Abstract
This study presents a comprehensive investigation into the electronic properties of Hydroxyapatite (HAp) doped with Zinc (Zn) and Cobalt (Co). Five distinct compositions, denoted as 0.15Zn-HAp, 0.15Co-0.15Zn-HAp, 0.30Co-0.15Zn-HAp, 0.45Co-0.15Zn-HAp, and 0.6Co-0.15Zn-HAp (at%,) have been systematically studied employing Density of States (DOS) and band structure calculations. The computed band gap values for these compositions were determined to be 4.6663, 4.6888, 4.7049, 4.7159, and 4.7082 eV, respectively. These results illuminate the profound influence of Zn and Co doping on the electronic structure of Hydroxyapatite. These findings hold significant implications for the potential applications of these materials in diverse technological and biomedical domains. The systematic approach and precise electronic property characterizations presented in this study provide a robust foundation for further advancements in the realm of advanced materials, with particular relevance to the development of innovative materials for use in cutting-edge technologies and medical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydroxyapatite (HAp) is a natural calcium apatite with the chemical formula Ca10(PO4)6(OH)2 and molecular weight of 1004.6 g/mol [1]. HAp crystal exhibits a hexagonal structure with α = β = 90o and γ = 120o variables in the P63/m space group [2]. 70% of HAp is composed of bone and the remaining part is composed of collagen and water [3]. It was found that HAp has a fluid contact angle of 10o and is hydrophilic [4]. HAp is the most stable phase among medium-hard calcium phosphates with a density of 3.156 g/cm3, a refractive index of 1.64 to 1.65, a melting point of 1650 oC and a Mohs hardness of 5 [5].
The biocompatibility and bioactivity of HAp, resulting from the chemical similarity of the structure of human bones and teeth to mineral compounds have led to great interest in the biomedical field for various applications such as implants and tissue repair or replacement in orthopedics, maxillofacial and dental fields [6]. The first application of calcium phosphate was its use as a direct gap filling agent to improve bone formation [2]. Subsequently, its research has increased in the fields of fracture healing, bone defects, joint replacement and oral and maxillofacial surgery [7]. In the following years, with the application of different polymer materials to calcium phosphate, there has been a transition from bone defect repair to bone regeneration and functionalization [2].
Porous and nanocrystalline hydroxyapatite is used as a drug delivery system for bone tumors and bone diseases such as osteoporosis due to its non-toxicity and biocompatibility [8]. Hydroxyapatite is mainly used to deliver antibiotics, anticancer agents, anti-inflammatory drugs, anti-osteoporosis agents and other drugs such as vitamins, hormones, proteins and growth factors directly into hard tissue [6, 8]. Titanium alloys, which are used in the majority of implants due to their mechanical properties, excellent strength, low density and chemical stability in body fluids, have excellent hardness and mechanical strength, but have poor biocompatibility and poor direct contact between the implant and the material observed in light microscopy [6, 9]. HAp with high biocompatibility, chemical stability and osteoconductivity are used to improve the properties of metallic implants [10]. In this way, it has been observed that it provides rapid osseointegration in metallic implants and creates much faster implant stabilization [11]. HAp has become a new source of inspiration in the treatment of bone defects due to the limited autograft tissue supply and the risks of allografts [12]. Hydroxyapatite increases Ca2+ density and enables the growth and differentiation of mesenchymal stem cells [13]. Apart from such uses, it is used in water purification, ion conductors and gas sensors [14, 15].
Although there are many methods in hydroxyapatite synthesis, it is divided into three main classes as dry, wet and high temperature methods [16]. It is divided into intermediate classes as solid state and mechanochemical method in dry method, chemical precipitation, hydrothermal and hydrolysis the method in wet method, combustion and pyrolysis method in high temperature method [3]. HAp synthesized by these different methods give results in different sizes, morphologies, crystallinity and purity according to the conditions used during the processes [17]. This affects the biocompatibility, bioactivity, mechanical strength and biological properties of the synthesized HAp [3, 18].
