characteristics of patients at baseline (T0) were analysed descriptively using appropriate measures (absolute and relative frequencies for categorical variables and mean ± standard deviation for continuous variables). Since randomisation was not performed at the patient level, the patient characteristics were also compared by means of statistical tests, whereby categorical variables were analysed by means of Fisher’s exact test and continuous variables by means of a t-test.
All outcome parameters were described separately by measurement point (T0, T1, and T2) and treatment group using mean and standard deviation.
The scores were analysed as differences from the baseline using a mixed linear model with GP as a random intercept and treatment, time point, and respective baseline value as fixed factors. Estimators for the group difference and the estimated mean values of the individual groups with the respective 95% confidence intervals (95% CI) as well as a p-value for the group comparison are presented. In addition, the model was also calculated with an interaction between the measurement point and the treatment group in order to detect possible differences between the measurement points. The interaction p-value as well as estimated group means of the separate time points with the respective 95% CIs and the p-value for the group comparison at the two time points are presented. These are to be regarded as exploratory. All statistical analyses are two-sided with an alpha of 0.05. The focus of the interpretation, however, lies on the effect estimators, which provide information about the magnitude of the possible intervention effect.
Results
Clinical Outcomes
Table 2 shows an overview of the clinical outcomes at all measurement points in IG and CG. All clinical outcomes showed higher (MHI-5, PACIC) or lower (PAS, MIA, PHG-9, OASIS) values at T2 compared to T0, indicating improvement of the condition measured, especially in the IG. Notably, the OASIS was higher at T2 than T0 in the CG. This pattern is preserved for the complete cases (n = 21, see supplement table 1).
Results of the statistical modelling are shown in Table 3. There was a difference in the development of the MHI-5 index in the follow-up compared to the baseline between the treatment groups of 6.66 [−7.38; 20.70] score points in favour of the intervention group (see Table 3).
We found consistent mean effects in favour of the intervention group in all other outcomes as well except for the MIA (see Table 3). Regarding the PACIC, there was a distinct difference between IG and CG across all measurement points (Table 3). In total, this corresponds to a difference of 15.92 [4.58; 27.26] score points in favour of the IG. For both depression and anxiety symptom severity (as measured by PHQ-9 and OASIS), the modelling showed a clear intervention effect over time of −3.43 [−5.71; −1.14] and −2.89 [−5.41; −0.37], respectively.
Dropouts
On the patient level, we see that recruitment success is quite differentiated between the groups. There were 65 patients in the IG, but only 20 patients in the CG. Only 48/65 = 73.8% in the IG and 11/20 = 55.0% in the CG provided baseline data. After baseline, the dropout rate was quite high: 25/48 = 52.1% IG and 3/11 = 27.3% in the CG did not provide any further data.
Subgroup Analyses
Since the total patient collective consisted of patients diagnosed with depression or panic disorder, we additionally performed separate analyses of PHQ-9 and OASIS in the subgroups of patients diagnosed with depression and patients diagnosed with panic disorder, respectively (Table 4). Complete cases (n = 21) follow the same pattern (see suppl. Table 2).
Despite the smaller sample size, substantial intervention effects could be seen (Table 5). Regarding depression, both cohorts (IG and CG) of the subgroup of patients with depression at baseline show a more pronounced symptom reduction (indicated by a decrease in the PHQ-9 score) compared to the total sample. The intervention effect is comparable to that of the total sample (−3.45 [−6.31; −0.60] vs. −3.43 [−5.71; −1.14]), corresponding to a by more than 3 points more pronounced average reduction of symptoms in the intervention group compared to control.
For anxiety symptom severity (OASIS), the changes in the IG were consistently larger compared to the total sample. In the CG, effects are comparable to the total sample, which altogether results in a more pronounced intervention effect compared to the total sample (−4.24 [−8.69; 0.20] vs. −2.89 [−5.41; −0.37]).
