Abstract
The aim of this study was to assess the effect of cognitive behavioural therapy intervention on sleep and health improvement in patients with cardiometabolic syndrome and sleep problems. This study also aimed to assess the effect of different study designs to explain the overall intervention effect through subgroup analysis. Relevant randomized controlled trial studies were searched through six online databases. The PEDro scale was used to assess the quality of the included studies. The random effects model was used to assess the mean difference, effect size, and standard deviation of the outcome variables. The heterogeneity of the included studies was assessed using I2 and Q tests. Publication bias was assessed by the Egger test. Cognitive behavioural therapy intervention provided a significant effect in improving the Pittsburgh Sleep Quality Index, Insomnia Severity Index, total sleep time, sleep efficiency, depression, fatigue, and HbA1c. The effect of cognitive behavioural therapy is more significant when relaxation training and education components are included. Cognitive behavioural therapy is suitable for the treatment of sleep problems in patients with cardiometabolic syndrome. Cognitive behavioural therapy is also effective on depression and fatigue but has a limited effect on blood pressure and biomedical indicators related to cardiometabolic syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiometabolic syndrome (CMS) is a group of diseases characterized by insulin resistance, abdominal obesity, hypertension, and hyperlipidaemia (Ahmad & Shapiro, 2021). Typical examples of CMS include type 2 diabetes, heart failure, coronary heart disease, atrial fibrillation, hypertension, and chronic kidney disease (Lan et al., 2020). As there are various definition criteria for CMS, the exact global prevalence is hard to estimate. However, studies have mentioned that CMS is around three times more common than diabetes (Saklayen, 2018). Based on this information, the estimated global prevalence can be up to 25 to 45% of the population, which means over a billion people are affected by CMS across the world (Saklayen, 2018).
Studies have found that sleep problems, especially insomnia and obstructive sleep apnoea, are very common in patients with CMS (Koren & Taveras, 2018; Schuster et al., 2006); insomnia generally encompasses various forms (Chasens et al., 2021), including difficulty falling asleep (sleep onset insomnia), difficulty maintaining sleep (sleep maintenance insomnia), early morning awakening (terminal insomnia), and non-restorative sleep. It is reported that 76.8% of type 2 diabetes patients have experienced sleep problems regularly (Gupta & Wang, 2016). Previous studies also mentioned that sleep problems are closely linked to the alterations of cardiometabolic conditions (Bathgate & Fernandez-Mendoza, 2018; Cedernaes et al., 2015; Tan et al., 2018). Adults who report sleeping less than the recommended duration have a higher risk of developing hypertension, systematic insulin resistance, and increased sympathetic nervous system activity, which can exacerbate the pathophysiology of existing CMS (Chasens et al., 2021; Koren & Taveras, 2018; Schuster et al., 2006).
People with cardiometabolic syndrome experience insomnia more so than anyone else, for reasons including (1) physiological factors: cardiometabolic syndrome is characterized by a cluster of conditions such as obesity, high blood pressure, high blood sugar, and abnormal cholesterol levels. These conditions can lead to physiological changes in the body, such as inflammation (Syauqy et al., 2019) and hormonal imbalances (Chrousos & Kino, 2007), which can disrupt sleep patterns and contribute to insomnia. (2) Psychological factors: individuals with cardiometabolic syndrome may experience increased stress, anxiety, and depression due to the impact of their condition on their overall health and quality of life. These psychological factors can interfere with sleep and contribute to insomnia (Brugnera et al., 2022). (3) Medications: antipsychotic medications are often prescribed to patients with insomnia, which have been associated with a number of serious side effects, including metabolic disorders and cardiovascular disorders (Kennedy et al., 2006). (4) Sleep apnea: sleep apnea is a common condition among individuals with cardiometabolic syndrome. It is characterized by interruptions in breathing during sleep, leading to fragmented and poor-quality sleep. Sleep apnea can contribute to insomnia symptoms (Rodrigues et al., 2021). (5) Lifestyle factors: unhealthy lifestyle habits, such as a sedentary lifestyle, poor diet, and lack of physical activity, are common in individuals with cardiometabolic syndrome. These factors can negatively impact sleep quality and contribute to insomnia (Romero-Cabrera et al., 2022).
In contrast, the improvement of overall sleep quality has positive effects on enhancing glycaemic control (Carroll et al., 2015; Lee et al., 2017). As a result, improving sleep in CMS patients is very important for overall disease management. When considering the sleep management methods on CMS patients, there are usually pharmacological options and non-pharmacological options. Pharmacological options showed significant effects on acute sleep improvement (Chiu et al., 2021; De Crescenzo et al., 2022). However, existing studies also mentioned that sleep drugs sometimes have addictive problems and might provide adverse effects on glycaemic control (Gramaglia et al., 2014; Ma et al., 2014). In this case, non-pharmacological treatments, especially cognitive behavioural therapy (CBT), are preferable in the management of sleep problems in CMS patients.
CBT is recommended as the first-line treatment for insomnia with high efficacy and safety (Dopheide, 2020). CBT is based on the concept that psychological problems are greatly affected by unhelpful thinking and behaviour patterns, and psychological problems can be relieved when the patients recognize and change their thinking and behaviour patterns (Rachman, 2015). CBT intervention for insomnia (CBT-I) involves multiple components targeting to change the thinking and behaviour patterns towards sleep. The core components of CBT-I include (1) sleep restriction, which restricts and adjusts the total time in bed and generally establishes a homoeostatic drive to sleep; (2) stimulus control, which restricts the bed only for sleep and reduces the associations between the bed and stimulus behaviours; (3) cognitive restructure, which helps identify and change the unhelpful thoughts towards sleep; (4) sleep hygiene, which helps establish proper environment, behaviours, and habits that promote sleep; and (5) relaxation training, which provides skills about muscle relaxation and reduces cognitive arousal (Perlis et al., 2005). Despite the core components, the intervention design of CBT-I varies in different studies. For example, the total length of the intervention ranges from 4 weeks to 12 months; the intervention contents can be delivered online or face-to-face, the intervention can be delivered in groups or individually, and some of the studies include unique components such as homework, education on other diseases, and problem-solving sessions (van Straten et al., 2018). Existing studies have confirmed that CBT-I is an effective alternative for the treatment of insomnia in patients from all age groups (Dewald-Kaufmann et al., 2022; van Straten et al., 2018). Studies have also been carried out to assess the effect of CBT-I on sleep improvement in patients with more complex physical and mental conditions, including patients with comorbid mental disorders such as depression (Carney et al., 2017) and anxiety (Mason et al., 2023), and chronic diseases such as cardiovascular diseases (Johann et al., 2020) and cancer (Savard et al., 2021). These studies all found that CBT-I intervention has a significant effect on sleep and mental health improvement (Carney et al., 2017; Mason et al., 2023). However, the effect of CBT-I on physical health indicators varies in different studies. For example, CBT-I was reported to have a limited effect on biomedical markers of cardiovascular diseases (Johann et al., 2020) in one randomized controlled trial study, but a positive effect on blood glucose level was reported in another study (Alshehri et al., 2020).
Existing meta-analysis studies have confirmed that CBT intervention provides a significant effect in prolonging total sleep time, increasing sleep efficiency, reducing sleep onset latency, and waking after sleep onset (Ho et al., 2015; Trauer et al., 2015; van Straten et al., 2018). However, no existing meta-analysis studies were found focusing on the effect of CBT intervention in CMS patients. Also, the existing meta-analysis did not provide results on the effect of CBT for insomnia on metabolic profiles, such as blood glucose, blood pressure, and glycated haemoglobin (HbA1c). In addition, existing meta-analysis studies only provided results about the overall effect of CBT on sleep outcomes, and none of them assessed the effect of different study designs on the final effect of CBT intervention.
To fill the research gaps above, this study aims to evaluate the effect of CBT intervention on sleep, psychiatric diseases, fatigue, HbA1c, and blood pressure in CMS patients with sleep problems. This study also aims to assess the effect of different intervention designs on the final CBT intervention effect through subgroup analysis. The results from this study will contribute to a more comprehensive understanding of the effect of CBT on sleep and health improvement in the CMS population and provide evidence for a more efficient intervention design.