Properties such as low dissolution rate and low biological interaction rates are some of the limiting factors of HAp [19]. In addition, the occurrence of postoperative infections caused by bacterial contamination due to the attachment of bacterial cells to biomaterial surfaces in the implant area is observed as one of the major problems in this field [20]. To eliminate the limitations in these areas, many researchers have aimed to obtain a material with the desired properties by doping HAp with Ag [21], Mg [22], Mn [23], Li [24], Sr [25], Ce [26], Cu [27], Zn [28], Co [29]. It has been reported that zinc, the second most abundant mineral in the human body, is found in bone metabolism, has antibacterial properties and is effective in the proliferation of bone-forming cells [1, 30]. Negrila et al. [31] and Predoi et al. [30] reported that the Zn content in the synthesis of Zn-doped HAp caused a change in the stability of the material solution and showed an antibacterial property. Yang et al. [32] reported that the antibacterial property was detected in the biocompatibility test of a flame-sprayed Zn-HAp coating. Esfahani et al. [33] reported that Zn2+ ions have a better effect on BSA protein differentiation and adhesion to the surface. Venkatasubbu et al. [34] reported antibacterial properties and positive results in controlled drug release. Iconaru et al. [35] reported that Zn-doped HAp has antifungal properties and Ding et al. [36] reported cytocompatibility and corrosion resistance properties. Cobalt is one of the components used in orthopedic implants due to its high durability and wear resistance [37]. Cobalt is also the active site of cobalamin, known as vitamin B-12, and plays an important role in DNA production [38]. Kramer et al. [39] and Chandra et al. [40] reported that cobalt-doped HAp exhibits paramagnetic properties in contrast to the diamagnetic behavior of pure HAp. Ignjatovic et al. [41] reported that increasing the cobalt weight to 12% gave positive results in new bone formation and growth.
A review of the literature shows that there are studies on the synthesis of Zn and Co doped hydroxyapatites. It is understood that there are fewer studies on Co doped hydroxyapatite compared to Zn doped hydroxyapatite. However, no Zn-based Co-doped hydroxyapatite synthesis study was found. This study aims to bring innovation to the field by filling this gap in the literature.
Materials and methods
(50-x-y) mmol calcium nitrate tetrahydrate (Carlo-Erba), x mmol zinc acetate dihydrate (Merck), and y mmol cobalt (II) nitrate hexahydrate (Merck) were dissolved in a flask of 100 mL and poured into a beaker. Where x was kept at a constant value of 0.075 and y was varied to the values of 0, 0.075, 0.150, 0.225 and 0.300, respectively. 0.3 M di-ammonium hydrogen phosphate (Merck) was dissolved in distilled water in a flask of 100 mL and was added drop wisely to the first solution. The pH of the final mixture was adjusted to 9.0 by adding ammonia solution (Sigma-Aldrich), it was stirred at 65 ºC for 4 h, and it was dried in an oven at 120 ºC for 24 h. The as-obtained dried powder was calcined in an electric furnace at 900 ºC for 1 h.
X-ray diffraction (XRD) analysis was done by a Bruker D8 Advance diffractometer, Fourier transform infrared (FTIR) analysis was carried out by using a Perkin Elmer Spectrum One spectrophotometer using KBr pellets. Differential thermal analysis (DTA) and thermogravimetric analysis (TGA) analyses were done by using Schimadzu’s DTA-60 and TGA-60 series. To make the morphological investigations, an FEI Quanta 450 FEG scanning electron microscope having an energy dispersive X-ray (EDX, Amatek Octane Plus) analyzer was used.
In the biocompatibility tests, 0.1 g of different materials for each test was used by adding 1 mL of High Glucose DMEM solution enriched with 10% FBS and 1% Pen-Strep to each material sample. Subsequently, this mixture was placed within a 5% carbon dioxide incubator at a temperature of 37 °C allowing it to incubate for 3 days. Following this 72-hour incubation, the materials were extracted and excluded from the DMEM medium.
In the confines of a 96-well plate, we planted L-929 (Mouse Fibroblast Cell) at a density of 10,000 cells per well, giving them the opportunity allowing them to adhere as they resided in a carbon dioxide incubator. After secure adhesion of cells to the well plate’s base, it was eliminated the prior medium from the plate. To replenish it, 100 µl of DMEM medium from earlier, stored in conjunction with the material, were dispensed into each well. This plate was then subjected to another 24-hour incubation period. Following this timeframe, it was exchanged the medium within the well plate. Instead, it was introduced DMEM infused with a 10% MTT solution, concocted by mixing PBS with a concentration of 5 mg/mL and a pH level of 7.4. This revitalized plate was readmitted to the incubator for a duration of 4 h. Upon completion, it was discarded the MTT-containing medium, and in its place, added 100 µl of DMSO to each well. Thorough mixing ensued, and the spectrophotometer measured the absorbance of each well at 540 nm.