Discussion
The present trial aimed to evaluate the effect of a team-based intervention in primary care that includes cognitive behavioural therapy elements and case management supported by eHealth components in patients with PD ± AG and/or with depression, compared to standard care. The trial was confronted with problems due to the COVID-19 pandemic, and thus, the number of patients recruited was limited. Therefore, we presented descriptive results of the resulting proof-of-concept study.
On the premise that the intervention is carried out as planned, we see a medium effect for the primary endpoint (MHI-5) in favour of the intervention group. Exploratory promising tendencies were notable regarding PHQ-9, OASIS, and PACIC, indicating a reduction of depression and anxiety symptom severity as well as an improvement of patients’ assessment of chronic illness care.
It should be noted that there were certainly selection effects at both the practice and patient levels, but these cannot be investigated with the available data. In the sense of a proof-of-concept study, however, indications of positive effects could be found after implementation of the intervention. These should not be over-interpreted but can serve as a point of reference for further studies.
We identified several obstacles to recruitment of both practices and patients, which were largely attributable to the onset of the COVID-19 pandemic which required all the resources of the practices and prevented patients from visiting the practices. Further barriers might include potential existing reservations about eHealth components on the part of the practices and patients. The numerous measures taken by the study team to increase the number (opening the project to other health insurance companies, specific advertising measures for practices and patients, close support of practice teams) could not take effect due to the multi-year pandemic.
Although parts of the intervention were based on an online tool, this did not turn out to be an advantage during the COVID-19 pandemic. This might come as a surprise, since an eHealth component should have supported patients and GPs. However, we assume that the pandemic used up all the resources of the GPs, so sufficient familiarisation with the training material and the eHealth components as well as an adequate recruiting of patients was not possible. GPs seem to be reluctant to include eHealth components: A Dutch study could show that an increased uptake of eHealth in Dutch general practice during the COVID-19 pandemic was rather a temporary uptake of digital healthcare delivery than the accelerated implementation of digital processes (Keuper et al., 2021). We assume that many GPs are not yet comfortable with eHealth solutions since meaningful incorporation into medical education has been largely absent to date (Houwink et al., 2020). Uptake of eHealth could be encouraged by broadening the focus of eHealth education to encompass the entire primary care team, including nurse practitioners, practice assistants, GPs involved in continuing professional development, and trainees undergoing vocational training.
On the patients’ site, the higher PACIC levels despite the pandemic might indicate that patients felt comfortable with the intervention due to the eHealth component. However, user satisfaction with eHealth interactions is significantly associated with (e)Health Literacy (Duplaga & Turosz, 2022) which we did not assess.
Lessons To Be Learned
Based on these results, further study concepts are needed that make use of innovative recruiting strategies. Social media campaigns seem to be a promising way to reach potential participants (Brøgger-Mikkelsen et al., 2020; Reuter, 2020). Closer data monitoring should be considered as well as adaptive strategies in dealing with external context factors (such as the COVID-19 pandemic of the years 2020 to 2022).
The PREMA trial was an important step towards improving the care of patients with mental health disorders in primary care, in which some obstacles to a successful development and implementation were identified. Since implementation has not reached the expected level, we cannot make recommendations about the transferability of this new form of care to standard care.
Further research should focus on recruitment strategies and optimizing the intervention. Regarding the latter, a review by Granja et al. (Granja et al., 2018) identified six work-flow related barriers to successful implementation: (1) workload, (2) workflow disruption, (3) alignment with clinical processes, (4) undefined and changed roles, (5) undermined face-to-face communication, and (6) staff turnover (Granja et al., 2018). Our current intervention certainly contributed to the high workload of GPs and MFAs. Thus, in a further development of the PREMA intervention, workload should be reduced (e.g. fewer monitoring calls). Although our intention was that the intervention should not interrupt the GPs’ workflow (e.g. by its online components), we assume that at the beginning, workflow might have been disrupted by technical problems or by insufficient knowledge about the intervention. Moreover, the online components certainly undermined face-to-face communication, although we balanced this with regular appointments with the GP. Intervention roles were clearly defined and did not change over the course of the intervention. Moreover, by adhering to national guidelines, the study aligned with clinical processes. The last barrier—staff turnover—was certainly aggravated by COVID-related sick leaves.
