Abstract
In this work, highly ordered ZrO2 nanotube arrays were fabricated on commercial pure Zr substrates through anodic oxidation in the water-based electrolyte at various voltages (30 V, 40 V and 50 V) for 1 h. The monoclinic- and tetragonal-ZrO2 phases were obtained on ZrO2 nanotubes through anodic oxidation. 13 vibration modes have been observed for the samples grown at low voltages (30 V and 40 V), which are assigned to monoclinic symmetry (7Ag + 6Bg), while—with the increasing growth voltage, the dominant phonon peak intensities associated with the monoclinic symmetry 6 times are decreased, and Eg (268 and 645 cm − 1) mode corresponding to tetragonal symmetry is observed. The nanotube array surfaces exhibited hydrophilic and super-hydrophilic behavior compared to the bare Zr surface. The elastic modulus values of ZrO2 nanotube surfaces (14.41 GPa) were highly similar to those of bone structure (10–30 GPa) compared to bare Zr substrate (120.5 GPa). Moreover, hardness values of ZrO2 nanotube surfaces were measured between ∼76.1 MPa and ∼ 283.0 MPa. The critical load values required to separate the nanotubes from the metal surface were measured between ∼1.6 N and ∼26.3 N. The wear resistance of the ZrO2 nanotube arrays was improved compared to that of plain Zr substrate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The clinical use of zirconium (Zr) implants has been increasing due to the general trend towards metal-free solutions and tooth-colored reconstruction materials [1]. Since Zr and its alloys have superior chemical and mechanical properties such as good corrosion resistance, chemical stability, high fracture toughness, high bending and bending strength and low toxicity; it has been one of the top choices for dental and orthopedic implant materials at the last few years [2,3,4,5]. Moreover, thanks to the presence of a natural passive oxide layer on its surface, Zr releases fewer ions than titanium (Ti) in biological fluids such as Hanks' physiological solution [6]. Additionally, despite a few nm thick natural oxide layer (ZrO2) on the Zr protects it against corrosion, it is bio-inert [7, 8]. Hence, as Zr and its alloys lack bioactivity, they are not chemically or biologically bonded to the bone structure. To address this issue, ZrO2/hydroxyapatite structures were coated onto Zr and its alloys using various coating methods [9,10,11,12].
ZrO2-coated Zr and its alloys are promising alternative materials for orthopedic implant applications such as dental roots and femoral components of artificial knee joints. This is due to the excellent properties of ZrO2, including its great hardness, high wear resistance, strong corrosion resistance, and good biocompatibility [13]. To produced ZrO2 surfaces on Zr and its alloys, different surface modification methods have been employed, including plasma spray coating [14], chemical or physical vapor deposition [15], sol–gel [16], plasma electrolytic oxidation [12] and anodic oxidation (AO) [17].
Anodic oxidation is a relatively effective and convenient method for fabricating of ZrO2 / TiO2 nanotubes arrays on Zr / Ti surfaces [18,19,20]. The AO technique offers several advantages, including production of a homogenous nanoscale surface structure with a well-ordered repeating pattern, high purity, an ideal porosity for bone integration, low equipment costs, and straightforward production. Therefore, it stands out compared to other coating methods. The well-ordered oxide nanotube surfaces possess a great surface area and they generally indicate superior interfacial adhesion to metal substrates. Furthermore, ZrO2-based nanotubes have suitable bioactivity [21]. Thus, it is vital to evaluate the mechanical properties of these types of materials for implants.
In this work, well-ordered ZrO2 nanotube surfaces were fabricated on Zr substrates using AO method in water-based electrolyte at various voltages. The influence of the applied voltages on structure, surface and cross-sectional morphology as well as thickness of the nanotube arrays, were examined. Additionally, Raman spectroscopy technique was employed to support XRD analysis. Wettability and PL spectrum analyses of all surfaces were also investigated. Finally, mechanical properties such as hardness-elastic modules and adhesions strength and tribological properties of surfaces were determined by nanoindentation, scratch tester and tribometer, respectively.
Experimental details
The commercial pure Zr (Zr 702) rectangular sheets with the dimensions of 10 mm × 10 mm × 1 mm were used as the substrates and were ground up to 2000# SiC sandpapers. After metallographic preparations, all sheets were ultrasonically cleaned in an acetone bath, followed by rinsing in isopropyl alcohol, and drying in warm air.
A DC (direct current) power supply (GW-Instek PSU 400) was used for the AO process. During the process, the Zr and Pt sheets were served as an anode (working electrode) and a cathode, respectively. The water-based electrolyte was prepared by dissolving 1 M (NH4)2SO4 and 0.2 M NH4F into de-ionized water. The AO process was performed at 30 V, 40 V and 50 V under a constant current (0.02 A) for 1 h and the process temperature was kept below 35 °C. After completing the AO process, all coated-Zr substrates were cleaned in an ultrasonic bath and dried with warm air. The nanotube arrays on Zr substrate are an amorphous form at post-fabrication AO process. Thus, in order to transform amorphous to crystalline form without any morphological changes, the heat treatment was applied to nanotube coated-Zr substrates at 450 °C during 1 h under air atmosphere, which are known as optimum parameters for the treatment in the literature [22,23,24].