Results and discussion
Theoretical results
Hydroxyapatite (HAp) possesses a complex crystal structure predominantly comprised of calcium, phosphorous, and oxygen ions. Its immense potential spans various fields, from biomedical implants and drug delivery systems to electronic devices. Within this theoretical framework, we delve deeply into understanding the core principles guiding our exploration of HAp’s electronic properties, achieved by strategically introducing Zinc (Zn) and Cobalt (Co) through controlled doping.
Pure HAp, with its inherent electronic properties, naturally piques our interest, particularly in the realm of biocompatible applications. However, when it comes to fulfilling the specific requirements of electronic devices and tailored functionalities, the need to manipulate its electronic characteristics through doping becomes paramount. These meticulous calculations, firmly rooted in the realm of quantum mechanics, empower us to predict crucial electronic behaviors such as band gaps and energy levels, essential for comprehending the electronic nature of this material.
Our research takes a focused approach towards Cobalt’s inclusion within the HAp matrix. We aim to uncover how the presence of Co shapes the material’s electronic structure and influences the distribution of energy within it. The quantification of states at each energy level is expressed through the concept of the density of states.
Here, δE represents the number of states in the system with volume V, whose energies fall within the range from E to E + δE.
In this work, we conducted a comprehensive investigation into the bandgap characteristics of the material, employing highly precise first-principles calculations rooted in Density Functional Theory (DFT). To perform these computations, we leveraged the CASTEP software package [42], a renowned and trusted platform known for its expertise in ab initio simulations. Our examination was centered on the electronic structure in the vicinity of the high-symmetry (G-H) points, yielding intricate revelations regarding the energy band dispersion of the material. The bandgap value, ascertained from these calculations, stands as a pivotal metric elucidating the material’s optical and electronic attributes and calculated by using the CASTEP package.
Calculation of bandgaps and density of states
In recent years, the exploration of novel materials with tailored electronic properties has gained immense importance, driven by their potential applications across a wide spectrum of technological and biomedical domains. In this context, the study presented here delves into a comprehensive investigation of the electronic properties of Hydroxyapatite (HAp) doped with Zinc (Zn) and Cobalt (Co). This study examines five distinct compositions, each denoted as CZ1, CZ2, CZ3, CZ4, and CZ5 employing advanced computational techniques such as Density of States (DOS) and band structure calculations.
One of the key findings of this investigation is the precise determination of band gap values for each of these compositions, as shown in Fig. 1, measured at 4.6663 eV, 4.6888 eV, 4.7049 eV, 4.7159 eV, and 4.7082 eV, respectively. These results signify a remarkable shift in the electronic structure of Hydroxyapatite due to the incorporation of Zn and Co dopants. Understanding and manipulating the electronic properties of materials at such a fundamental level are pivotal in designing materials with tailored characteristics for specific applications.
The profound influence of Zn and Co doping on the electronic structure of Hydroxyapatite, as revealed by this study, holds significant implications for various technological and biomedical domains. These findings not only contribute to our fundamental understanding of material science but also pave the way for potential breakthroughs in fields ranging from advanced technologies to innovative medical applications. The dopant of Zn and Co may change the electronic structure of the system that can alter the band structure and band gap energy. The systematic approach and precise electronic property characterizations presented in this study provide a robust foundation for further advancements in the realm of advanced materials, emphasizing their relevance in the development of cutting-edge technologies and materials for medical applications. In this pursuit, the study bridges the gap between fundamental research and practical applications, offering new possibilities for the design and utilization of materials with finely tuned electronic properties.
In Fig. 2, the LAC vs. photon energy plot is shown. The LAC has a decreasing trend with the increasing energy. Because the increasing amount of both co-dopants causes a continuous increase in the LAC, the addition of Co and Zn dopants to the HAp may be useful for using it in the radiation shielding applications [43].
Experimental results
XRD analysis
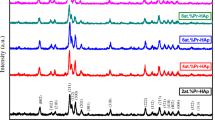
The XRD patterns of the Co/Zn co-doped HAps are plotted in Fig. 3 and these patterns support the existence of the HAp phase (JCPDS no: 09-0432) for each sample. As a secondary phase, beta-tricalcium phosphate (β-TCP) was detected and its percentage was found to be in the range from 13.5 to 21.1% (Table 1).