The review by Granja et al. (Granja et al., 2018) also identified the quality of healthcare, such as improved diagnosis, clinical management, and patient-centred care, as the major facilitator of eHealth interventions. The PREMA intervention supported GPs in diagnosis-making by providing established and validated instruments. However, on the downside, this might have contributed to GPs’ workload (see above). On patients’ side, the level of empowerment and self-management were major contributors to the success of an intervention (Granja et al., 2018). Our intervention put great emphasis on patient self-management, which might have been overwhelming to some in times of COVID. Last but not least, costs also determine whether an intervention can be successfully implemented or not. The PREMA study attempted to determine differences in healthcare costs between the study groups. Unfortunately, no reliable cost data is available due to the small and likely biased sample. Intervention costs were not collected. Consequently, there are no indications so far on the possible implementation costs that could also differ from the intervention costs (Gold et al., 2022).
In subsequent studies, therefore, the differences in healthcare costs and, based on these, the cost-effectiveness of the PREMA intervention should first be determined, including the intervention costs. In a subsequent implementation study, the actual implementation costs can be determined, taking into account the associated fixed and variable costs
Conclusion
Based on the given limited sample size, this proof-of-concept study shows promising effects of a team-based intervention, combining cognitive behavioural therapy and case management supported by eHealth components in primary care patients with PD ± AG or with depression, compared to standard care. Further research should focus on recruitment strategies and optimizing the intervention for GPs and MFAs, especially regarding workflow and workload, and for patients, e.g. regarding eHealth literacy and self-management.
Data availability
Data cannot be shared publicly because of German data protection laws. The data that support the findings of this study are available from the consortia of the PREMA-study, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available upon reasonable request. Data requests may be directed at “Stiftung Allgemeinmedizin - The Primary Health care Foundation” ("http://www.stiftung-allgemeinmedizin.de"). Mail: office@stiftung-allgemeinmedizin.de.
References
Archer, C., Kessler, D., Wiles, N., & Turner, K. (2021). GPs’ and patients’ views on the value of diagnosing anxiety disorders in primary care: a qualitative interview study. British Journal of General Practice, 71(707), e450–e457.
Bandelow, B. (1997). Panik- und Agoraphobie-Skala (PAS). Hogrefe.
Bandelow, B., Aden, I., Alpers, G. W., Benecke, A., Benecke, C., Beutel, M. E., Deckert, J., Domschke, K., Eckhardt-Henn, A., Geiser, F., Gerlach, A. L., Harfst, T. Hau, S., Hoffmann, S., Hoyer, J., Hunger-Schoppe, C., Kellner, M., Köllner, V., Kopp, I. B., Langs, G., Liebeck, H., Matzat, J., Ohly, M., Rüddel, H. P., Rudolf, S., Scheufele, E., Simon, R., Staats, H., Ströhle, A., Waldherr, B., Wedekind, D., Werner, A. M., Wiltink, J., Wolters, J. P., Beutel M. E. (2021) Deutsche S3-Leitlinie Behandlung von Angststörungen, Version 2. Retrieved from: www.awmf.org/leitlinien/de-tail/ll/051-028.html
Barrio-Martínez, S., Cano-Vindel, A., Muñoz-Navarro, R., Moriana, J. A., Ruíz-Rodríguez, P., Medrano, L. A., Ventura, L., & González-Blanch, C. (2021). Factors differentiating panic disorder with and without comorbid symptoms of depression. Psychology and Psychotherapy: Theory, Research and Practice, 94(3), 523–540.
Berwick, D. M., Murphy, J. M., Goldman, P. A., Ware, J. E., Jr., Barsky, A. J., & Weinstein, M. C. (1991). Performance of a five-item mental health screening test. Medical Care, 29(2), 169–176.