phase structures of the surfaces were analyzed by XRD (Rigaku Dmax 2200) with Cu-Kα radiation at a scanning speed of 1° min−1 from 10° to 90°. A custom-built micro-Raman and micro-PL setup were used to excite the samples with a 532 nm CW laser (Novanta Photonics gem532) and collect the signal from the samples, via a 50 × NIR objective (NA = 0.42). The excitation laser beam is focused to a spot of ~ 1.2 μm in diameter on the sample placed on a XYZ piezo stage. Laser power on the sample is 2 mW (Power density ≈ 1.77 × 105 W/cm2 and \(0.2 {\text{mW}}\) (Power density ≈ 1.77 × 104 W/cm2) for the Raman and PL measurements, respectively. Emission is collected by the same objective. An 1800 g/mm grating used provides a spectral resolution of ≈ 0.6 cm−1. The surface and cross-sectional morphologies of the samples were analyzed using surface SEM (Philips XL30S FEG) and cross-sectional SEM (FEI Versa 3D Dual Beam system equipped with an EDAX Octone Super detector). The elemental composition and quantities through the surface were investigated using energy-dispersive X-ray spectroscopy (EDX) attached to SEM. The surface roughness and topography were evaluated over 2.5 μm × 2.5 μm area using AFM (Veeco Nanoscope IV). To measure contact angles, 1 μL de-ionized water was initially dropped onto the surfaces. The average contact angle values were examined within 10 s using sessile drop technique with a contact angle goniometer (Dataphysics OCA 15EC). The hardness-elastic modulus values of bare Zr and ZrO2 nanotube arrays were investigated using a nanoindentation device (Hysitron TI 950). The tests applied a maximum load of 500 µN and were carried out on the surfaces, measuring up to 5 tracks by a Berkovich indenter. To investigate adhesion behaviors such as critical loads (Lc1, Lc2 and Lc3), the micro/macro scratch tests (CSM Instrument) were performed on nanotube surfaces using Rockwell C diamond indenter of 50 µm. Lc values of the nanotube coatings were obtained from load – distance graphs. A progressive load ranging from 0.03 N to 30 N was applied along 8.5 mm on nanotube-coated surfaces. Friction tests were conducted using a WC (tungsten carbide; the diameter of 6 mm) ball-on-disc type tribometer (CSM Instruments). The linear reciprocating tests were performed under 1 N normal load with a sliding speed of 3 × 10–2 m.s−1 corresponding to a sliding distance of 30 m.
Results and discussion
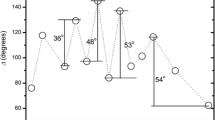
Figure 1 shows the XRD spectrum of the nanotube surfaces produced on Zr substrates by the AO process followed by annealing. To convert the amorphous form into a crystalline form, it is essential to apply heat treatment without altering surface morphology. It is also employed to eliminate any remaining fluoride as reported in the literature [25]. After annealing at 450 °C in air, the phases of zirconium (Zr, # 005–0665), baddeleyite-ZrO2 (monoclinic (m-ZrO2), # 037–1484) and zirconium oxide-ZrO2 (tetragonal (t-ZrO2), # 050–1089) were observed on the surfaces. The baddeleyite-ZrO2 and zirconium oxide-ZrO2 refer to crystallographic forms of monoclinic (m-ZrO2) and tetragonal (t-ZrO2), respectively. The XRD peaks located at 2θ = 35.31°, 36.55°, 48.05°, 63.64°, and 73.64° correspond to the crystallographic orientations (002), (101), (102), (103), (004) of the Zr (# 005–0665; space group: P63/mmc; lattice parameters: 3.23 Å × 3.23 Å × 5.15 Å < 90.0 × 90.0 × 120.0 >). The XRD peaks of the annealed nanotube surfaces at 2θ = 24.08°, 28.21°, 31.52°, 33.94°, 34.05°, 38.62°, 40.77°, 45.45°, 49.28°, 50.52°, 55.41°, 60.05°, and 65.51 correspond to the (110), (− 111), (111), (200), (020), (− 210), (− 112), (− 202), (− 212), (022), (221), (131) and (230) crystallographic orientations of the m-ZrO2 (# 037–1484; space group: P21/a; lattice parameters: 5.31 Å × 5.21 Å × 5.15 Å < 90.0 × 99.218 × 90.0), respectively. In addition, the XRD peaks at 2θ = 30.42°, 35.31°, 43.32°, 50.52°, 59.72° and 60.09° are attributed to the (011), (110), (012), (020), (013) and (121) crystallographic orientations of the t-ZrO2 phase, respectively (# 050–1089; space group: P42/nmc; lattice parameters: 3.60 Å × 3.60 Å × 5.15 Å < 90.0 × 90.0 × 90.0 >).
The phase of t-ZrO2 forms at temperatures above 1170 °C as m-ZrO2 occurs at temperatures below 1170 °C. The phase of Zr obtained from XRD spectrum is originated from the substrate. The amount of detected Zr on nanotubes produced at 30 V is greater than those at 40 V and 50 V, attributed to the increased nanotube thicknesses with higher anodic oxidation (AO) potential, as depicted in Figs. 2 and 3. Similarly, the nanotubes produced at 40 V exhibit the highest quantity of m-ZrO2 compared to those formed at 30 V and 50 V. However, their wall thickness is lower than that observed in nanotubes produced at 50 V. Furthermore, the nanotubes produced at 50 V exhibit the highest quantity of t-ZrO2 compared to those formed at 30 V and 40 V.
Raman spectroscopy is a powerful complementary technique to X-ray diffraction for structural characterisations of the crystals [26,27,28]. It is particularly useful for differentiating between various crystal phases for the same materials due to their characteristic lattice vibrations bands. Raman spectroscopy is used to justify the crystal phase of ZrO2. As mentioned above, ZrO2 can exhibit various crystalline phases, namely monoclinic (M), tetragonal (T) and cubic (C), depending on the specific growth methods and conditions employed [29, 30]. Based on the group theory and first-principles studies 13 (7Ag + 6Bg), 3 (2Eg, A2g) and 1 (T1g) modes are expected for M, T and C phases, respectively [31, 32].
Figure 2a shows the Raman spectrum of the ZrO2 samples. On the samples grown at low voltages (30 V and 40 V), 13 bands corresponding to monoclinic symmetry (7Ag + 6Bg) were observed, while no bands related to higher symmetry phases (T or C) detected. This result supports the XRD findings, indicating the single crystal structure in the low voltage grown samples. On the other hand, as the growth voltage increases, the dominant phonon peak intensities associated with the monoclinic symmetry decrease by six times and Eg (268 and 645 cm−1) mode corresponding to tetragonal symmetry is observed.
The bandgap and the emission wavelength of the ZrO2 are also dependent on the specific growth methods and conditions. Chauhan et al. found that the bandgap of ZrO2 thin film is 4.2 eV and Kumari et al. showed that microstructured form of ZrO2 has a bandgap of 3 eV [33]. Emeline et al. showed that bulk ZrO2 has two emission peaks (5 eV and 2.21 eV), which are originated from direct band gap emission and defect states [34]. Figure 2b shows the PL spectrum of the ZrO2 samples. The maximum PL intensities are observed at wavelengths of 575 nm (2.15 eV), 587 nm (2.11 eV) and 603 nm (2.05 eV) for the samples grown at 30 V, 40 V and 50 V, respectively. The redshift of the emission energies can be attributed to the increments on the tube diameters as applied voltage during the process increases. The low energy shoulder at PL spectrum at 625 nm can be attributed to the defect states-related radiative transition that became dominant with increasing growth voltage. It can be concluded that the optimum growth voltage for ZrO2 is 30 V with respect to optical properties.