Using the following relations, the calculation of the lattice parameters (a and c), unit cell volume (V) and crystallite size (D) were done [44]:
here d is the interplanar spacing, λ is the wavelength, β is the full-width at half maximum and θ is the Bragg’s angle. The lattice parameters, unit cell volume and crystallite size are affected by the amount of both co-dopants. Also, the crystallinity percent (XC%) values calculated from the relation derived by Landi et al. [45] are given in Table 1. The composition affects the crystallinity of the HAp.
FTIR analysis
Fig. 4 shows the FTIR analysis results of the samples. For all the samples, the as-observed bands are belonged to the hydroxyl and phosphate groups. The hydroxyl bands centered at 630 (libration mode) and 3572 cm− 1 (stretching mode) are detected [46]. The phosphate-group related bands are observed at 474 (υ2), 566 (υ4), 599 (υ4), 963 (υ1), 1025 (υ3), and 1087 (υ3) cm− 1 [47, 48]. The existence of hydroxyl and phosphate groups in the samples supports the HAp structure for all the samples [49].
Thermal analysis
DTA thermograms of the samples are shown in Fig. 5. These results imply that all the samples are thermally stable from room temperature to 700 °C. At higher temperatures, the peak observed in the range of 700–900 °C can be related to the decomposition of HAp phase to the β–TCP [50]. In Fig. 6, TGA results show that there are mass losses from room temperature to 1000 °C, but these losses remain below the value of 1.50%. The as detected mass losses are found to be 0.88, 0.85, 1.42, 1.40 and 1.23 for CZ1, CZ2, CZ3, CZ4 and CZ5, respectively.
SEM observations
Fig. 7 shows the SEM images and EDX analyses of the samples. The morphology shows some variations in the shape and size of the nanoparticles with the compositions of the samples. In the EDX spectra, there is not any element related to the impurity for all the samples. The stoichiometric ratios of (Ca + Zn + Co)/P are found as 1.70, 1.60, 1.57, 1.68 and 1.65 for CZ1, CZ2, CZ3, CZ4 and CZ5, respectively. The introduction of Zn and Co is detected, but the influence of Co on the HAp structure is seen as limited.
Cell viability results
Formulations created through an indirect method underwent biocompatibility assessment utilizing L-929 mouse fibroblast cells. The outcomes highlighted that the materials’ biocompatibility surpassed 80%. Assessing the biocompatibility of formulations prepared indirectly and tested with L-929 mouse fibroblast cells demonstrated a noteworthy level of biocompatibility, evident in Figs. 8 and 9; Table 2 detailing cell viability results. As per ISO-10993-5 standards, a biomaterial is classified as cytotoxic if it triggers a cell viability reduction of more than 30% [51].
Analysis of biocompatibility test findings for materials generated via the indirect method unveiled the absence of cytotoxic impacts, as the decline in cell viability remained below the 30% threshold. Nevertheless, the CZ1 material exhibited an interfering effect. Photographic evidence of L-929 cells exposed to the samples utilized in the biocompatibility assessment illustrated the CZ material’s unfavorable influence on cell viability.
Conclusions
This study has provided a comprehensive exploration of the electronic properties of Hydroxyapatite (HAp) doped with Zinc (Zn) and Cobalt (Co). Through the meticulous examination of five distinct compositions, denoted as CZ1, CZ2, CZ3, CZ4 and CZ5, using advanced computational techniques like Density of States (DOS) and band structure calculations, we have gained valuable insights into the impact of Zn and Co doping on the electronic structure of Hydroxyapatite. Notably, the determination of precise band gap values for each composition, measured at 4.6663 eV, 4.6888 eV, 4.7049 eV, 4.7159 eV, and 4.7082 eV, respectively, highlights the significant alterations in the material’s electronic properties due to dopant incorporation. These findings underscore the potential for tailoring Hydroxyapatite’s electronic structure to meet specific application requirements, ranging from advanced technologies to innovative medical applications.
The profound influence of Zn and Co doping on Hydroxyapatite’s electronic structure unveiled in this study holds great promise for diverse technological and biomedical domains. It not only advances our fundamental understanding of materials but also opens new avenues for designing and utilizing materials with finely tuned electronic properties. This systematic approach and precise electronic property characterizations serve as a robust foundation for future advancements in advanced materials science, with substantial implications for the development of cutting-edge technologies and materials for medical applications. In closing, this study exemplifies the synergy between fundamental research and practical applications, offering a path towards the development of advanced materials with tailored electronic characteristics to meet the demands of tomorrow’s technology and healthcare industries.