Methods
The protocol of this study was developed based on the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines. This study was registered at the International Prospective Register of Systematic Reviews (PROSPERO), and the registration ID was CRD42023405451. The research protocol could be found at the following website: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=405451.
Database Search
The database search and study selection process were completed by two researchers independently. In cases of disagreement, a third researcher was consulted until a consensus was reached. Relevant studies were searched in six electronic databases: PubMed, Scopus, EMBASE, Cochrane Library, PsycINFO, and China National Knowledge Infrastructure. Grey literatures were also searched from ProQuest central: Dissertations and Thesis. The Boolean approach was used in the title and abstract search using the following keywords: “cognitive behavior therapy” OR “cognitive behavioural therapy” OR “CBT” AND “cardiometabolic syndrome” OR “type-2 diabetes” OR “hypertension” OR “heart failure” OR “coronary heart disease” OR “metabolic syndrome” OR “atrial fibrillation” OR “chronic kidney disease” AND “sleep” OR “insomnia”. Search results from the six databases were exported, and duplicates were removed in the EndNote X9 software.
Study Selection
The titles and abstracts of the exported articles were examined by two researchers, and articles that did not focus on CBT, cardiac metabolic syndrome, or sleep problems were excluded from the study. A full-text review was then conducted to screen the studies available for data extraction and data analysis according to the designed inclusion and exclusion criteria. Inclusion and exclusion criteria used in full-text screening in this study were developed based on the population, intervention, control, and outcomes (PICO) approach. If the full text of the articles was not available online, reasonable attempts such as sending direct request emails to the corresponding author would be made to acquire the full article.
Inclusion Criteria
P: The target population of the studies must be adults over 18 years old. Patients must be diagnosed with at least one of the cardiometabolic diseases, including type 2 diabetes, heart failure, coronary heart disease, atrial fibrillation, hypertension, metabolic syndrome, and chronic kidney disease. Patients should also be diagnosed with insomnia or have self-reported sleep problems.
I: The intervention delivered in the articles must be either CBT, based on CBT principles, or related cognitive or behavioural interventions. The intervention must utilize at least one of the core CBT components, including behavioural therapy (sleep restriction and stimulus control) and cognitive therapy. The design of other intervention components in the intervention plan is not limited.
C: Participants in the control group received non-CBT interventions such as sleep hygiene education only and sleep medication, or treatment as usual, or were put on a wait list and receive the same intervention as the intervention group later (wait-list control).
O: Articles reporting at least one of the designed outcomes were included in this study. The primary outcomes in this study include overall sleep quality measured by the Pittsburgh Sleep Quality Index (Buysse et al., 1989) and insomnia severity measured by the Insomnia Severity Index (Morin, 1993). Secondary outcomes include total sleep time, sleep efficiency, sleep onset latency, wake after sleep onset, depression measured by depression scales Beck et al., 1996; Eaton et al., 2004; Hamilton, 1986; Yesavage, 1988; Zung, 1965), anxiety measured by anxiety scales (Beck et al., 1988; Bieling et al., 1998; Zigmond & Snaith, 1983), fatigue measured by fatigue scales (Krupp et al., 1989; Smets et al., 1995), glycated haemoglobin (HbA1c), systolic blood pressure, and diastolic blood pressure.
Other requirements: The studies should involve a randomized controlled trial (RCT) design, in which participants were randomly assigned to intervention groups or control groups with no restrictions on sample size. The publication date and publication language of the articles were not limited.
Exclusion Criteria
Studies meeting either of the following criteria were excluded from this study, including studies targeting patients with severe mental disorders with a high possibility of cognitive or behavioural impairment, such as schizophrenia, bipolar disorder, severe depression, severe anxiety, and personality disorders; studies with no control groups or patients in the control group receiving CBT or CBT-related intervention; and studies that could not provide complete data for at least one of the designed research outcomes. Systematic reviews, study protocols, case reports, and studies that did not involve a randomized controlled trial design were not included in this study. Journal articles that were not peer-reviewed and low-quality studies scoring 3 points or less on the PEDro scale (Verhagen et al., 1998) were also excluded.
Quality Assessment
The quality assessment of studies was completed by two researchers independently using the Physiotherapy Evidence Database (PEDro) scale (Verhagen et al., 1998). The PEDro scale includes 10 independent marking criteria, with a score range of 0 to 10 points. The marking criteria include random allocation of subjects, concealed allocation of subjects, similar baseline characteristics, blinding to all subjects, blinding to all therapists, blinding to assessors who assessed the key outcomes, enough outcome measures obtained, subjects receiving intervention or control condition in the study designs, the presence of between-group comparison analysis, and the presence of both point measures and measures of variability. For every single criterion, the study would receive one point only if it completely met the criteria; otherwise, it would receive zero points. The overall quality of RCT studies could be categorized into four levels: low quality (0–3 points), moderate quality (4–5 points), good quality (6–8 points), and excellent quality (9–10 points) (Cashin & McAuley, 2020).
Data Extraction
Data extraction process was also completed by two researchers independently. In cases of disagreement, a third researcher was also invited to review and confirm the extracted data. When the included studies did not provide enough data, reasonable attempts such as direct email requests to the corresponding authors were made to acquire the full datasets.
Study Characteristics
For each of the included studies, the following information was collected and presented in one table: author, year of publication, study location, sample size, average age, gender (number and percentage of female), type of disease including comorbidity, intervention format and contents, duration of treatment, therapist support, percentage of completion, description of control condition, outcome variables reported, and PEDro score.
Primary and Secondary Outcomes
Means and standard deviations for primary and secondary outcomes in intervention groups and control groups before and after intervention were extracted. The extracted data was exported into separate Excel spreadsheets for every single outcome. The mean difference was then calculated by subtracting the mean value before the intervention from the mean value after the intervention. If the article provided results for both post-intervention and follow-up, the results for the longest follow-up time were extracted.
Subgroup Variables
Due to the different intervention designs in different studies, we manually coded the study characteristics into subgroup variables. The subgroup variables included the length of intervention (0 for ≤ 6 weeks and 1 for > 6 weeks), CMS disease type (0 for type 2 diabetes, 1 for hypertension, 2 for heart diseases, and 3 for kidney failure), delivery format (0 for face-to-face delivered, 1 for remotely delivered, and 3 for mixed delivery), and the presence of certain intervention components, including sleep hygiene, relapse prevention, relaxation training, sleep education, homework, CMS education, use of self-help intervention manual, use of sleep diary, problem reporting and solving, individual counselling, use of sleep medication, and use of CMS medication (0 for not presence and 1 for presence).
Statistical Analysis
All the data analysis processes in this study were completed in the STATA 17.0 software. The significance level was set at 0.05 (two-sided), and a p value < 0.05 was statistically significant. A separate meta-analysis using a random effects model was performed for each of the research outcomes. Pooled mean differences (MD) with 95% confidence intervals (CI) were used in the results presentation because all outcome variables in this study were continuous variables. Standardized mean difference (SMD) with 95% CIs was also used to measure the effect size of the variables. SMD was calculated by dividing the mean difference by the pooled standard deviation (Hedges & Olkin, 2014). A SMD between 0.2 and 0.5 indicates a small effect size; a SMD between 0.5 and 0.8 is a medium effect size, and a SMD greater than 0.8 is a large effect size (Hedges & Olkin, 2014). The forest plots were also plotted to give a straightforward overview of the meta-analysis results. In subgroup analysis, p values between groups were also presented to indicate the difference within a subgroup variable.
Heterogeneity among the studies was assessed by I2 values (Higgins et al., 2003) and Q tests (Huedo-Medina et al., 2006). The I2 value ranged from 0 to 100%. An I2 value greater than 50% indicated that there was high heterogeneity, and a subgroup analysis was required to examine the possible causes. A greater number of Q-test value also indicated a higher level of heterogeneity.