Figure 3 indicates surface SEM morphologies and the distribution of outer diameter and wall thicknesses of ZrO2 nanotubes produced on Zr substrates at various voltage values. The outer diameter and wall thicknesses of ZrO2 nanotubes were examined by Fiji ImageJ software. As shown in Figs. 3a, c and e, nanotube arrays on Zr were clearly observed. Anodic layers with the 2-D (two dimensional) network possess vertically aligned and relatively well-ordered nanotubes/nanopores. However, the dimensions of nanotubes (outer diameter and wall thickness) vary significantly across the Zr surface and depend on applied voltage values. The outer diameters and wall thicknesses of nanotubes usually increased with increasing AO voltage values as given in Table 1. The most probable outer diameters of the nanotubes were approximately examined between 77.8 nm and 113.0 nm. However, the ordered nanotubes transitioned to disordered arrays with increasing voltage. Furthermore, the surfaces of nanotubes produced at 50 V were covered by secondary structures and the majority of nanotube morphology disappeared at this voltage.
A thin and tightly adhering ZrO2 passive oxide layer forms spontaneously on the Zr surface when exposed to atmospheric air [35]. While the naturally formed oxide layer enhances corrosion resistance, it may not provide sufficient mechanical, tribological, and bioactive properties. This causes accumulation of adsorbed bone tissue around the implant. Eventually, this can result in slow healing and weaken the implant-bone interface [36]. Texturing a Zr implant surface with nanoporous or nanotubes features increases its surface area, roughness, and wettability, enhancing bioactivity [36, 37]. Thus, to improve bioactivity (cell attachment and growth), the fabrication of ZrO2 nanotubes on Zr could be proposed. The formation mechanism of ZrO2 nanotube arrays on Zr develops in four main stages [24]. Rapid growth occurs in the barrier oxide layer due to high current followed by a gradual reduction in ionic current and an increase in electronic current with the thickness of the oxide layer. The anions, influenced by electric field, penetrate the barrier oxide layer, forming the anion-contaminated layer. Simultaneously, small bubbles and anion-contaminated layers disperse in the barrier oxide layer (Step I) [38]. The probability of forming of oxygen bubbles increases as the electric current gradually increases. Thus, the dense small oxygen bubbles in the oxide structure expand with each other. This results in forming a pattern of oxygen bubbles (Step II) [39,40,41,42]. Meanwhile, the barrier oxide grows around the oxygen bubble. Simultaneously, the oxide layer laterally deforms under the nanotubes [43]. Consequently, greater nanotube nuclei are occurred within the anodic oxide film. It is reported that the electronic current of ZrO2 nanotubes consistently increases but slows down over time compared to the traditional growth mechanism of TiO2 nanotubes. Eventually, ZrO2 nanotubes produce significantly more oxygen bubbles than TiO2 nanotubes. When the internal oxygen pressure exceeds atmospheric and the anion-contaminated layer on the pressures, the oxygen is released (Step III). Thanks to the stable oxygen release, the electrolyte gently travels to the bottom of the nuclei hole, resulting in a decrease in the concentration of anionic F− in the nanotubes.. F− ions are responsible for the dissolution of the oxide layer and the formation of nanotubes on Zr substrate [5]. This results in a continuous reduction in the total current. During Step IV, the transformation from nuclei pores to nanotubes begins, with adjacent nanotubes being squeezed towards each other due to the expansion of the gaseous. As a result, the nanotube walls grow around the oxygen bubble. The continuous development of oxygen at the bottom of the nanotubes serves as a driving force for "viscous flow" [24]. According to the field assisted dissolution (FAD) theory, electrolyte/oxide interfacing FAD reaction which exists in the entire AO process is as Eq. 3 [44, 45].
Figure 4 shows the cross-sectional SEM structures of ZrO2 nanotubes produced on Zr substrates at various voltage values. The length of the nanotubes increase with higher voltage values as mentioned above. The average thicknesses of the nanotubes were measured as as 10.05 ± 0.5 µm for 30 V, 12.66 ± 0.16 µm for 40 V and 39.66 ± 1.86 µm for 50 V. However, as observed in Figs. 4d and f, the nanotubes arrays appear discontinuous compared to Fig. 4b. It can be inferred that the coating integrity was destroyed above 30 V throughout cross-sectional area, in contrast to surface images. This situation negatively affected the adhesion strength of the coatings whereas nanotube lengths increased with increasing voltage value as seen in adhesion test results. Moreover, the nanotube structures produced at 40 V and 50 V were extended in layers in a broken state as seen at high magnification SEM images. It is thought that during tube formation, stress-induced fractures occur in the tube layer.
Figure 5 indicates surface EDX mapping images of ZrO2 nanotube arrays produced on Zr at different voltages. The green and red regions correspond to Zr and O elements on nanotube surfaces, respectively. Both Zr and O elements exhibit uniform distribution across the nanotube arrays at different voltage values. As expected, the Zr and O come from the ZrO2 nanotube structure on Zr substrate. Detailed measurements of the amounts of Zr and O elements in terms of atomic and weight percentages are provided in Table 2.
The roughness and topography of all surfaces were analyzed by AFM, as shown in Fig. 6. The surface roughness of ZrO2 nanotube arrays produced at 30 V, 40 V and 50 V, as well as plain Zr, were measured. The surface roughness of plain Zr was determined to be 2.00 nm, while that of the ZrO2 nanotube arrays at 30 V, 40 V and 50 V were measured at 3.57 nm, 6.98 nm and 8.21 nm, respectively. In Fig. 3, it is evident that the most probable outer diameter of nanotubes increases with increasing potential value. Additionally, the top of some nanotubes produced at 50 V were filled and covered with an additional layer. Thus, the roughness value increased proportionally with the potential values.