References
Panda, S., Biswas, C.K., Paul, S.: A comprehensive review on the preparation and application of calcium hydroxyapatite: A special focus on atomic doping methods for bone tissue engineering. Ceram. Int. 47(20), 28122–28144 (2021). https://doi.org/10.1016/j.ceramint.2021.07.100
Shavandi, A., Bekhit, A.E.D.A., Sun, Z.F., Ali, A.: A review of synthesis methods, properties and use of hydroxyapatite as a substitute of bone. J. Biomim. Biomater. Biomed. Eng. 25, 98–117 (2015). https://doi.org/10.4028/www.scientific.net/JBBBE.25.98
Pu’ad N.A.S.M., Haq R.H.A., Noh H.M., Abdullah H.Z., Idris M.I., Lee T.C.: Synthesis method of hydroxyapatite: A review. Mater. Today: Proc. 291, 233–239 (2020). https://doi.org/10.1016/j.matpr.2020.05.536
Jarcho, M., Bolen, C.H., Thomas, M.B., Bobick, J., Kay, J.F., Doremus, R.H.: Hydroxylapatite synthesis and characterization in dense polycrystalline form. J. Mater. Sci. 11, 2027–2035 (1976). https://doi.org/10.1007/BF02403350
Lu, Y., Dong, W., Ding, J., Wang, W., Wang, A.: 10-Hydroxyapatite nanomaterials: synthesis, properties, and functional applications. In: Wang, A., Wang, W. (eds.) Nanomaterials from Clay Minerals, In Micro and Nano Technologies, pp. 485–536. Elsevier (2019). https://doi.org/10.1016/B978-0-12-814533-3.00010-7
Gomes, D.S., Santos, A.M.C., Neves, G.A., Menezes, R.R.: A brief review on hydroxyapatite production and use in biomedicine. Cerâmica. 65, 282–302 (2019). https://doi.org/10.1590/0366-69132019653742706
Hing, K.A.: Bone repair in the twenty–first century: Biology, chemistry or engineering? Philos. Trans. Math. Phys. Eng. Sci. 362(1825), 2821–2850 (2004). https://doi.org/10.1098/rsta.2004.1466
Szcześ, A., Hołysz, L., Chibowski, E.: Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 249, 321–330 (2017). https://doi.org/10.1016/j.cis.2017.04.007
Beig, B., Liaqat, U., Niazi, M.F.K., Douna, I., Zahoor, M., Niazi, M.B.K.: Current challenges and innovative developments in hydroxyapatite-based coatings on metallic materials for bone implantation: A review. Coatings. 10(12), 1249 (2020). https://doi.org/10.3390/coatings10121249
Yuan, Q., Qin, C., Wu, J., Xu, A., Zhang, Z., Liao, J., Lin, S., Ren, X., Zhang, P.: Synthesis and characterization of cerium-doped hydroxyapatite/polylactic acid composite coatings on metal substrate. Mater. Chem. Phys. 182, 365–371 (2016). https://doi.org/10.1016/j.matchemphys.2016.07.044
Sandukas, S., Yamamoto, A., Rabiei, A.: Osteoblast adhesion to functionally graded hydroxyapatite coatings doped with silver. J. Biomed. Mater. Res. - A. 97A(4), 490–497 (2011). https://doi.org/10.1002/jbm.a.33081
Shi, H., Zhou, Z., Li, W., Fan, Y., Li, Z., Wei, J.: Hydroxyapatite based materials for bone tissue engineering: A brief and comprehensive introduction. Crystals. 11(2), 149 (2021). https://doi.org/10.3390/cryst11020149
Zhao, R., Xie, P., Zhang, K., Tang, Z., Chen, X., Zhu, X., Fan, Y., Yang, X., Zhang, X.: Selective effect of hydroxyapatite nanoparticles on osteoporotic and healthy bone formation correlates with intracellular calcium homeostasis regulation. Acta Biomater. 59, 338–350 (2017). https://doi.org/10.1016/j.actbio.2017.07.009
Gómez-Morales, J., Torrent‐Burgues, J., Boix, T., Fraile, J., Rodríguez‐Clemente, R.: Precipitation of stoichiometric hydroxyapatite by a continuous method. Cryst. Res. Technol. 36(1), 15–26 (2001). https://doi.org/10.1002/1521-4079(200101)36:1<15::AID-CRAT15>3.0.CO;2-E