A regression-based Egger test (Lin & Chu, 2018) was used to investigate the potential publication bias among the studies for each of the primary and secondary outcomes. The results were presented as Egger’s t values with 95% CIs and p values. A p value greater than 0.05 indicated that the publication bias was not statistically significant. Funnel plots were also presented to assist in the evaluation of publication bias. Sensitivity analysis was also conducted by removing one or multiple studies at a time from the meta-analysis to examine the stability of the pooled results and the potential source of publication bias.
Results
Database Search and Study Selection
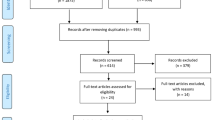
A total of 1563 articles were identified through the database search process. After removing 892 duplicates, the title and abstract of 671 articles were reviewed, and 519 articles were excluded because they did not meet the inclusion criteria. The full text of the rest of the 152 articles was reviewed. Among the 152 articles, 68 studies were not RCTs, 28 studies were not focusing on CMS, 7 studies did not use CBT intervention, 18 studies could not provide sufficient data, and 16 studies scored 3 or less on the PEDro scale. A total of 137 studies were excluded during full-text screening. The final number of studies available for data extraction and analysis was 15. The PRISMA flow chart for the database search and screening process is presented in Fig. 1.
Characteristics of Included Studies
A total of 15 studies (Alshehri et al., 2021; Alshehri et al., 2020; Chen et al., 2011; Chen et al., 2008; Doering et al., 2016; Harris et al., 2019; Hartescu et al., 2022; Johann et al., 2020; Liu and Wang, 2018; McGrath et al., 2017; Picariello et al., 2021; Redeker et al., 2015; Redeker et al., 2022; Zhang et al., 2021; Zuo et al., 2020) were included in data extraction and analysis, and the number of participants in these studies was 2174. The average age of the participants was 61.1 years old, and the percentage of females was 59.5%. All participants reported sleep problems along with cardiometabolic diseases, including type 2 diabetes (Alshehri et al., 2020, 2021; Zhang et al., 2021; Zuo et al., 2020), hypertension (Hartescu et al., 2022; Johann et al., 2020; Liu & Wang, 2018; McGrath et al., 2017), heart failure (Harris et al., 2019; Redeker et al., 2015, 2022), coronary heart disease (Doering et al., 2016), and chronic kidney disease (Chen et al., 2008, 2011; Picariello et al., 2021). Participants in the intervention group all received CBT or CBT-related intervention, and all intervention designs included sleep restriction and stimulus control components. The interventions were face-to-face delivered (Alshehri et al., 2021; Alshehri et al., 2020; Doering et al., 2016; Johann et al., 2020; Liu & Wang, 2018; Redeker et al., 2015; Redeker et al., 2022; Zhang et al., 2021; Zuo et al., 2020), through the internet (Chen et al., 2008, 2011; McGrath et al., 2017), or with a mixed delivery method (Harris et al., 2019; Hartescu et al., 2022; Picariello et al., 2021). The duration of the intervention ranged from 4 to 8 weeks, and all studies reported high completion rates of over 90%. The control group designs included treatment as usual, wait-list control, and sleep education. In addition, all 15 studies reported at least one of the research outcomes. The studies had an overall high PEDro score, with 8 good quality studies (Alshehri et al., 2020; Chen et al., 2008, 2011; Doering et al., 2016; Hartescu et al., 2022; Redeker et al., 2015; Zhang et al., 2021; Zuo et al., 2020) and 7 excellent quality studies (Alshehri et al., 2021; Harris et al., 2019; Johann et al., 2020; Liu & Wang, 2018; McGrath et al., 2017; Picariello et al., 2021; Redeker et al., 2022). Detailed information about the studies is presented in Table 1.
Effect of CBT on Sleep Outcomes
The meta-analysis results for all research outcomes are presented in Table 2. The forest plots for meta-analysis are also presented in Figs. 2 and 3.
The Pittsburgh Sleep Quality Index was reported in 13 studies, with a total of 1992 participants. There was a significant improvement in sleep quality for patients in the CBT intervention group compared with the control group, as the average Pittsburgh Sleep Quality Index score in the intervention group was significantly lower than in the control group (MD = − 2.02, 95% CI − 2.60 to − 1.45, p < 0.001, I2 = 69.56%) with a medium effect size (SMD = − 0.66, 95% CI − 0.86 to − 0.46, p < 0.001, I2 = 65.37%). Subgroup analysis was conducted on the Pittsburgh Sleep Quality Index because there was a significantly high level of heterogeneity (I2 = 69.56%, p < 0.001). The results of the subgroup analysis on the Pittsburgh Sleep Quality Index are presented in Table 3. Subgroup analysis found that CBT intervention with a sleep hygiene component (MD = − 2.09, 95% CI − 2.66 to − 1.52, p < 0.001, I2 = 74.99%) has a greater effect on the Pittsburgh Sleep Quality Index compared with intervention designs that did not include sleep hygiene (MD = − 1.76, 95% CI − 5.03 to 1.51, p > 0.05, I2 = 44.20%). It was also found that the Pittsburgh Sleep Quality Index was more significantly improved in studies that did not provide self-help manual (MD = − 2.27, 95% CI − 2.92 to − 1.62, p < 0.001, I2 = 68.30%) compared with studies that provided self-help manual (MD = − 1.12, 95% CI − 2.65 to 0.40, p > 0.05, I2 = 76.40%).
The Insomnia Severity Index was reported in 6 studies, and 441 participants were included in these studies. The meta-analysis showed that there was a more significant decrease in Insomnia Severity Index scores in patients in the CBT intervention group compared with patients in the control group (MD = − 4.69, 95% CI − 6.48 to − 2.91, p < 0.001, I2 = 66.13%) and a large effect size (SMD = − 0.90, 95% CI − 1.25 to − 0.55, p < 0.001, I2 = 66.16%).
For the four sleep diary outcomes, we found that CBT intervention provided significant in improving total sleep time and sleep efficiency. Participants in the CBT intervention group showed a more significant increase in total sleep time compared with participants in the control group (MD = 28.45, 95% CI 6.61 to 74.99, p < 0.05, I2 = 82.05%) with a large effect size (SMD = 1.97, 95% CI − 1.75 to 5.68, p < 0.05, I2 = 99.68%). Also, we found that sleep efficiency was more significantly increased in patients in the CBT intervention group compared with the control group (MD = 4.40, 95% CI 1.06 to 7.80, p < 0.05, I2 = 72.06%), and there was a small effect size (SMD = 0.45, 95% CI 0.26 to 0.65, p < 0.001, I2 = 0.00%). In contrast, CBT intervention did not show a significant effect on improving sleep onset latency and wake after sleep onset. The mean difference improvement in sleep onset latency between the CBT intervention group and the control group was not significant (MD = − 1.15, 95% CI − 8.33 to 6.02, p > 0.05, I2 = 59.08%), and the effect size was very small (SMD = − 0.04, 95% CI − 0.43 to 0.35, p > 0.05, I2 = 65.63%). A similar result was observed in wake after sleep onset. No significant difference in wake after sleep onset was found between the CBT intervention group and the control group (MD = v0.31, 95% CI − 12.13 to 11.51, p > 0.05, I2 = 85.93%), with a very small effect size (SMD = 0.08, 95% CI − 0.66 to 0.82, p > 0.05, I2 = 88.82%).