The wettability properties of bare Zr and ZrO2 nanotube arrays on Zr were investigated using the sessile drop contact angle goniometer technique. Figure 7 shows the contact angle (CA) images of all surfaces within 10 s at post-contacted droplet. A CA with 65° is considered the frontier for distinguishing between hydrophilic and hydrophobic surfaces [46], while super hydrophilic surfaces exhibit CA values lower than 10° [47]. According to the results, the Zr substrate exhibits a hydrophobic property because the CA value is higher than 65°. The ZrO2 nanotube surface fabricated at 30 V indicates hydrophilic character, whereas both nanotube surfaces produced at 40 V and 50 V exhibit super hydrophilic behaviors within 10 s. Moreover, the nanotube surface produced at 50 V completely adsorbed water molecules, making it impossible to measure the CA. The nanotube surfaces readily absorb water molecules due to their porous surfaces compared to bare Zr substrate. With increasing applied AO voltage, the diameter of nanotubes increased, and the interface exists between water and air in the nanotube surfaces. The entrance of water into nanotube structures is influenced by capillary and gravitational forces [48]. The capillary forces of ZrO2 nanotubes reduced with increasing voltage for annealed surfaces, resulting in improved hydrophilic properties of the nanotube surfaces.
Several investigations have explored the wetting behavior of ZrO2 nanotubes in the literature. Raghu and Killian studied ZrO2 nanotubes modified with a non-fuorinated substance and achieved a CA value 160°, indicating super hydrophobic properties [49]. Raghu et al. fabricated super hydrophobic ZrO2 nanotube surfaces [50]. Wang et al. examined variation on wettability of anodic zirconium oxide nanotube surface from 10 to 25 V. The increased CA values were obtained from about 10.6° to 22.8° and the CA values of their produced surfaces increased with increasing voltage (from 10 to 25 V) [51]. Nezhad et al. investigated adhesion properties of ZrO2 nanotube coatings produced at 80 V for 120 min within ethylene glycol-based electrolyte up to 400 °C various annealing temperatures and obtained that the CA values of water were measured between 5.6° and 11.5° [48]. In this work, the CA values of ZrO2 nanotube surfaces at increasing voltage values were importantly reduced compared to above literature studies and the surfaces indicate hydrophilic and super hydrophilic behaviors.
The hardness and elastic modulus values measured on bare Zr and ZrO2 nanotube arrays are presented in Table 3. The average hardness and elastic modulus values of bare Zr were determined to be approximately 2225.8 ± 416.3 MPa and 120.5 ± 11.7 GPa, respectively. It's noteworthy that the elastic modulus of Zr and monoclinic polycrystalline ZrO2 was reported as 92 GPa and 241 GPa at room temperature, respectively [52,53,54]. The elastic modulus value (120.5 GPa) of plain Zr is a bit much compared to previous literature works (92 GPa). One of the possible reasons of difference between elastic modulus values could be measurement techniques. Wheeler et at investigated mechanical properties of cerium and a cerium–5 wt% lanthanum alloy by nanoindentation and ultrasonic velocity measurements and reported that the mean nanoindentation elastic modulus was on average 14% higher than that determined from the ultrasonic velocity measurements [55]. Another possible reason could be that the nanoindenter initially contacted with naturally formed passive oxide layer with high elastic modulus on Zr surfaces. Moreover, the elastic modulus of bone structure was approximately measured as 15–30 GPa in the literature [56,57,58]. Thus, elastic modulus of bare Zr is considerably higher than that of bone structure. In terms of Zr metal, this situation refers to elastic mismatching for implant application. The elastic modulus of the nanotube surfaces produced on Zr at 30 V was measured as 14.41 GPa. This promising result value is highly comparable to the bone structure [56,57,58].
The lengths of nanotubes indicated that nanoindenter contacted only the tubes without contacting Zr substrate. Consequently, mechanical data such as hardness and elastic modulus were measured for all nanotube arrays. It is well known that the hardness of bulk ZrO2 is higher than that of plain metallic Zr. However, it was observed that the hardness values of ZrO2 nanotubes are lower than those of plain Zr, as expected, since the nanoindenter easily penetrates into oxide nanotubes compared to bulk ZrO2. The most significant issue here is the elastic mismatch for dental and orthopedic implant applications. Therefore, to prevent implant loss, reducing the elastic mismatch effect is vital for implant applications. The elastic modulus of nanotube arrays produced at 30 V (14.41 GPa) is very close to that of bone structure (15–30 GPa) and indicated lower elastic modulus values respect to the plain Zr substrate. The elastic modulus of ZrO2 nanotubes at 30 V was ultimate compared to high potentials. This value was close to bone structure for biomaterials application. A porous nanotube array characteristically exhibits a low elastic modulus. This leads to better biomechanical compatibility with bone. It is well known that this diminishes the stress shielding problem in the literature [59]. The nanotube arrays on Zr surface are well-ordered porous structures. However, the well-ordered nanotube structures with small diameter produced at low voltage values, such as 30 V, are uniformly separated across whole surface. In this case, the nanotube surfaces can behave like a compact structure. Thus, the nanoindenter cannot penetrate deeper by encountering resistance. Eventually, mechanical measurements can be taken along nanotube arrays at lower voltage values. However, above 30 V, the hardness and elastic modulus of the nanotube arrays decreased despite the growth of nanotubes with increased voltages. The surface densities significantly decrease whereas the length and the diameter of nanotube arrays increase with increasing potential. Therefore, the nanoindenter penetrate more easily onto nanotube structures produced above 30 V. Eventually, the load carrying capability of the nanotube arrays above 30 V is not sufficient due to their porous structure. Thus, it can be claimed that the ZrO2 nanotube arrays at 30 V on Zr is appropriate for load carrying.
The adhesion strengths of ZrO2 nanotubes on Zr at different voltage values were measured using a progressive mode micro/macro scratch tester device. The scratch tester data including the frictional coefficient, frictional force and penetration depth obtained at 50 µm radius diamond indenter for nanotube surfaces, are shown in Fig. 8 and Table 4. The critical load (Lc) was measured as a drastic alteration point in the penetration depth profile coincided with the coating detachment. The Lc values were also evaluated according to the scratch curves of the normal load via distance. The Lc1, Lc2 and Lc3 loads, which were considered as the coating failure, refer to critical loads for various failures on the nanotube arrays. The Lc1 is the first failure on the nanotube surfaces refers to initial cracking. The Lc2 is the second failure refers to extensive cracking. The Lc3 is the final failure refers to delamination of the nanotube surfaces from the Zr surface. Furthermore, the Lc1, Lc2 and Lc3 are used to characterize the cohesive failure, cohesive—adhesive failures and total detachment [48, 60]. After scratch test, the microstructure images correspond to the Lc1, Lc2 and Lc3 were also monitored by external optical microscope as seen in Fig. 9. The characteristic value Lc for nanotube surfaces depends on some parameters such as microstructure, phase structure, thickness, hardness, etc.[61, 62]. Lc values are detected using methods such as microscopic imaging evaluation, evaluation of the diffusion depth of the scratch point, acoustic reflection mode scanning microscopy, acoustic emission inspection and 3D force recording as proposed in literature [63]. Thus, the higher Lc value means the greater adhesion and bonding strength between substrate and coating layers.