Brøgger-Mikkelsen, M., Ali, Z., Zibert, J. R., Andersen, A. D., & Thomsen, S. F. (2020). Online patient recruitment in clinical trials: Systematic review and meta-analysis. Journal of medical Internet research, 22(11), e22179.
Bystritsky, A., Kerwin, L., Niv, N., Natoli, J. L., Abrahami, N., Klap, R., Wells, K., & Young, A. S. (2010). Clinical and subthreshold panic disorder. Depression and anxiety, 27(4), 381–389.
Chambless, D. L., Caputo, G. C., Jasin, S. E., Gracely, E. J., & Williams, C. (1985). The mobility inventory for agoraphobia. Behaviour Research and Therapy, 23(1), 35–44.
Dahli, M. P., Haavet, O. R., Ruud, T., & Brekke, M. (2022). Ps’ identification of patients with mental distress: A coupled questionnaire and cohort study from norwegian urban general practice. Primary Care, 23(1), 1–7.
De Jonge, P., Roest, A. M., Lim, C. C., Florescu, S. E., Bromet, E. J., Stein, D. J., Harris, M., Nakov, V., Caldas-de-Almeida, J. M., & Levinson, D. (2016). Cross-national epidemiology of panic disorder and panic attacks in the world mental health surveys. Depression and anxiety, 33(12), 1155–1177.
Duplaga, M., & Turosz, N. (2022). User satisfaction and the readiness-to-use e-health applications in the future in Polish society in the early phase of the COVID-19 pandemic: A cross-sectional study. International Journal of Medical Informatics, 168, 104904.
Eysenbach, G. (2001). What is e-health. Journal of Medical Internet Research, 3(2), e20.
Gensichen, J., Hiller, T. S., Breitbart, J., Brettschneider, C., Teismann, T., Schumacher, U., Lukaschek, K., Schelle, M., Schneider, N., Sommer, M., Wensing, M., Konig, H. H., Margraf, J., & Jena, P. S. G. (2019). Panic disorder in primary care. Deutsches Ärzteblatt International, 116(10), 159–166.
Gensichen, J., Peitz, M., Torge, M., Mosig-Frey, J., Wendt-Hermainski, H., Rosemann, T., Gerlach, F. M., & Lowe, B. (2006). The "Depression Monitoring list" (DeMoL) with integrated PHQ-D-Rationale and design of a tool for the case management for depression in primary care. Zeitschrift fur Arztliche Fortbildung und Qualitatssicherung, 100(5), 375–382.
Gensichen, J., von Korff, M., Peitz, M., Muth, C., Beyer, M., Guthlin, C., Torge, M., Petersen, J. J., Rosemann, T., Konig, J., Gerlach, F. M., & ProMpt. (2009). Case management for depression by health care assistants in small primary care practices: A cluster randomized trial. Annals of Internal Medicine, 151(6), 369–378.
Gold, H. T., McDermott, C., Hoomans, T., & Wagner, T. H. (2022). Cost data in implementation science: Categories and approaches to costing. Implementation Science, 17(1), 11.
Granja, C., Janssen, W., & Johansen, M. A. (2018). Factors determining the success and failure of eHealth interventions: Systematic review of the literature. Journal of medical Internet research, 20(5), e10235.
Gugiu, P. C., Coryn, C., Clark, R., & Kuehn, A. (2009). Development and evaluation of the short version of the Patient Assessment of Chronic Illness Care instrument. Chronic Illness, 5(4), 268–276.
Hanel, G., Henningsen, P., Herzog, W., Sauer, N., Schaefert, R., Szecsenyi, J., & Löwe, B. (2009). Depression, anxiety, and somatoform disorders: vague or distinct categories in primary care? Results from a large cross-sectional study. Journal of Psychosomatic Research, 67(3), 189–197.
Hiller, T. S., Freytag, A., Breitbart, J., Teismann, T., Schöne, E., Blank, W., Schelle, M., Vollmar, H. C., Margraf, J., & Gensichen, J. (2018). Die Jena Angst-Monitoring-Liste (JAMoL)-ein Instrument zur evidenzbasierten Behandlung von Panikstörung mit oder ohne Agoraphobie in der Hausarztpraxis. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen, 131, 28–37.