Effect of CBT on Depression and Anxiety
A total of 10 studies with 608 participants reported depression scale scores as outcome variables. There was a more significant improvement in depression scale scores in the CBT intervention group compared with the control group (MD = − 2.73, 95% CI − 4.46 to − 1.00, p < 0.001, I2 = 75.51%) with a medium effect size (SMD = − 0.51, 95% CI − 0.80 to − 0.21, p < 0.001, I2 = 64.33%). A subgroup analysis was also conducted on the depression outcome, and the results are presented in Table 4. We found that CBT intervention delivered in less than 6 weeks (MD = − 3.09, 95% CI − 5.16 to − 1.02, p < 0.01, I2 = 52.15%) had a more significant effect on depression than intervention delivered longer than 6 weeks (MD = − 2.57, 95% CI − 6.17 to 1.04, p > 0.05, I2 = 88.68%). The effect of CBT on depression was more significant in studies that did not provide education on CMS (MD = − 3.73, 95% CI − 6.76 to − 0.69, p < 0.05, I2 = 81.45%) compared with studies providing CMS education (MD = − 1.99, 95% CI − 4.35 to 0.37, p > 0.05, I2 = 68.52%). CBT also showed a greater effect on depression when a self-help manual was not present (MD = − 3.45, 95% CI − 5.84 to − 1.06, p < 0.01, I2 = 75.17%) compared with studies that provided a self-help manual (MD = − 1.44, 95% CI − 3.94 to 1.05, p > 0.05, I2 = 68.35%).
In addition, we found that CBT intervention did not provide significant in improving anxiety symptoms. The difference in anxiety scale scores between the CBT intervention group and the control group was not significant (MD = − 1.08, 95% CI v2.31 to 0.15, p > 0.05, I2 = 72.68%), and the effect size result was also not significant (SMD = − 0.25, 95% CI − 0.50 to 0.01, p > 0.05, I2 = 64.36%).
Effect of CBT on Fatigue
Fatigue was reported in 7 studies, and 419 participants were included in these studies. The fatigue level measured by fatigue scales was more significantly reduced in the CBT intervention group compared with the control group (MD = − 1.12, 95% CI − 1.52 to − 0.71, p < 0.001, I2 = 0.00%) with a medium effect size (SMD = − 0.55, 95% CI − 0.79 to − 0.32, p < 0.001, I2 = 21.35%). The Q test results were consistent with the I2 result, showing a significantly low level of heterogeneity in fatigue.
Effect of CBT on HbA1c and Blood Pressure
HbA1c was reported in 4 studies with 1274 participants. A significant difference in the improvement of HbA1c between the CBT intervention group and the control group was observed (MD = − 0.57, 95% CI − 1.08 to − 0.07, p < 0.05, I2 = 76.75%), and there was a small effect size (SMD = − 0.43, 95% CI − 0.78 to − 0.08, p < 0.05, I2 = 72.08%).
Systolic blood pressure and diastolic blood pressure were reported in 4 studies, which included 369 participants. The effect of CBT intervention on both systolic blood pressure and diastolic blood pressure was not significant. The difference in systolic blood pressure improvement between the CBT intervention group and the control group was not significant (MD = − 5.32, 95% CI − 11.01 to 0.43, p > 0.05, I2 = 88.57%), and the effect size was also not significant (SMD = − 0.93, 95% CI − 2.37 to 0.52, p > 0.05, I2 = 97.32%). A similar result was found for diastolic blood pressure (MD = − 5.36, 95% CI − 12.51 to 1.80, p > 0.05, I2 = 95.72%), with an insignificant effect size result (MD = − 1.23, 95% CI − 3.22 to 0.76, p > 0.05, I2 = 98.53%).
Publication Bias
Publication bias for all outcomes was assessed by regression-based Egger test, and the results are presented in Table 2. The Funnel plots were also presented in Figs. 4 and 5. No significant publication bias was found for the Pittsburgh Sleep Quality Index (t = − 0.52, p > 0.05), Insomnia Severity Index (t = − 1.39, p > 0.05), total sleep time (t = − 1.13, p > 0.05), sleep efficiency (t = 2.62, p > 0.05), sleep onset latency (t = − 1.68, p > 0.05), wake after sleep onset (t = − 1.60, p > 0.05), depression (t = − 2.22, p > 0.05), fatigue (t = − 2.65, p > 0.05), HbA1c (t = − 0.03, p > 0.05), systolic blood pressure (t = 1.73, p > 0.05), and diastolic blood pressure (t = 1.21, p > 0.05). Originally, there was a significant publication bias in the anxiety outcome, so we conducted sensitivity analysis and found that the source of publication bias was from 3 studies (Chen et al., 2011; Harris et al., 2019; Picariello et al., 2021). Therefore, these three studies were removed from the meta-analysis process for the anxiety outcome, and the publication bias result for the rest of the studies for anxiety outcome turned out to be not significant (t = − 2.45, p > 0.05).
Discussion
This study included 15 RCT studies with a total of 2174 participants. The meta-analysis results found that CBT intervention had a significant effect on improving overall sleep quality as measured by the Pittsburgh Sleep Quality Index, overall insomnia severity as measured by the Insomnia Severity Index, total sleep time, sleep efficiency, depression, fatigue, and HbA1c. The meta-analysis also found that CBT intervention did not provide a significant effect on improving sleep onset latency, wake after sleep onset, anxiety, and blood pressure.
Effect of CBT Intervention on Sleep Outcomes
The meta-analysis results showed that overall sleep quality measured by the Pittsburgh Sleep Quality Index, overall insomnia severity measured by the Insomnia Severity Index, total sleep time, and sleep efficiency were more significantly improved in the CBT intervention group compared with the control group, which was consistent with the results from previous studies (van Straten et al., 2018). Similar results were also found in other studies analysing the effect of CBT on sleep problems in different disease groups (Climent-Sanz et al., 2022; Ma et al., 2021; Selvanathan et al., 2021), which suggested that CBT intervention provides a similar positive effect on improving sleep quality and prolonging sleep duration in CMS patients by changing their cognitive and behavioural patterns. However, the effect of CBT on sleep onset latency and wake after sleep onset was not significant, and this was quite different from the results in existing studies (van Straten et al., 2018). A possible explanation for this could be the limited number of studies that reported these two outcomes, as sleep onset latency was only presented in five studies and wake after sleep onset was only reported in four studies. For now, we could not conclude that CBT intervention was not effective in improving the continuity of sleep in CMS patients.
Subgroup analysis was conducted on the Pittsburgh Sleep Quality Index to identify the specific intervention components that have the greatest effect on overall sleep quality. We found that the inclusion of the sleep hygiene component was especially important in improving the efficiency of CBT intervention, and this finding was consistent with existing studies (Chung et al., 2018). Existing studies have mentioned that sleep hygiene education can be used alone as a non-pharmacological treatment for insomnia, and the independent use of sleep hygiene education had already showed a significant effect on sleep improvement (Chung et al., 2018). In CBT designs, the main contents of behavioural therapy were sleep restriction and stimulus control. The target of these two components was limited to the behaviours in bed (Baillargeon et al., 1998; Miller et al., 2014), while the overall sleep quality was also greatly affected by factors outside of the bed, such as the environment of bedrooms and substance intake. As an important complement of behavioural treatment, sleep hygiene education provided comprehensive education on behaviours promoting sleep, including keeping a quiet sleep environment, maintaining regular sleep schedules, and limiting alcohol and tobacco intake (Morin & Espie, 2007).
Another effective component identified through subgroup analysis was the absence of self-help manuals. Studies providing self-help manuals showed a worse effect on sleep quality compared with studies without self-help manuals. Although some of the existing studies have concluded that fully self-help CBT intervention had a promising effect on improving sleep quality (Ho et al., 2015), intervention under the guidance of an experienced therapist would be a better choice because patients might have questions or misunderstandings on the intervention contents in fully self-help interventions, and the support from the therapists would be necessary to ensure the intervention contents are properly delivered.
In addition, the length of the intervention did not show significant effects on CBT intervention effects. The length of intervention in RCT included in this study ranged from 4 to 8 weeks, which means there were no studies providing extremely long or extremely short intervention. Further research is required to find out if the CBT intervention effects are affected by extremely long or brief intervention designs. Subgroup analysis also found similar effects in studies with different disease groups, delivery methods, and the use of medications, which was in line with the findings from previous studies (Morin et al., 2006; Trauer et al., 2015). This suggested that the effect of CBT intervention was not significantly affected by comorbid diseases, delivery methods, and medications.