The ZrO2 nanotube arrays on Zr substrate produced at 30 V indicated strong adhesion between the Zr substrate and nanotube layers compared to those produced at 40 V and 50 V. The nanotube layers fabricated at 50 V resulted in the weakest adhesion despite being the thickest coating. This situation is closely related to the continuity of the nanotube structures. The amount of oxide phases increased with increasing potential as seen in Fig. 1. However, the coating integrity was destroyed above 30 V as shown in Fig. 4. Thus, Lc values decreased with increasing AO voltage values.
A few works have investigated the adhesion analyses of ZrO2 nanotube arrays on Zr in the literature. Nezhad et al. investigated adhesion properties of ZrO2 nanotube coatings produced at 80 V for 120 min using an ethylene glycol-based electrolyte at various annealing temperatures up to 400 °C, obtaining an Lc value of approximately 2.5 N [48]. Zhang et al. assessed adhesion between anodized monoclinic- and cubic-ZrO2 nanotube films and Zr substrates across variying electrolyte concentrations of fluoride ions. Their findings indicated that the critical load (Lc) ranged between 4.5 N and 24.5 N [13]. In this work, adhesion strength of monoclinic- and tetragonal-ZrO2 nanotube surfaces at all voltage values were significantly improved compared to the findings reported in the literature.
The friction coefficients of both plain Zr and ZrO2 nanotubes were investigated under dry environment conditions using a standard tribometer. The friction coefficients of ZrO2 nanotubes produced at 30 V, 40 V and 50 V were measured as 0.682, 0.675 and 0.647, respectively while the friction coefficients of plain Zr substrate was 0.652, as illustrated in Fig. 10. It is widely acknowledged that the friction coefficient and the wear rate are directly proportional. However, although this is widely accepted, no one has managed to establish a mathematical relationship between the two quantities as reported in the literature [64]. The Zr substrate possesses the greatest hardness value compared to ZrO2 nanotube arrays surfaces as given in Table 3. However, as seen in Fig. 10, the friction coefficient values of ZrO2 nanotubes coated Zr surfaces are nearly close to that of plain Zr substrate. The friction coefficient of plain Zr substrate remained almost stable through wear test as shown in Fig. 10a. The friction coefficients of ZrO2 nanotubes produced at 30 V and 40 V sharply increased to fabricate a worn trace as seen in Figs. 10b and c. After that, the friction coefficients of ZrO2 nanotubes produced at 30 V and 40 V decreased rapidly and carried out stable through the wear test. Furthermore, after a worn trace created on the surface, the friction coefficient curve of ZrO2 nanotubes produced at 50 V remains stable through the test as shown in Fig. 10d. The possible reason of this situation could be that the particles detached from the outer layer act as solid lubricants between the ball and the inner layer. Thus, the friction coefficient exhibits stable behavior.
The wear tracks of plain Zr and ZrO2 nanotubes were monitored by SEM as seen in Fig. 11. Many plastic deformations were observed on the worn Zr substrate (Fig. 11a), as expected. However, there were no plastic deformations on ZrO2 nanotubes at post-wear test. It has been observed that the wear track regions of nanotube arrays gradually become smaller from 30 to 50 V. The wear track region of ZrO2 nanotubes at 50 V is smaller than that of other nanotube arrays surfaces and the plain Zr substrate despite the fact that ZrO2 nanotubes at 50 V have lowest hardness compared to other surfaces. However, the tube length and diameter values of ZrO2 nanotubes produced at 50 V are the highest compared to the others, as shown in Table 1 and Fig. 4. A WC counterface contacts much smaller area on the coating surface produced at 50 V compared to the those at 30 V and 40 V. In addition, the longer tube length of ZrO2 nanotubes at 50 V can take an advantage for wear test. Thus, the tube lengths and diameters could be dominant parameter compared to hardness for tribology test of ZrO2 nanotube surfaces.
Conclusions
In this study, the well-ordered ZrO2 nanotube arrays were formed on Zr surface at 30 V, 40 V and 50 V using water-based electrolyte via AO process. Since the ZrO2 nanotubes were amorphous after the AO process, all nanotube surfaces were transformed from amorphous to crystalline at 450 °C for 1 h without any morphological changing. Monoclinic- and tetragonal-based ZrO2 phases were observed on nanotube surfaces. The Zr and O elements were detected across nanotube-coated surfaces. 13 vibration modes were observed for the samples grown at low voltages (30 V and 40 V), which were assigned to monoclinic symmetry (7Ag + 6Bg), while—with the increasing growth voltage, the dominant phonon peak intensities associated with the monoclinic symmetry 6 times are decreased, and Eg (268 and 645 cm−1) mode corresponding to tetragonal symmetry was observed. The most probable tube length, wall thickness and outer diameter of the nanotube surfaces were evaluated as 10 μm – 40 μm, 20 nm – 28 nm and 78 nm – 113 nm, respectively. The nanotube array surfaces exhibited hydrophilic and super hydrophilic behavior compared to bare Zr surface. The elastic modulus values of ZrO2 nanotube surfaces (14.41 GPa) were highly similar to bone structure (10–30 GPa) compared to bare Zr substrate (120.5 GPa). Moreover, hardness values of ZrO2 nanotube surfaces were measured between ∼76.1 MPa and ∼ 283.0 MPa. The critical load values required to separate the nanotubes from the metal surface were measured between ∼1.6 N and ∼26.3 N. The friction coefficient of the nanotube surfaces were measured between 0.647 and 0.687 under 1 N normal load in a dry environment conditions.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Osman, R.B., Swain, M.V.: A critical review of dental implant materials with an emphasis on titanium versus zirconia. Materials (Basel) 8, 932–958 (2015). https://doi.org/10.3390/ma8030932