Prakash, V.C.A., Venda, I., Thamizharasi, V.: Synthesis and characterization of surfactant assisted hydroxyapatite powder using microemulsion method. Mater. Today: Proc. 51(4), 1788–1792 (2022). https://doi.org/10.1016/j.matpr.2021.05.059
Sadat-Shojai, M., Khorasani, M.T., Dinpanah-Khoshdargi, E., Jamshidi, A.: Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 9(8), 7591–7621 (2013). https://doi.org/10.1016/j.actbio.2013.04.012
Varadavenkatesan, T., Vinayagam, R., Pai, S., Brindhadevi, K., Pugazhendhi, A., Selvaraj, R.: Synthesis, biological and environmental applications of hydroxyapatite and its composites with organic and inorganic coatings. Prog Org. Coat. 151, 106056 (2021). https://doi.org/10.1016/j.porgcoat.2020.106056
Sathiyavimal, S., Vasantharaj, S., LewisOscar, F., Selvaraj, R., Brindhadevi, K., Pugazhendhi, A.: Natural organic and inorganic–hydroxyapatite biopolymer composite for biomedical applications. Prog Org. Coat. 147, 105858 (2020). https://doi.org/10.1016/j.porgcoat.2020.105858
Saxena, V., Hasan, A., Pandey, L.M.: Effect of Zn/ZnO integration with hydroxyapatite: A review. Mater. Technol. 33(2), 79–92 (2018). https://doi.org/10.1080/10667857.2017.1377972
Samani, S., Hossainalipour, S.M., Tamizifar, M., Rezaie, H.R.: In vitro antibacterial evaluation of sol–gel-derived Zn‐, Ag‐, and (zn + Ag)‐doped hydroxyapatite coatings against methicillin‐resistant Staphylococcus aureus. J. Biomed. Mater. Res. - A. 101A(1), 222–230 (2013). https://doi.org/10.1002/jbm.a.34322
Riaz, M., Zia, R., Ijaz, A., Hussain, T., Mohsin, M., Malik, A.: Synthesis of monophasic ag doped hydroxyapatite and evaluation of antibacterial activity. Mater. Sci. Eng. C. 90, 308–313 (2018). https://doi.org/10.1016/j.msec.2018.04.076
Dasgupta, S., Banerjee, S.S., Bandyopadhyay, A., Bose, S.: Zn-and Mg-doped hydroxyapatite nanoparticles for controlled release of protein. Langmuir. 26(7), 4958–4964 (2010). https://doi.org/10.1021/la903617e
Paluszkiewicz, C., Ślósarczyk, A., Pijocha, D., Sitarz, M., Bućko, M., Zima, A., Chróścicka, A., Lewandowska-Szumieł, M.: Synthesis, structural properties and thermal stability of Mn-doped hydroxyapatite. J. Mol. Struct. 976(1–3), 301–309 (2010). https://doi.org/10.1016/j.molstruc.2010.04.001
Wang, Y., Yang, X., Gu, Z., Qin, H., Li, L., Liu, J., Yu, X.: In vitro study on the degradation of lithium-doped hydroxyapatite for bone tissue engineering scaffold. Mater. Sci. Eng. C. 66, 185–192 (2016). https://doi.org/10.1016/j.msec.2016.04.065
Zhu, H., Guo, D., Sun, L., Li, H., Hanaor, D.A.H., Schmidt, F., Xu, K.: Nanostructural insights into the dissolution behavior of Sr-doped hydroxyapatite. J. Eur. Ceram. Soc. 38(16), 5554–5562 (2018). https://doi.org/10.1016/j.jeurceramsoc.2018.07.056
Ciobanu, G., Harja, M.: Cerium-doped hydroxyapatite/collagen coatings on titanium for bone implants. Ceram. Int. 45(2B), 2852–2857 (2019). https://doi.org/10.1016/j.ceramint.2018.07.290
Bazin, T., Magnaudeix, A., Mayet, R., Carles, P., Julien, I., Demourgues, A., Gaudon, M., Champion, E.: Sintering and biocompatibility of copper-doped hydroxyapatite bioceramics. Ceram. Int. 47(10A), 13644–13654 (2021). https://doi.org/10.1016/j.ceramint.2021.01.225