Effect of CBT Intervention on Depression and Anxiety
The meta-analysis found that CBT intervention had a significant effect on depression, which was similar to the findings in existing research (Carney et al., 2017; Lattie et al., 2019). Studies have mentioned that there was a close relationship between low sleep quality and depression, and the improvement of sleep quality and amount could also lead to the improvement of depression symptoms (Roberts & Duong, 2014).
Subgroup analysis was also conducted on depression, and the results showed that the effect of CBT intervention was more significant when the length of intervention was shorter than 6 weeks. A possible explanation would be patients might lose concentration and enthusiasm to participate in the intervention sessions across a longer intervention period. The subgroup analysis also found that relapse prevention, homework, and problem-solving components did not provide a significant effect on depression, which was out of our expectation. The possible reason would be the limited number of studies, so more studies are required to evaluate the effect of CBT on depression.
The meta-analysis also found that CBT intervention did not have a significant effect on anxiety, which was different from the findings in existing studies (Mason et al., 2023; Thun et al., 2019). This is possibly because the main target of CBT interventions in these studies was sleep problems instead of anxiety, so the information related to insomnia management was relatively limited. But we also need to mention that anxiety was reported in only seven studies, and the results might be different when more studies are included.
Effect of CBT Intervention on Fatigue
We found that CBT intervention had a significant effect on daytime fatigue. Fatigue is an important measurement of daytime functioning in patients with sleep problems, and studies have mentioned that the insufficiency of sleep was the greatest cause of daytime fatigue (Caldwell et al., 2019). The CBT intervention successfully created a regulated sleep pattern, which enabled the patients to be fully rested in bed, and this directly reduced the daytime fatigue level 97.
Effect of CBT Intervention on HbA1c and Blood Pressure
While the direct pathways linking sleep to specific medical factors in CMS are still being studied, research suggests several possible mechanisms. Insufficient sleep duration and poor sleep quality have been associated with impaired glucose metabolism (Liu et al., 2022) and insulin resistance (Wang et al., 2023); by improving sleep, it is hypothesized that there may be a positive impact on glucose regulation, leading to improved HbA1c levels. Sleep plays a role in regulating various hormones involved in metabolic processes, including insulin, cortisol, and appetite-regulating hormones like leptin and ghrelin (Brochard et al., 2016); disrupted sleep can lead to dysregulation of these hormones, which can impact blood sugar control, appetite, and weight management. By improving sleep, it is possible that hormonal balance may be restored, which could positively influence HbA1c levels and weight management. Sleep disturbances, particularly sleep apnea, have been associated with increased blood pressure and hypertension (Bathgate & Fernandez-Mendoza, 2018). Sleep apnea is characterized by repeated interruptions in breathing during sleep, leading to fragmented sleep and increased sympathetic nervous system activity. Treating sleep apnea or improving sleep quality may help reduce blood pressure levels (Van Ryswyk et al., 2018). Regarding behavioural patterns and medication compliance, improved sleep may indirectly influence these factors. When individuals experience better sleep, they may have increased energy levels, an improved mood, and reduced daytime sleepiness, which can positively impact their motivation and ability to engage in healthy behaviours, such as regular exercise and adherence to dietary recommendations (Mead & Irish, 2020). Additionally, better sleep quality may lead to improved cognitive function and decision-making, potentially enhancing medication compliance (Taillard et al., 2021).
Consistent with the findings in previous studies (Carroll et al., 2015), CBT intervention was effective in the improvement of metabolic profiles, especially HbA1c, in CMS patients. It was reported that the loss of sleep could lead to heavy metabolic burdens, and some CBT components, such as sleep restriction, have been confirmed to compromise metabolic control in healthy individuals (Reynolds et al., 2012; Schmid et al., 2015). However, further research is required to provide a more detailed overview of the effect of CBT intervention and sleep improvement on metabolic profiles.
In addition, we found that the effect of CBT was not significant on blood pressure, even though previous studies have reported that poor sleep quality was related to higher blood pressure (Lo et al., 2018). This can be explained by the time required to reflect the CBT intervention’s effects on blood pressure. The patients experience sleep improvement immediately during or after CBT intervention, but it might take more time for the effect to be showed on blood pressure reduction. Also, the number of studies reporting blood pressure was limited, and the results might be different when more studies with bigger sample size are included.
Strength, Limitations, and Implications
While nutrition adjustment and exercise involvement are indeed key components of CMS management (Schiavon et al., 2020), addressing sleep as part of a comprehensive treatment approach may provide additional benefits and optimize overall outcomes. It is important for medical and behavioural clinicians to consider the multidimensional nature of CMS and take into account the potential impact of sleep on metabolic health. This study included 15 RCT studies focusing on using CBT intervention in the management of sleep problems in patients with cardiometabolic syndrome. All included studies had high-quality study designs and provided representative data on sleep, psychiatric disease, fatigue, biomedical, and blood pressure outcomes. This study also conducted a subgroup analysis to identify the possible factors in the research designs that might affect the overall effect of the CBT intervention. This study adapted a well-developed study design and minimized the publication bias in the final results.
However, there are still some limitations to this study. Firstly, the meta-analysis results showed that the effect of CBT intervention is not significant in terms of sleep onset latency, wake after sleep onset, anxiety, and blood pressure, which was inconsistent with previous studies. These outcomes were provided in a small number of studies, so results might be changed if the number of studies and participants is increased. Secondly, the number of studies providing results on some important biomedical indicators related to cardiometabolic disease, such as blood glucose and blood lipid indicators, is very limited. As a result, the effect of CBT intervention on overall physical health remains unclear. Finally, the subgroup analysis results found some CBT components being not effective, which might also be due to the limited number of studies. Therefore, further studies that include more RCT studies with a larger sample size and more biomedical outcomes are needed to support the results of this study.
Most patients with cardiometabolic syndrome not only have cardiometabolic syndrome symptoms but also have comorbidities with sleep problems, depression, and anxiety. The present meta-analysis found CBT-I and CBT principles were beneficial not only to sleep problems but also to depression and anxiety, and this is consistent with other previous studies (Cunningham & Shapiro, 2018). It is highly recommended that CBT-I can be used as the priority treatment and be implemented in medical and clinical settings to treat underlying psychological and behavioural problems. The improvement of sleep problem, depression, and anxiety would result in more improvement of metabolic health and overall outcomes in patients with cardiometabolic syndrome.
Conclusion
In conclusion, CBT intervention provides a positive effect on improving overall sleep quality measured by the Pittsburgh Sleep Quality Index, total sleep time, sleep efficiency, depression symptoms, and reducing insomnia severity measured by the Insomnia Severity Index, daytime fatigue, and HbA1c in CMS patients with sleep problems. The effect of CBT intervention is more significant when sleep hygiene education is included and delivered without self-help manuals. Further research with more studies including randomized controlled trials with more biomedical indicators and different intervention designs is required to confirm the findings.
Data Availability
Raw data were generated at the School of Medicine and Dentistry, Griffith University, Australia. Derived data supporting the findings of this study are available from the corresponding author, Jing Sun, on request.
References
Ahmad, M. I., & Shapiro, M. D. (2021). Preventing diabetes and atherosclerosis in the cardiometabolic syndrome. Current Atherosclerosis Reports, 23(4), 16. https://doi.org/10.1007/s11883-021-00913-8
*Alshehri, M. M., Alenazi, A. M., Alothman, S. A., Rucker, J. L., Phadnis, M. A., Miles, J. M., … Kluding, P. M. (2021). Using cognitive behavioral therapy for insomnia in people with type 2 diabetes, pilot RCT part I: Sleep and concomitant symptom. Behavioral Sleep Medicine, 19(5), 652–671. https://doi.org/10.1080/15402002.2020.1831501
*Alshehri, M. M., Alothman, S. A., Alenazi, A. M., Rucker, J. L., Phadnis, M. A., Miles, J. M., … Kluding, P. M. (2020). The effects of cognitive behavioral therapy for insomnia in people with type 2 diabetes mellitus, pilot RCT part II: Diabetes health outcomes. BMC Endocrine Disorders, 20(1), 136. https://doi.org/10.1186/s12902-020-00612-6
Baillargeon, L., Demers, M., & Ladouceur, R. (1998). Stimulus-control: Nonpharmacologic treatment for insomnia. Canadian Family Physician, 44, 73–79.