Sollazzo, V., Pezzetti, F., Scarano, A., et al.: Zirconium oxide coating improves implant osseointegration in vivo. Dent. Mater. 24, 357–361 (2008). https://doi.org/10.1016/j.dental.2007.06.003
Sanchez, A.G., Schreiner, W., Duffo, G., Cere, S.: Surface characterization of anodized zirconium for biomedical applications. Appl. Surf. Sci. 257, 6397–6405 (2011). https://doi.org/10.1016/j.apsusc.2011.02.005
Sanchez, A.G., Ballarre, J., Orellano, J.C., Duffo, G., Cere, S.: Surface modification of zirconium by anodisation as material for permanent implants: in vitro and in vivo study. J. Mater. Sci-Mater. Med. 24, 161–169 (2013). https://doi.org/10.1007/s10856-012-4770-8
Hosseini, M.G., Daneshvari-Esfahlan, V., Maleki-Ghaleh, H.: Effect of water and fluoride content of anodizing electrolyte on morphology and corrosion behavior of ZrO2-nanotubes developed on zirconium implant. J. Mater. Eng. Perform. 25, 1129–1135 (2016). https://doi.org/10.1007/s11665-016-1904-z
Tsutsumi, Y., Nishimura, D., Doi, H., Nomura, N., Hanawa, T.: Difference in surface reactions between titanium and zirconium in Hanks’ solution to elucidate mechanism of calcium phosphate formation on titanium using XPS and cathodic polarization. Mater. Sci. Eng. C 29, 1702–1708 (2009). https://doi.org/10.1016/j.msec.2009.01.016
Katunar, M.R., Sanchez, A.G., Ballarre, J., et al.: Can anodised zirconium implants stimulate bone formation? Preliminary study in rat model. Prog. Biomater. 3, 1–10 (2014). https://doi.org/10.1007/s40204-014-0024-9
Hanawa, T.: In vivo metallic biomaterials and surface modification. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 267, 260–266 (1999). https://doi.org/10.1016/s0921-5093(99)00101-x
Yildiz, T., Durdu, S., Ozcan, K., Usta, M.: Characterization and investigation of biological properties of silver nanoparticle-doped hydroxyapatite-based surfaces on zirconium. Sci. Rep. 13, 6773 (2023). https://doi.org/10.1038/s41598-023-33992-5
Chopra, D., Jayasree, A., Guo, T., Gulati, K., Ivanovski, S.: Advancing dental implants: Bioactive and therapeutic modifications of zirconia. Bioactive Materials 13, 161–178 (2022). https://doi.org/10.1016/j.bioactmat.2021.10.010
Molaei, M., Attarzadeh, N., Fattah-alhosseini, A.: Tailoring the biological response of zirconium implants using zirconia bioceramic coatings: A systematic review. J. Trace Elem. Med. Biol. 66, 1–8 (2021). https://doi.org/10.1016/j.jtemb.2021.126756
Fidan, S., Muhaffel, F., Riool, M., et al.: Fabrication of oxide layer on zirconium by micro-arc oxidation: Structural and antimicrobial characteristics. Mater. Sci. Eng. C-Mater. Biol. Appl. 71, 565–569 (2017). https://doi.org/10.1016/j.msec.2016.11.035
Zhang, L., Wang, S., Han, Y.: Interfacial structure and enhanced adhesion between anodized ZrO2 nanotube films and Zr substrates by sedimentation of fluoride ions. Surf. Coat. Technol. 212, 192–198 (2012). https://doi.org/10.1016/j.surfcoat.2012.09.049
Shanmugavelayutham, G., Yano, S., Kobayashi, A.: Microstructural characterization and properties of ZrO2/Al2O3 thermal barrier coatings by gas tunnel-type plasma spraying. Vacuum 80, 1336–1340 (2006). https://doi.org/10.1016/j.vacuum.2006.01.056
Leushake, U., Krell, T., Schulz, U., Peters, M., Kaysser, W.A., Rabin, B.H.: Microstructure and phase stability of EB-PVD alumina and alumina/zirconia for thermal barrier coating applications. Surf. Coat. Technol. 94–95, 131–136 (1997). https://doi.org/10.1016/S0257-8972(97)00490-8
Brenier, R., Mugnier, J., Mirica, E.: XPS study of amorphous zirconium oxide films prepared by sol-gel. Appl. Surf. Sci. 143, 85–91 (1999). https://doi.org/10.1016/s0169-4332(98)00901-5
Li, P., Wang, H., Ni, Y., et al.: Unraveling the six stages of the current-time curve and the bilayer nanotubes obtained by one-step anodization of Zr. Nanoscale Adv. 4, 582–589 (2022). https://doi.org/10.1039/d1na00692d
Durdu, S., Cihan, G., Yalcin, E., Altinkok, A.: Characterization and mechanical properties of TiO2 nanotubes formed on titanium by anodic oxidation. Ceram. Int. 47, 10972–10979 (2021). https://doi.org/10.1016/j.ceramint.2020.12.218
Durdu, S., Sancak, M., Yalcin, E., Usta, M., Akagunduz, E., Altinkok, A.: Surface characterization of TiO2 nanotube arrays produced on Ti6Al4V alloy by anodic oxidation. Surf. Coat. Technol. 428, 127903 (2021). https://doi.org/10.1016/j.surfcoat.2021.127903
Bashirom, N., Tan, W.K., Kawamura, G., Matsuda, A., Lockman. Z.: Comparison of ZrO2, TiO2, and alpha-Fe2O3 nanotube arrays on Cr(VI) photoreduction fabricated by anodization of Zr, Ti, and Fe foils. Mater. Res. Express 7, 1–9 (2020). https://doi.org/10.1088/2053-1591/ab8ee3
Wang, C., Wang, Y., Zhang, G., et al.: Preparation of a novel transplant material, zirconium oxide (ZrO2) nanotubes, and characterizations research. Ann. Transplant. 25, e924272 (2020). https://doi.org/10.12659/aot.924272