Hirschfeld, R. M. (2001). The comorbidity of major depression and anxiety disorders: recognition and management in primary care. Primary Care Companion to the Journal of Clinical Psychiatry, 3(6), 244.
Hofmann, S. G., Asnaani, A., Vonk, I. J., Sawyer, A. T., & Fang, A. (2012). The efficacy of cognitive behavioral therapy: A review of meta-analyses. Cognitive Therapy and Research, 36(5), 427–440.
Houwink, E. J., Kasteleyn, M. J., Alpay, L., Pearce, C., Butler-Henderson, K., Meijer, E., van Kampen, S., Versluis, A., Bonten, T. N., & van Dalfsen, J. H. (2020). SERIES: eHealth in primary care. Part 3: eHealth education in primary care. European Journal of General Practice, 26(1), 108–118.
Hudon, C., Chouinard, M.-C., Bisson, M., Brousselle, A., Lambert, M., Danish, A., Rodriguez, C., & Sabourin, V. (2022). Case management programs for improving integrated care for frequent users of healthcare services: An implementation analysis. International Journal of Integrated Care, 22(1), 1–13.
Hudon, C., Chouinard, M.-C., Pluye, P., El Sherif, R., Bush, P. L., Rihoux, B., Poitras, M.-E., Lambert, M., Zomahoun, H. T. V., & Légaré, F. (2019). Characteristics of case management in primary care associated with positive outcomes for frequent users of health care: a systematic review. The Annals of Family Medicine, 17(5), 448–458.
Jackson, J. L., Kuriyama, A., Bernstein, J., & Demchuk, C. (2022). Depression in primary care, 2010-2018. The American Journal of Medicine, 135(12), 1505–1508.
Keuper, J., Batenburg, R., Verheij, R., & Van Tuyl, L. (2021). Use of E-health in Dutch general practice during the COVID-19 pandemic. International Journal of Environmental Research and Public Health, 18(23), 12479.
Kroenke, K., Spitzer, R. L., Williams, J. B., Monahan, P. O., & Lowe, B. (2007). Anxiety disorders in primary care: Prevalence, impairment, comorbidity, and detection. Annals of Internal Medicine, 146(5), 317–325.
Kuehner, C. (2017). Why is depression more common among women than among men? The Lancet Psychiatry, 4(2), 146–158.
Lamers, F., van Oppen, P., Comijs, H. C., Smit, J. H., Spinhoven, P., van Balkom, A. J., Nolen, W. A., Zitman, F. G., Beekman, A. T., & Penninx, B. W. (2011). Comorbidity patterns of anxiety and depressive disorders in a large cohort study: The Netherlands Study of Depression and Anxiety (NESDA). The Journal of Clinical Psychiatry, 72(3), 341–348.
Lepping, P., Whittington, R., Sambhi, R. S., Lane, S., Poole, R., Leucht, S., Cuijpers, P., McCabe, R., & Waheed, W. (2017). Clinical relevance of findings in trials of CBT for depression. European Psychiatry, 45, 207–211.
Lim, G. Y., Tam, W. W., Lu, Y., Ho, C. S., Zhang, M. W., & Ho, R. C. (2018). Prevalence of depression in the community from 30 countries between 1994 and 2014. Scientific Reports, 8(1), 2861.
López-López, J. A., Davies, S. R., Caldwell, D. M., Churchill, R., Peters, T. J., Tallon, D., Dawson, S., Wu, Q., Li, J., & Taylor, A. (2019). The process and delivery of CBT for depression in adults: A systematic review and network meta-analysis. Psychological Medicine, 49(12), 1937–1947.
Lowe, B., Grafe, K., Zipfel, S., Spitzer, R. L., Herrmann-Lingen, C., Witte, S., & Herzog, W. (2003). Detecting panic disorder in medical and psychosomatic outpatients: Comparative validation of the hospital anxiety and depression scale, the patient health questionnaire, a screening question, and physicians’ diagnosis. The Journal of Psychosomatic Research, 55(6), 515–519.