Bathgate, C. J., & Fernandez-Mendoza, J. (2018). Insomnia, short sleep duration, and high blood pressure: Recent evidence and future directions for the prevention and management of hypertension. Current Hypertension Reports, 20(6), 52. https://doi.org/10.1007/s11906-018-0850-6
Beck, A. T., Epstein, N., Brown, G., & Steer, R. A. (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. https://doi.org/10.1037//0022-006x.56.6.893
Beck, A. T., Steer, R. A., & Brown, G. (1996). Beck depression inventory–II. Psychological assessment. American Psychological Association.
Bieling, P. J., Antony, M. M., & Swinson, R. P. (1998). The state-trait anxiety inventory, trait version: Structure and content re-examined. Behaviour Research and Therapy, 36(7–8), 777–788.
Brochard, H., Boudebesse, C., Henry, C., Godin, O., Leboyer, M., & Étain, B. (2016). Metabolic syndrome and bipolar disorder: Is sleep the missing link? Encephale, 42(6), 562–567. https://doi.org/10.1016/j.encep.2015.08.007
Brugnera, A., Compare, A., Omboni, S., Greco, A., Carrara, S., Tasca, G. A., … Parati, G. (2022). Psychological covariates of blood pressure among patients with hypertension and metabolic syndrome. Health Psychology, 41(12), 946–954. https://doi.org/10.1037/hea0001205
Buysse, D. J., Reynolds, C. F., 3rd., Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. https://doi.org/10.1016/0165-1781(89)90047-4
Caldwell, J. A., Caldwell, J. L., Thompson, L. A., & Lieberman, H. R. (2019). Fatigue and its management in the workplace. Neuroscience and Biobehavioral Reviews, 96, 272–289. https://doi.org/10.1016/j.neubiorev.2018.10.024
Carney, C. E., Edinger, J. D., Kuchibhatla, M., Lachowski, A. M., Bogouslavsky, O., Krystal, A. D., & Shapiro, C. M. (2017). Cognitive behavioral insomnia therapy for those with insomnia and depression: A randomized controlled clinical trial. Sleep, 40(4). https://doi.org/10.1093/sleep/zsx019
Carroll, J. E., Seeman, T. E., Olmstead, R., Melendez, G., Sadakane, R., Bootzin, R., … Irwin, M. R. (2015). Improved sleep quality in older adults with insomnia reduces biomarkers of disease risk: Pilot results from a randomized controlled comparative efficacy trial. Psychoneuroendocrinology, 55, 184–192. https://doi.org/10.1016/j.psyneuen.2015.02.010
Cashin, A. G., & McAuley, J. H. (2020). Clinimetrics: Physiotherapy Evidence Database (PEDro) scale. Journal of Physiotherapy, 66(1), 59. https://doi.org/10.1016/j.jphys.2019.08.005
Cedernaes, J., Schiöth, H. B., & Benedict, C. (2015). Determinants of shortened, disrupted, and mistimed sleep and associated metabolic health consequences in healthy humans. Diabetes, 64(4), 1073–1080. https://doi.org/10.2337/db14-1475
Chasens, E. R., Imes, C. C., Kariuki, J. K., Luyster, F. S., Morris, J. L., DiNardo, M. M., … Yang, K. (2021). Sleep and metabolic syndrome. Nurs Clin North Am, 56(2), 203–217. https://doi.org/10.1016/j.cnur.2020.10.012
*Chen, H. Y., Cheng, I. C., Pan, Y. J., Chiu, Y. L., Hsu, S. P., Pai, M. F., … Wu, K. D. (2011). Cognitive-behavioral therapy for sleep disturbance decreases inflammatory cytokines and oxidative stress in hemodialysis patients. Kidney Int, 80(4), 415–422. https://doi.org/10.1038/ki.2011.151
*Chen, H. Y., Chiang, C. K., Wang, H. H., Hung, K. Y., Lee, Y. J., Peng, Y. S., … Tsai, T. J. (2008). Cognitive-behavioral therapy for sleep disturbance in patients undergoing peritoneal dialysis: A pilot randomized controlled trial. Am J Kidney Dis, 52(2), 314–323. https://doi.org/10.1053/j.ajkd.2008.03.012
Chiu, H. Y., Lee, H. C., Liu, J. W., Hua, S. J., Chen, P. Y., Tsai, P. S., & Tu, Y. K. (2021). Comparative efficacy and safety of hypnotics for insomnia in older adults: A systematic review and network meta-analysis. Sleep, 44(5). https://doi.org/10.1093/sleep/zsaa260
Chrousos, G. P., & Kino, T. (2007). Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress, 10(2), 213–219. https://doi.org/10.1080/10253890701292119
Chung, K. F., Lee, C. T., Yeung, W. F., Chan, M. S., Chung, E. W., & Lin, W. L. (2018). Sleep hygiene education as a treatment of insomnia: A systematic review and meta-analysis. Family Practice, 35(4), 365–375. https://doi.org/10.1093/fampra/cmx122
Climent-Sanz, C., Valenzuela-Pascual, F., Martínez-Navarro, O., Blanco-Blanco, J., Rubí-Carnacea, F., García-Martínez, E., … Gea-Sánchez, M. (2022). Cognitive behavioral therapy for insomnia (CBT-i) in patients with fibromyalgia: A systematic review and meta-analysis. Disabil Rehabil, 44(20), 5770–5783. https://doi.org/10.1080/09638288.2021.1954706
Cunningham, J. E. A., & Shapiro, C. M. (2018). Cognitive behavioural therapy for insomnia (CBT-I) to treat depression: A systematic review. Journal of Psychosomatic Research, 106, 1–12. https://doi.org/10.1016/j.jpsychores.2017.12.012
De Crescenzo, F., D'Alò, G. L., Ostinelli, E. G., Ciabattini, M., Di Franco, V., Watanabe, N., … Cipriani, A. (2022). Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: A systematic review and network meta-analysis. Lancet, 400(10347), 170–184. https://doi.org/10.1016/s0140-6736(22)00878-9
Dewald-Kaufmann, J., de Bruin, E., & Michael, G. (2022). Cognitive behavioral therapy for insomnia in school-aged children and adolescents. Sleep Medicine Clinics, 17(3), 355–365. https://doi.org/10.1016/j.jsmc.2022.06.003
*Doering, L. V., McGuire, A., Eastwood, J. A., Chen, B., Bodán, R. C., Czer, L. S., & Irwin, M. R. (2016). Cognitive behavioral therapy for depression improves pain and perceived control in cardiac surgery patients. European Journal of Cardiovascular Nursing, 15(6), 417–424. https://doi.org/10.1177/1474515115592292
Dopheide, J. A. (2020). Insomnia overview: Epidemiology, pathophysiology, diagnosis and monitoring, and nonpharmacologic therapy. The American Journal of Managed Care, 26(4 Suppl), S76-s84. https://doi.org/10.37765/ajmc.2020.42769
Eaton, W. W., Muntaner, C., Smith, C., Tien, A., & Ybarra, M. (2004). Center for epidemiologic studies depression scale: Review and revision. The use of psychological testing for treatment planning and outcomes assessment. Routledge.
Gramaglia, E., Ramella Gigliardi, V., Olivetti, I., Tomelini, M., Belcastro, S., Calvi, E., … Broglio, F. (2014). Impact of short-term treatment with benzodiazepines and imidazopyridines on glucose metabolism in healthy subjects. Journal of Endocrinological Investigation, 37(2), 203–206. https://doi.org/10.1007/s40618-013-0016-y
Gupta, S., & Wang, Z. (2016). Predictors of sleep disorders among patients with type 2 diabetes mellitus. Diabetes and Metabolic Syndrome: Clinical Research and Reviews, 10(4), 213–220. https://doi.org/10.1016/j.dsx.2016.06.009
Hamilton, M. (1986). The Hamilton rating scale for depression. In Assessment of depression (pp. 143–152). Springer.