Maleki-Ghaleh, H., Siadati, M.H., Fallah, A., Zarrabi, A., Afghah, F., Koc, B., Abdolahinia, E.D., Omidi, Y., Barar, J., Akbari-Fakhrabadi, A., Beygi-Khosrowshahi, Y., Adibkia, K.: Effect of zinc-doped hydroxyapatite/graphene nanocomposite on the physicochemical properties and osteogenesis differentiation of 3D-printed polycaprolactone scaffolds for bone tissue engineering. Chem. Eng. J. 426, 131321 (2021). https://doi.org/10.1016/j.cej.2021.131321
Pang, Y., Kong, L., Chen, D., Yuvaraja, G., Mehmood, S.: Facilely synthesized cobalt doped hydroxyapatite as hydroxyl promoted peroxymonosulfate activator for degradation of rhodamine B. J. Hazard. Mater. 384, 121447 (2020). https://doi.org/10.1016/j.jhazmat.2019.121447
Predoi, D., Iconaru, S.L., Predoi, M.V., Motelica-Heino, M., Guegan, R., Buton, N.: Evaluation of antibacterial activity of zinc-doped hydroxyapatite colloids and dispersion stability using ultrasounds. Nanomaterials. 9(4), 515 (2019). https://doi.org/10.3390/nano9040515
Negrila, C.C., Predoi, M.V., Iconaru, S.L., Predoi, D.: Development of zinc-doped hydroxyapatite by sol-gel method for medical applications. Molecules. 23(11), 2986 (2018). https://doi.org/10.3390/molecules23112986
Yang, Y.-C., Chen, C.-C., Wang, J.-B., Wang, Y.-C., Lin, F.-H.: Flame sprayed zinc doped hydroxyapatite coating with antibacterial and biocompatible properties. Ceram. Int. 43(1), S829–S835 (2017). https://doi.org/10.1016/j.ceramint.2017.05.318
Esfahani, H., Prabhakaran, M.P., Salahi, E., Tayebifard, A., Rahimipour, M.R., Keyanpour-Rad, M., Ramakrishna, S.: Electrospun nylon 6/zinc doped hydroxyapatite membrane for protein separation: Mechanism of fouling and blocking model. Mater. Sci. Eng. C. 59, 420–428 (2016). https://doi.org/10.1016/j.msec.2015.09.100
Venkatasubbu, G.D., Ramasamy, S., Ramakrishnan, V., Kumar, J.: Nanocrystalline hydroxyapatite and zinc-doped hydroxyapatite as carrier material for controlled delivery of ciprofloxacin. 3 Biotech. 1, 173–186 (2011). https://doi.org/10.1007/s13205-011-0021-9
Iconaru, S.L., Prodan, A.M., Buton, N., Predoi, D.: Structural characterization and antifungal studies of zinc-doped hydroxyapatite coatings. Molecules. 22(4), 604 (2017). https://doi.org/10.3390/molecules22040604
Ding, Q., Zhang, X., Huang, Y., Yan, Y., Pang, X.: In vitro cytocompatibility and corrosion resistance of zinc-doped hydroxyapatite coatings on a titanium substrate. J. Mater. Sci. 50, 189–202 (2015). https://doi.org/10.1007/s10853-014-8578-4
Czarnek, K., Terpiłowska, S., Siwicki, A.K.: Selected aspects of the action of cobalt ions in the human body. Centr Eur. J. Immunol. 40(2), 236–242 (2015). https://doi.org/10.5114/ceji.2015.52837
Herrmann, W., Obeid, R.: Cobalamin Deficiency. In: Stanger, O. (ed.) Water Soluble Vitamins. Subcellular Biochemistry, pp. 301–322. Springer, Dordrecht (2012). https://doi.org/10.1007/978-94-007-2199-9_16
Kramer, E., Itzkowitz, E., Wei, M.: Synthesis and characterization of cobalt-substituted hydroxyapatite powders. Ceram. Int. 40(8B), 13471–13480 (2014). https://doi.org/10.1016/j.ceramint.2014.05.072
Chandra, V.S., Elayaraja, K., Arul, K.T., Ferraris, S., Spriano, S., Ferraris, M., Asokan, K., Kalkura, S.N.: Synthesis of magnetic hydroxyapatite by hydrothermal–microwave technique: Dielectric, protein adsorption, blood compatibility and drug release studies. Ceram. Int. 41(10A), 13153–13163 (2015). https://doi.org/10.1016/j.ceramint.2015.07.088