Lukaschek, K., Mergenthal, K., Heider, D., Hanke, A., Munski, K., Moschner, A., Emig, M., van den Akker, M., Zapf, A., & Wegscheider, K. (2019). eHealth-supported case management for patients with panic disorder or depression in primary care: Study protocol for a cRCT (PREMA). Trials, 20, 1–11.
Margraf, J., Barlow, D. H., Clark, D. M., & Telch, M. J. (1993). Psychological treatment of panic: Work in progress on outcome, active ingredients, and follow-up. Behaviour Research and Therapy, 31(1), 1–8.
Means-Christensen, A. J., Arnau, R. C., Tonidandel, A. M., Bramson, R., & Meagher, M. W. (2005). An efficient method of identifying major depression and panic disorder in primary care. Journal of Behavioral Medicine, 28(6), 565–572.
Meier, C. A., Fitzgerald, M. C., & Smith, J. M. (2013). eHealth: extending, enhancing, and evolving health care. Annual Review of Biomedical Engineering, 15, 359–382.
Mitchell, A. J., Rao, S., & Vaze, A. (2011). International comparison of clinicians' ability to identify depression in primary care: Meta-analysis and meta-regression of predictors. British Journal of General Practice, 61(583), e72–e80.
Mitchell, A. J., Vaze, A., & Rao, S. (2009). Clinical diagnosis of depression in primary care: A meta-analysis. The Lancet, 374(9690), 609–619.
Morisky, D. E., Green, L. W., & Levine, D. M. (1986). Concurrent and predictive validity of a self-reported measure of medication adherence. Medical Care, 24(1), 67–74.
Nationale VersorgungsLeitlinie Unipolare Depression – Leitlinienreport, Version 3.0. 2022. (2022). Retrieved [cited: 2023-02-28], from "http://www.leitlinien.de/depression"
Nay, W., Brown, R., & Roberson-Nay, R. (2013). Longitudinal course of panic disorder with and without agoraphobia using the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Psychiatry Research, 208(1), 54–61.
Norman, S. B., Campbell-Sills, L., Hitchcock, C. A., Sullivan, S., Rochlin, A., Wilkins, K. C., & Stein, M. B. (2011). Psychometrics of a brief measure of anxiety to detect severity and impairment: The Overall Anxiety Severity and Impairment Scale (OASIS). Journal of Psychiatric Research, 45(2), 262–268.
Norris, S. L., Nichols, P. J., Caspersen, C. J., Glasgow, R. E., Engelgau, M. M., Jack, L., Jr., Isham, G., Snyder, S. R., Carande-Kulis, V. G., & Garfield, S. (2002). The effectiveness of disease and case management for people with diabetes: A systematic review. American Journal of Preventive Medicine, 22(4), 15–38.
Olariu, E., Forero, C. G., Castro-Rodriguez, J. I., Rodrigo-Calvo, M. T., Álvarez, P., Martín-López, L. M., Sánchez-Toto, A., Adroher, N. D., Blasco-Cubedo, M. J., & Vilagut, G. (2015). Detection of anxiety disorders in primary care: A meta-analysis of assisted and unassisted diagnoses. Depression and Anxiety, 32(7), 471–484.
Parker, E. L., Banfield, M., Fassnacht, D. B., Hatfield, T., & Kyrios, M. (2021). Contemporary treatment of anxiety in primary care: a systematic review and meta-analysis of outcomes in countries with universal healthcare. BMC family practice, 22(1), 1–15.
Pompoli, A., Furukawa, T. A., Imai, H., Tajika, A., Efthimiou, O., & Salanti, G. (2016). Psychological therapies for panic disorder with or without agoraphobia in adults: A network meta-analysis. Cochrane Database of Systematic Reviews, 4, CD011004. https://doi.org/10.1002/14651858.CD011004.pub2
Reuter, K. (2020). Social media for clinical trial recruitment: How real is the potential? Innovations Published Online First: 2020.