Stępień, M., Handzlik, P., Fitzner, K.: Synthesis of ZrO2 nanotubes in inorganic and organic electrolytes by anodic oxidation of zirconium. J. Solid State Electrochem. 18, 3081–3090 (2014). https://doi.org/10.1007/s10008-014-2422-2
Raghu, S.N.V., Hartwich, P., Patalas, A., et al.: Nanodentistry aspects explored towards nanostructured ZrO2: Immobilizing zirconium-oxide nanotube coatings onto zirconia ceramic implant surfaces. Open Ceram. 14, 100340 (2023). https://doi.org/10.1016/j.oceram.2023.100340
Ni, Y.L., Li, C.Y., Chen, J.D., et al.: Formation process of zirconia nanotubes and porous structures and model of oxygen bubble growth. Ceram. Int. 48, 495–502 (2022). https://doi.org/10.1016/j.ceramint.2021.09.125
Zalnezhad, E., Hamouda, A.M.S., Jaworski, J., Do Kim, Y.: From zirconium nanograins to zirconia nanoneedles. Sci. Rep. 6, 33282 (2016). https://doi.org/10.1038/srep33282
Sarcan, F., Dönmez, Ö., Kara, K., et al.: Bismuth-induced effects on optical, lattice vibrational, and structural properties of bulk GaAsBi alloys. Nanoscale Res. Lett. 9, 119 (2014). https://doi.org/10.1186/1556-276X-9-119
Sarcan, F., Fairbairn, N.J., Zotev, P., et al.: Understanding the impact of heavy ions and tailoring the optical properties of large-area monolayer WS2 using focused ion beam. npj 2D Mater. Appl. 7, 23 (2023). https://doi.org/10.1038/s41699-023-00386-0
Erol, A., Akalin, E., Sarcan, F., et al.: Excitation energy-dependent nature of Raman scattering spectrum in GaInNAs/GaAs quantum well structures. Nanoscale Res. Lett. 7, 656 (2012). https://doi.org/10.1186/1556-276X-7-656
Zhao, X., Vanderbilt, D.: Phonons and lattice dielectric properties of zirconia. Phys. Rev. B 65, 075105 (2002). https://doi.org/10.1103/PhysRevB.65.075105
Ciszak, C., Mermoux, M., Gutierrez, G., et al.: Raman spectra analysis of ZrO2 thermally grown on Zircaloy substrates irradiated with heavy ion: Effects of oxygen isotopic substitution. J. Raman Spectrosc. 50, 425–435 (2019). https://doi.org/10.1002/jrs.5513
Singh, F., Rawat, M., Gautam, S.K., Ojha, S.: Micro-Raman investigations on zirconium oxide film during swift heavy ion irradiation to study crystalline-to-crystalline phase transformation kinetics by cascade overlap model. J. Appl. Phys. 126, 1–7 (2019). https://doi.org/10.1063/1.5090309
Rawat, M., Das, A., Shukla, D.K., et al.: Micro-Raman and electronic structure study on kinetics of electronic excitations induced monoclinic-to-tetragonal phase transition in zirconium oxide films. RSC Adv. 6, 104425–104432 (2016). https://doi.org/10.1039/c6ra14199d
Chauhan, V., Gupta, D., Upadhyay, S., et al.: Influence of high dose gamma radiation on optical, physico-chemical and surface morphology properties of nanocrystalline ZrO2 thin films. Opt. Mater. 126, 112125 (2022). https://doi.org/10.1016/j.optmat.2022.112125
Emeline, A., Kataeva, G.V., Litke, A.S., Rudakova, A.V., Ryabchuk, V.K., Serpone, N.: Spectroscopic and photoluminescence studies of a wide band gap insulating material: powdered and colloidal ZrO2 sols. Langmuir 14, 5011–5022 (1998). https://doi.org/10.1021/la980083l
Sowa, M., Łastówka, D., Kukharenko, A.I., et al.: Characterisation of anodic oxide films on zirconium formed in sulphuric acid: XPS and corrosion resistance investigations. J. Solid State Electrochem. 21, 203–210 (2017). https://doi.org/10.1007/s10008-016-3369-2
Noothongkaew, S., Ariyachaokun, K., Pansri, S.: Enhanced bioactivity and antibacterial properties of anodized ZrO2 implant coatings via optimized nanoscale morphology and timed antibiotic release through PLGA overcoat. Ceram. Int. 47, 33775–33787 (2021). https://doi.org/10.1016/j.ceramint.2021.08.289
Wang, L.N., Luo, J.L.: Electrochemical behaviour of anodic zirconium oxide nanotubes in simulated body fluid. Appl. Surf. Sci. 258, 4830–4833 (2012). https://doi.org/10.1016/j.apsusc.2012.01.043
Yu, M.S., Li, C., Yang, Y.B., et al.: Cavities between the double walls of nanotubes: Evidence of oxygen evolution beneath an anion-contaminated layer. Electrochem. Commun. 90, 34–38 (2018). https://doi.org/10.1016/j.elecom.2018.03.009
Zhu, X.F., Song, Y., Liu, L., et al.: Electronic currents and the formation of nanopores in porous anodic alumina. Nanotechnology 20, 1–7 (2009). https://doi.org/10.1088/0957-4484/20/47/475303
Zhu, X.F., Song, Y., Yu, D.L., Zhang, C.S., Yao, W.: A novel nanostructure fabricated by an improved two-step anodizing technology. Electrochem. Commun. 29, 71–74 (2013). https://doi.org/10.1016/j.elecom.2013.01.018
Deen, K.M., Farooq, A., Raza, M.A., Haider, W.: Effect of electrolyte composition on TiO2 nanotubular structure formation and its electrochemical evaluation. Electrochim. Acta 117, 329–335 (2014). https://doi.org/10.1016/j.electacta.2013.11.108
Li, X., Li, C.Y., Gong, T.L., et al.: Comparative study on the anodizing process of Ti and Zr and oxide morphology. Ceram. Int. 47, 23332–23337 (2021). https://doi.org/10.1016/j.ceramint.2021.05.046
Zhang, S.Y., Yu, D.H., Li, D.D., et al.: Forming Process of Anodic TiO2 Nanotubes under a Preformed Compact Surface Layer. J. Electrochem. Soc. 161, E135–E141 (2014). https://doi.org/10.1149/2.0661410jes
Sulka, G.D., Kapusta-Kolodziej, J., Brzozka, A., Jaskula, M.: Anodic growth of TiO2 nanopore arrays at various temperatures. Electrochim. Acta 104, 526–535 (2013). https://doi.org/10.1016/j.electacta.2012.12.121
Chen, C.Y., Ozasa, K., Kitamura, F., et al.: Self-organization of TiO2 Nanobamboos by Anodization with Deep Eutectic Solvent. Electrochim. Acta 153, 409–415 (2015). https://doi.org/10.1016/j.electacta.2014.11.084