*Harris, K. M., Schiele, S. E., & Emery, C. F. (2019). Pilot randomized trial of brief behavioral treatment for insomnia in patients with heart failure. Heart Lung, 48(5), 373–380. https://doi.org/10.1016/j.hrtlng.2019.06.003
*Hartescu, I., Stensel, D. J., Thackray, A. E., King, J. A., Dorling, J. L., Rogers, E. N., … Morgan, K. (2022). Sleep extension and metabolic health in male overweight/obese short sleepers: A randomised controlled trial. Journal of Sleep Research, 31(2). https://doi.org/10.1111/jsr.13469
Hedges, L. V., & Olkin, I. (2014). Statistical methods for meta-analysis. Academic Press.
Higgins, J. P., Thompson, S. G., Deeks, J. J., & Altman, D. G. (2003). Measuring Inconsistency in Meta-Analyses. BMJ, 327(7414), 557–560. https://doi.org/10.1136/bmj.327.7414.557
Ho, F.Y.-Y., Chung, K.-F., Yeung, W.-F., Ng, T. H., Kwan, K.-S., Yung, K.-P., & Cheng, S. K. (2015). Self-help cognitive-behavioral therapy for insomnia: A meta-analysis of randomized controlled trials. Sleep Medicine Reviews, 19, 17–28. https://doi.org/10.1016/j.smrv.2014.06.010
Huedo-Medina, T. B., Sánchez-Meca, J., Marín-Martínez, F., & Botella, J. (2006). Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological Methods, 11(2), 193–206. https://doi.org/10.1037/1082-989x.11.2.193
*Johann, A. F., Hertenstein, E., Feige, B., Akram, U., Holub, F., Baglioni, C., … Spiegelhalder, K. (2020). Cognitive behavioural therapy for insomnia does not appear to have a substantial impact on early markers of cardiovascular disease: A preliminary randomized controlled trial. J Sleep Res, 29(5), e13102. https://doi.org/10.1111/jsr.13102
Kennedy, A., Tapp, A., Kelly, W. S., Kilzieh, N., & Wood, A. E. (2006). Abstinence, anticipation, reduction, and treatment (AART): A stepwise approach to the management of atypical antipsychotic side effects. Essential Psychopharmacology, 7(1), 1–14.
Koren, D., & Taveras, E. M. (2018). Association of sleep disturbances with obesity, insulin resistance and the metabolic syndrome. Metabolism, 84, 67–75. https://doi.org/10.1016/j.metabol.2018.04.001
Krupp, L. B., LaRocca, N. G., Muir-Nash, J., & Steinberg, A. D. (1989). The fatigue severity scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology, 46(10), 1121–1123. https://doi.org/10.1001/archneur.1989.00520460115022
Lan, Q., Wu, H., Zhou, X., Zheng, L., Lin, F., Meng, Q., … Fan, H. (2020). Predictive value of uric acid regarding cardiometabolic disease in a community-dwelling older population in Shanghai: A cohort study. Frontiers in Medicine, 7. https://doi.org/10.3389/fmed.2020.00024
Lattie, E. G., Adkins, E. C., Winquist, N., Stiles-Shields, C., Wafford, Q. E., & Graham, A. K. (2019). Digital mental health interventions for depression, anxiety, and enhancement of psychological well-being among college students: Systematic review. Journal of Medical Internet Research, 21(7), e12869. https://doi.org/10.2196/12869
Lee, S. W. H., Ng, K. Y., & Chin, W. K. (2017). The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: A systematic review and meta-analysis. Sleep Medicine Reviews, 31, 91–101. https://doi.org/10.1016/j.smrv.2016.02.001
Lin, L., & Chu, H. (2018). Quantifying publication bias in meta-analysis. Biometrics, 74(3), 785–794. https://doi.org/10.1111/biom.12817
Liu, J., Richmond, R. C., Bowden, J., Barry, C., Dashti, H. S., Daghlas, I., … Rutter, M. K. (2022). Assessing the causal role of sleep traits on glycated hemoglobin: A Mendelian randomization study. Diabetes Care, 45(4), 772–781. https://doi.org/10.2337/dc21-0089
*Liu, Y. B. X., Wang, J. H. (2018). Effect evaluation of sleep intervention on elderly patients with hypertension. Preventive Medicine, 30(8), 776–779. https://doi.org/10.19485/j.cnki.issn2096-5087.2018.08.005
Lo, K., Woo, B., Wong, M., & Tam, W. (2018). Subjective sleep quality, blood pressure, and hypertension: A meta-analysis. Journal of Clinical Hypertension (Greenwich, Conn.), 20(3), 592–605. https://doi.org/10.1111/jch.13220
Ma, J., Svetnik, V., Snyder, E., Lines, C., Roth, T., & Herring, W. J. (2014). Electroencephalographic power spectral density profile of the orexin receptor antagonist suvorexant in patients with primary insomnia and healthy subjects. Sleep, 37(10), 1609–1619. https://doi.org/10.5665/sleep.4068
Ma, Y., Hall, D. L., Ngo, L. H., Liu, Q., Bain, P. A., & Yeh, G. Y. (2021). Efficacy of cognitive behavioral therapy for insomnia in breast cancer: A meta-analysis. Sleep Medicine Reviews, 55, 101376. https://doi.org/10.1016/j.smrv.2020.101376
Mason, E. C., Grierson, A. B., Sie, A., Sharrock, M. J., Li, I., Chen, A. Z., & Newby, J. M. (2023). Co-occurring insomnia and anxiety: A randomized controlled trial of internet cognitive behavioral therapy for insomnia versus internet cognitive behavioral therapy for anxiety. Sleep, 46(2). https://doi.org/10.1093/sleep/zsac205
*McGrath, E. R., Espie, C. A., Power, A., Murphy, A. W., Newell, J., Kelly, C., … O'Donnell, M. J. (2017). Sleep to lower elevated blood pressure: A randomized controlled trial (SLEPT). Am J Hypertens, 30(3), 319–327. https://doi.org/10.1093/ajh/hpw132
Mead, M. P., & Irish, L. A. (2020). Application of health behaviour theory to sleep health improvement. Journal of Sleep Research, 29(5), e12950. https://doi.org/10.1111/jsr.12950
Miller, C. B., Espie, C. A., Epstein, D. R., Friedman, L., Morin, C. M., Pigeon, W. R., … Kyle, S. D. (2014). The evidence base of sleep restriction therapy for treating insomnia disorder. Sleep Med Rev, 18(5), 415–424. https://doi.org/10.1016/j.smrv.2014.01.006
Morin, C. M. (1993). Insomnia: Psychological Assessment and Management. Guilford.
Morin, C. M., Bootzin, R. R., Buysse, D. J., Edinger, J. D., Espie, C. A., & Lichstein, K. L. (2006). Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004). Sleep, 29(11), 1398–1414. https://doi.org/10.1093/sleep/29.11.1398
Morin, C. M., & Espie, C. A. (2007). Insomnia: A clinical guide to assessment and treatment. Springer Science & Business Media.
Perlis, M. L., Jungquist, C., Smith, M. T., & Posner, D. (2005). Cognitive behavioral treatment of insomnia: A session-by-session guide (Vol. 1). Springer Science & Business Media.