Roy-Byrne, P. P., Craske, M. G., & Stein, M. B. (2006). Panic disorder. Lancet, 368(9540), 1023–1032.
Roy-Byrne, P. P., Wagner, A. W., & Schraufnagel, T. J. (2005). Understanding and treating panic disorder in the primary care setting. Journal of Clinical Psychiatry, 66(Suppl 4), 16–22.
Rudden, M., Busch, F. N., Milrod, B., Singer, M., Aronson, A., Roiphe, J., & Shapiro, T. (2003). Panic disorder and depression: A psychodynamic exploration of comorbidity. The International Journal of Psychoanalysis, 84(4), 997–1015.
Saddichha, S., Al-Desouki, M., Lamia, A., Linden, I. A., & Krausz, M. (2014). Online interventions for depression and anxiety–A systematic review. Health Psychology and Behavioral Medicine: An Open Access Journal, 2(1), 841–881.
Shaw, T., McGregor, D., Brunner, M., Keep, M., Janssen, A., & Barnet, S. (2017). What is eHealth (6)? Development of a conceptual model for eHealth: qualitative study with key informants. Journal of Medical Internet Research, 19(10), e324.
Simon, N. M., & Fischmann, D. (2005). The implications of medical and psychiatric comorbidity with panic disorder. J Clin Psychiatry, 66(Suppl 4), 8–15.
Teper, M. H., Vedel, I., Yang, X. Q., Margo-Dermer, E., & Hudon, C. (2020). Understanding barriers to and facilitators of case management in primary care: A Systematic review and thematic synthesis. The Annals of Family Medicine, 18(4), 355–363.
Tilli, V., Suominen, K., & Karlsson, H. (2012). Panic disorder in primary care: Comorbid psychiatric disorders and their persistence. Scandinavian Journal of Primary Health Care, 30(4), 247–253.
Tylee, A., & Walters, P. (2007). Underrecognition of anxiety and mood disorders in primary care: Why does the problem exist and what can be done? Journal of Clinical Psychiatry, 68(supplement 2), 27–30.
van der Kleij, R. M., Kasteleyn, M. J., Meijer, E., Bonten, T. N., Houwink, E. J., Teichert, M., van Luenen, S., Vedanthan, R., Evers, A., & Car, J. (2019). SERIES: eHealth in primary care. Part 1: Concepts, conditions and challenges. European Journal of General Practice, 25(4), 179–189.
Van Dis, E. A., Van Veen, S. C., Hagenaars, M. A., Batelaan, N. M., Bockting, C. L., Van Den Heuvel, R. M., Cuijpers, P., & Engelhard, I. M. (2020). Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders: A systematic review and meta-analysis. JAMA Psychiatry, 77(3), 265–273.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization: KL, JG; data curation: AZ, SL, DH, MH, HHK; formal analysis: AZ, SL, DH; methodology: AZ, HHK; project administration: KL, MvdA; resources: HHK, JG, MvdA; supervision: JG, AZ, MvdA, HHK; validation: SL, AZ; visualisation: SL; writing - original draft: KL; writing - review and editing: AZ, SL, DH, HHK, MH, MvdA, JG.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The ethics committee of the Goethe-University (Frankfurt/Main, Germany) approved the study protocol on April 24th, 2019 (approval number 432/18). Written informed consent was obtained from all participating patients and GPs.
Consent for Publication
Consent forms for the trial include consent for publication of results in peer-reviewed journals.
Strength and Limitations
As limitation, an evaluation of the results can only be done with caution due to the small number of participants. As strength, the present study is based on evidence-based concepts regarding CBT and case management in primary care for patients with depression and anxiety disorders.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 539 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lukaschek, K., Lezius, S., van den Akker, M. et al. CBT-Based and eHealth-Supported Case Management for Patients with Panic Disorder or Depression in Primary Care: Results of a Proof of Concept. J Cogn Ther (2023). https://doi.org/10.1007/s41811-023-00195-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s41811-023-00195-9