Kwon, Y.S., Park, J.W.: Osteogenic differentiation of mesenchymal stem cells modulated by a chemically modified super-hydrophilic titanium implant surface. J. Biomater. Appl. 33, 205–215 (2018). https://doi.org/10.1177/0885328218786873
Nie, Y., Kalapos, C., Nie, X., Murphy, M., Hussein, R., Zhang, J.: Superhydrophilicity and antibacterial property of a Cu-dotted oxide coating surface. Ann. Clin. Microbiol. Antimicrob. 9, 25 (2010). https://doi.org/10.1186/1476-0711-9-25
Nezhad, E.Z., Sarraf, M., Musharavati, F., et al.: Effect of zirconia nanotube coating on the hydrophilicity and mechanochemical behavior of zirconium for biomedical applications. Surf. Interfaces 28, 101623 (2022). https://doi.org/10.1016/j.surfin.2021.101623
Vakamulla Raghu, S.N., Killian, M.S.: Wetting behavior of zirconia nanotubes. RSC Adv. 11, 29585–29589 (2021). https://doi.org/10.1039/D1RA04751E
Raghu, S.N.V., Chuluunbandi, K., Killian, M.S.: Zirconia nanotube coatings - UV-resistant superhydrophobic surfaces. Surf. Interfaces 26, 101357 (2021). https://doi.org/10.1016/j.surfin.2021.101357
Wang, L.N., Shen, C., Shinbine, A., Luo, J.L.: Variation on wettability of anodic zirconium oxide nanotube surface. Thin. Solid Films 531, 277–283 (2013). https://doi.org/10.1016/j.tsf.2013.01.066
Zhao, Y., Zhang, J., Pantea, C., et al.: Thermal equations of state of the $\ensuremath{\alpha}$, $\ensuremath{\beta}$, and $\ensuremath{\omega}$ phases of zirconium. Phys. Rev. B 71, 184119 (2005). https://doi.org/10.1103/PhysRevB.71.184119
Shiraishi, T., Yubuta, K., Shishido, T., Shinozaki, N.: Elastic Properties of As-Solidified Ti-Zr Binary Alloys for Biomedical Applications. Mater. Trans. 57, 1986–1992 (2016). https://doi.org/10.2320/matertrans.MI201501
Fogaing, E.Y., Lorgouilloux, Y., Huger, M., Gault, C.P.: Young’s modulus of zirconia at high temperature. J. Mater. Sci. 41, 7663–7666 (2006). https://doi.org/10.1007/s10853-006-0593-7
Wheeler, D.W., Zekonyte, J., Wood, R.J.K.: Mechanical properties of cerium and a cerium–5wt% lanthanum alloy by nanoindentation and ultrasonic velocity measurements. Mater. Sci. Eng. A 578, 294–302 (2013). https://doi.org/10.1016/j.msea.2013.04.083
Patel, N.R.G.P.P.: A review on biomaterials: Scope, applications & human anatomy significance. Int. J. Emerg. Technol. Adv. Eng. 2, 91–101 (2012)
Rho, J.Y., Ashman, R.B., Turner, C.H.: Young’s modulus of trabecular and cortical bone material: ultrasonic and microtensile measurements. J. Biomech. 26, 111–119 (1993). https://doi.org/10.1016/0021-9290(93)90042-d
Long, M., Rack, H.J.: Titanium alloys in total joint replacement - a materials science perspective. Biomaterials 19, 1621–1639 (1998). https://doi.org/10.1016/s0142-9612(97)00146-4
Almeida, A.C.C., Fontes, L.S., Laurindo, C.A.H., Torres, R.D., Popat, K.C., Soares, P.: Annealing temperature efect on tribocorrosion and biocompatibility properties of TiO2 nanotubes. J. Bio. Tribo-Corros. 64, 1–12 (2020)
Sarraf, M., Zalnezhad, E., Bushroa, A.R., et al.: Structural and mechanical characterization of Al/Al2O3 nanotube thin film on TiV alloy. Appl. Surf. Sci. 321, 511–519 (2014). https://doi.org/10.1016/j.apsusc.2014.10.040
Durdu, S., Usta, M.: Characterization and mechanical properties of coatings on magnesium by micro arc oxidation. Appl. Surf. Sci. 261, 774–782 (2012). https://doi.org/10.1016/j.apsusc.2012.08.099
Polat, A., Makaraci, M., Usta, M.: Influence of sodium silicate concentration on structural and tribological properties of microarc oxidation coatings on 2017A aluminum alloy substrate. J. Alloy. Compd. 504, 519–526 (2010). https://doi.org/10.1016/j.jallcom.2010.06.008
Sekler, J., Steinmann, P.A., Hintermann, H.E.: The scratch test: Different critical load determination techniques. Surf. Coat. Technol. 36, 519–529 (1988). https://doi.org/10.1016/0257-8972(88)90179-X
Rus, D., Capitanu, L., Badita, L.-L.: A qualitative correlation between friction coefficient and steel surface wear in linear dry sliding contact to polymers with SGF. Friction 2, 47–57 (2014). https://doi.org/10.1007/s40544-014-0038-2
Acknowledgements
This work was completely supported by the Scientific and Technological Research Council of Turkey (TUBITAK), Turkey (Project Number – 122M329). S.D. and M.U. acknowledge the support from TUBITAK project (122M329). For Raman spectroscopy, PL and cross sectional SEM analyses, F.S. and A.E. acknowledge the support from the Scientific Research Projects Coordination Unit of Istanbul University (FBA-2023-39412 and FBG-2022-38573) and TUBITAK project (121F169).
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
S.D: conceptualization; investigation; methodology; data curation; software; formal analysis; visualization; writing-review and editing and supervising. S.A: formal analysis; data curation; software; writing-review and editing. F.S: investigation; formal analysis; data curation; software; writing-review and editing. E.A: formal analysis; data curation. B.G: formal analysis; data curation. A.E: formal analysis; data curation; writing-review and editing. M.U: formal analysis; data curation; writing-review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Durdu, S., Aktas, S., Sarcan, F. et al. Effect of water-based electrolyte on surface, mechanical and tribological properties of ZrO2 nanotube arrays produced on zirconium. J Aust Ceram Soc (2024). https://doi.org/10.1007/s41779-024-01030-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41779-024-01030-w