Ignjatović, N., Ajduković, Z., Savić, V., Najman, S., Mihailović, D., Vasiljević, P., Stojanović, Z., Uskoković, V., Uskoković, D.: Nanoparticles of cobalt-substituted hydroxyapatite in regeneration of mandibular osteoporotic bones. J. Mater. Sci: Mater. Med. 24, 343–354 (2013). https://doi.org/10.1007/s10856-012-4793-1
Clark, S.J., Segall, M.D., Pickard, C.J., Hasnip, P.J., Probert, M.I.J., Refson, K.: Payne. First principles methods using CASTEP. Z. für Kristallogr. - Cryst. Mater. 220, 5–6 (2005). https://doi.org/10.1524/zkri.220.5.567.65075
Sahin, B., Ates, T., Karaca Acari, I., Barzinjy, A.A., Ates, B., Özcan, İ., Bulut, N., Keser, S., Kaygili, O.: Tuning electronic properties of hydroxyapatite through controlled doping using zinc, silver, and praseodymium: A density of states and experimental study. Ceram. Int. 50(5), 7919–7929 (2024). https://doi.org/10.1016/j.ceramint.2023.12.120
B.D. Cullity. Elements of X-ray Diffraction, (2nd edn), Addison–Wesley Publishing Company, Massachusetts pp. 127–131. (1978)
Landi, E., Tampieri, A., Celotti, G., Sprio, S.: Densification behaviour and mechanisms of synthetic hydroxyapatites. J. Eur. Ceram. Soc. 20(14–15), 2377–2387 (2000). https://doi.org/10.1016/S0955-2219(00)00154-0
Ayhan, Y.M., Ates, T., Seçkin, T., Özcan, İ., Bulut, N., Kuruçay, A., Kaygili, O.: The effects of Zn and Yb co-dopants on the electronic, radiation shielding, structural, thermal and spectroscopic properties of hydroxyapatite. Chem. Phys. Impact. 8, 100488 (2024). https://doi.org/10.1016/j.chphi.2024.100488
Keser, S., Efe, H.: Investigation of in vitro bioactivities of Zn-based hydroxyapatite samples doped with chitosan. J. Aust Ceram. Soc. 57, 117–124 (2021). https://doi.org/10.1007/s41779-020-00519-4
İsen, F., Kaygili, O., Bulut, N., Ates, T., Osmanlıoğlu, F., Keser, S., Tatar, B., Özcan, İ., Ates, B., Ercan, F., Ercan, I., Kareem, R.O.: Experimental and theoretical characterization of Dy-doped hydroxyapatites. J. Aust Ceram. Soc. 59, 849–864 (2023). https://doi.org/10.1007/s41779-023-00878-8
Hssain, A.H., Bulut, N., Ates, T., Koytepe, S., Kuruçay, A., Kebiroglu, H., Kaygili, O.: Sr/Smco-doped hydroxyapatites: Experimental characterization and theoretical research. J. Aust Ceram. Soc. 58, 1491–1507 (2022). https://doi.org/10.1007/s41779-022-00788-1
Ercan, F., Kayed, T.S., Kaygili, O., Bulut, N., Almohazey, D., Ates, T., Al-Ahmari, F.S., Ay, I., Demirci, T., Kirat, G., Flemban, T., İnce, T., Ghrib, T., Al-Suhaimi, E.A., Ercan, I.: Investigation of structural, spectroscopic, dielectric, magnetic, and in vitro biocompatibility properties of Sr/Ni co-doped hydroxyapatites. Ceram. Int. 48(18), 26585–26607 (2022). https://doi.org/10.1016/j.ceramint.2022.05.354
Nedeljkovic, I., Doulabi, B.Z., Abdelaziz, M., Feilzer, A.J., Exterkate, R.A.M., Szafert, S., Gulia, N., Krejci, I., Kleverlaan, C.J.: Cytotoxicity and anti-biofilm properties of novel hybrid-glass-based caries infiltrant. Dent. Mater. 38(12), 2052–2061 (2022). https://doi.org/10.1016/j.dental.2022.11.018
Acknowledgements
The study was supported by Malatya Turgut Özal University Scientific Research Projects Coordination Unit with project number 23Y06.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tekin, Y.Ş., Ates, T. Comprehensive investigation of the electronic properties of zinc and cobalt doped hydroxyapatite. J Aust Ceram Soc (2024). https://doi.org/10.1007/s41779-024-01024-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41779-024-01024-8