*Picariello, F., Moss-Morris, R., Norton, S., Macdougall, I. C., Da Silva-Gane, M., Farrington, K., … Chilcot, J. (2021). Feasibility trial of cognitive behavioral therapy for fatigue in hemodialysis (BReF intervention). Journal of Pain and Symptom Management, 61(6), 1234–1246.e1235. https://doi.org/10.1016/j.jpainsymman.2020.10.005
Rachman, S. (2015). The evolution of behaviour therapy and cognitive behaviour therapy. Behaviour Research and Therapy, 64, 1–8. https://doi.org/10.1016/j.brat.2014.10.006
*Redeker, N. S., Jeon, S., Andrews, L., Cline, J., Jacoby, D., & Mohsenin, V. (2015). Feasibility and efficacy of a self-management intervention for insomnia in stable heart failure. Journal of Clinical Sleep Medicine, 11(10), 1109–1119. https://doi.org/10.5664/jcsm.5082
*Redeker, N. S., Yaggi, H. K., Jacoby, D., Hollenbeak, C. S., Breazeale, S., Conley, S., … Jeon, S. (2022). Cognitive behavioral therapy for insomnia has sustained effects on insomnia, fatigue, and function among people with chronic heart failure and insomnia: The HeartSleep Study. Sleep, 45(1). https://doi.org/10.1093/sleep/zsab252
Reynolds, A. C., Dorrian, J., Liu, P. Y., Van Dongen, H. P., Wittert, G. A., Harmer, L. J., & Banks, S. (2012). Impact of five nights of sleep restriction on glucose metabolism, leptin and testosterone in young adult men. PLoS ONE, 7(7), e41218. https://doi.org/10.1371/journal.pone.0041218
Roberts, R. E., & Duong, H. T. (2014). The prospective association between sleep deprivation and depression among adolescents. Sleep, 37(2), 239–244. https://doi.org/10.5665/sleep.3388
Rodrigues, G. D., Fiorelli, E. M., Furlan, L., Montano, N., & Tobaldini, E. (2021). Obesity and sleep disturbances: The “chicken or the egg” question. European Journal of Internal Medicine, 92, 11–16. https://doi.org/10.1016/j.ejim.2021.04.017
Romero-Cabrera, J. L., Garaulet, M., Jimenez-Torres, J., Alcala-Diaz, J. F., Quintana Navarro, G. M., Martin-Piedra, L., … García-Rios, A. (2022). Chronodisruption and diet associated with increased cardiometabolic risk in coronary heart disease patients: The CORDIOPREV study. Translational Research, 242, 79–92. https://doi.org/10.1016/j.trsl.2021.11.001
Saklayen, M. G. (2018). The global epidemic of the metabolic syndrome. Current Hypertension Reports, 20(2), 12. https://doi.org/10.1007/s11906-018-0812-z
Savard, J., Ivers, H., Morin, C. M., & Lacroix, G. (2021). Video cognitive-behavioral therapy for insomnia in cancer patients: A cost-effective alternative. Psycho-Oncology, 30(1), 44–51. https://doi.org/10.1002/pon.5532
Schiavon, C. C., Marchetti, E., Ayala, F. O., Loewe, G., Bauer, J., Busnello, F. M., & Reppold, C. T. (2020). Positive psychological characteristics in patients with metabolic syndrome associated with prospective changes in diet and anthropometric factors. PLoS ONE, 15(9), e0236693. https://doi.org/10.1371/journal.pone.0236693
Schmid, S. M., Hallschmid, M., & Schultes, B. (2015). The metabolic burden of sleep loss. The Lancet Diabetes and Endocrinology, 3(1), 52–62. https://doi.org/10.1016/s2213-8587(14)70012-9
Schuster, S. R., Tabba, M., & Sahota, P. (2006). Relationship between the cardiometabolic syndrome and obstructive sleep apnea. Journal of the Cardiometabolic Syndrome, 1(3), 204–208. https://doi.org/10.1111/j.1559-4564.2006.05846.x
Selvanathan, J., Pham, C., Nagappa, M., Peng, P. W. H., Englesakis, M., Espie, C. A., … Chung, F. (2021). Cognitive behavioral therapy for insomnia in patients with chronic pain - a systematic review and meta-analysis of randomized controlled trials. Sleep Medicine Reviews, 60, 101460. https://doi.org/10.1016/j.smrv.2021.101460
Smets, E., Garssen, B., Bonke, B. D., & De Haes, J. (1995). The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of Psychosomatic Research, 39(3), 315–325.
Syauqy, A., Hsu, C. Y., Rau, H. H., Kurniawan, A. L., & Chao, J. C. (2019). Association of sleep duration and insomnia symptoms with components of metabolic syndrome and inflammation in middle-aged and older adults with metabolic syndrome in Taiwan. Nutrients, 11(8). https://doi.org/10.3390/nu11081848
Taillard, J., Sagaspe, P., Philip, P., & Bioulac, S. (2021). Sleep timing, chronotype and social jetlag: Impact on cognitive abilities and psychiatric disorders. Biochemical Pharmacology, 191, 114438. https://doi.org/10.1016/j.bcp.2021.114438
Tan, X., van Egmond, L., Chapman, C. D., Cedernaes, J., & Benedict, C. (2018). Aiding sleep in type 2 diabetes: Therapeutic considerations. The Lancet Diabetes and Endocrinology, 6(1), 60–68. https://doi.org/10.1016/s2213-8587(17)30233-4
Thun, E., Sivertsen, B., Knapstad, M., & Smith, O. R. F. (2019). Unravelling the prospective associations between mixed anxiety-depression and insomnia during the course of cognitive behavioral therapy. Psychosomatic Medicine, 81(4), 333–340. https://doi.org/10.1097/psy.0000000000000676
Trauer, J. M., Qian, M. Y., Doyle, J. S., Rajaratnam, S. M., & Cunnington, D. (2015). Cognitive behavioral therapy for chronic insomnia: A systematic review and meta-analysis. Annals of Internal Medicine, 163(3), 191–204. https://doi.org/10.7326/m14-2841
Van Ryswyk, E., Mukherjee, S., Chai-Coetzer, C. L., Vakulin, A., & McEvoy, R. D. (2018). Sleep disorders, including sleep apnea and hypertension. American Journal of Hypertension, 31(8), 857–864. https://doi.org/10.1093/ajh/hpy082
van Straten, A., van der Zweerde, T., Kleiboer, A., Cuijpers, P., Morin, C. M., & Lancee, J. (2018). Cognitive and behavioral therapies in the treatment of insomnia: A meta-analysis. Sleep Medicine Reviews, 38, 3–16. https://doi.org/10.1016/j.smrv.2017.02.001
Verhagen, A. P., de Vet, H. C., de Bie, R. A., Kessels, A. G., Boers, M., Bouter, L. M., & Knipschild, P. G. (1998). The Delphi list: A criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. Journal of Clinical Epidemiology, 51(12), 1235–1241. https://doi.org/10.1016/s0895-4356(98)00131-0
Wang, X., Zhao, C., Feng, H., Li, G., He, L., Yang, L., … Ai, S. (2023). Associations of insomnia with insulin resistance traits: A cross-sectional and Mendelian randomization study. The Journal of Clinical Endocrinology & Metabolism, 108(8), e574–e582. https://doi.org/10.1210/clinem/dgad089
Yesavage, J. A. (1988). Geriatric depression scale. Psychopharmacology Bulletin, 24(4), 709–711.
*Zhang, H. Z., Zhang, P., Chang, G. Q., Xiang, Q. Y., Cao, H., Zhou, J. Y., … Qin, Y. (2021). Effectiveness of cognitive behavior therapy for sleep disturbance and glycemic control in persons with type 2 diabetes mellitus: A community-based randomized controlled trial in China. World Journal of Diabetes, 12(3), 292.
Zigmond, A. S., & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370.
Zung, W. W. (1965). A self-rating depression scale. Archives of General Psychiatry, 12(1), 63–70.
*Zuo, X., Dong, Z., Zhang, P., Zhang, P., Chang, G., Xiang, Q., … Lou, P. (2020). Effects of cognitive behavioral therapy on sleep disturbances and quality of life among adults with type 2 diabetes mellitus: A randomized controlled trial. Nutr Metab Cardiovasc Dis, 30(11), 1980–1988. https://doi.org/10.1016/j.numecd.2020.06.024
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dawei Xu and Miao Xu are parallel first authors and contributed equally to this work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, D., Cardell, E., Xu, M. et al. Effect of Cognitive Behavioural Therapy in Improving Sleep and Health Status in Patients with Cardiometabolic Syndrome: a Meta-Analysis. J Cogn Ther 17, 122–159 (2024). https://doi.org/10.1007/s41811-023-00189-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41811-023-00189-7