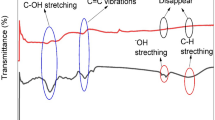
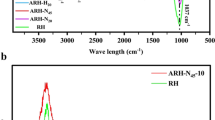
Abstract
Hydrogen (H2), a renewable energy source with a high energy density and a reputation for being environmentally benign, is being lauded for its potential in various future applications. In the present context, the catalytic methanolysis of sodium borohydride (NaBH4) is of considerable importance due to its provision of a pathway for the efficient production of hydrogen gas (H2). The main aim of this research attempt was to assess the viability of utilizing refuse defatted sumac seeds as an unusual precursor in microwave-assisted K2CO3 activation to produce a biocatalyst.
The primary objective that motivated the synthesis of the biocatalyst was to facilitate the generation of hydrogen via the catalytic methanolysis of NaBH4. With the aim of developing a biocatalyst characterized by enhanced catalytic performance, we conducted an exhaustive investigation of a wide range of experimental parameters. The activation agent-to-sample ratio (IR), impregnation time, microwave power, and irradiation time were among these parameters.
Significantly enhanced in catalytic activity, the biocatalyst produced under particular conditions achieved a peak hydrogen production efficiency of 10,941 mL min− 1 g.cat− 1. In particular, it was determined that the ideal conditions were as follows: 0.5 IR, 24 h of impregnation, 500 W of microwave power, and 10 min of irradiation. This novel strategy not only demonstrates the impressive potential of eco-friendly biocatalysts, but also positions them as a viable alternative material for the sustainable production of hydrogen via NaBH4 methanolysis.
Three significant parameters contribute to the value and renewability of this study. The first is that waste is used as the primary material; the second is that the activator is less hazardous than other activators; and the third is that microwave activation is a green chemistry technique.
Graphical Abstract

Similar content being viewed by others
Introduction
Fossil fuels are widely recognized environmental threat that is depleting resources, leading to range of harmful consequences. A global shift to cleaner and more sustainable renewable energy sources is necessary to mitigate the risks of climate change and its associated health impacts, requiring a move away from fossil fuels [1, 2]. Renewable energy refers to the energy derived from natural sources that are regenerated over time, such as solar radiation, wind, geothermal heat, hydropower, and biomass. Renewable energy is also characterized as energy obtained from sources that are continually replenished and do not contribute to the accumulation of greenhouse gases or other harmful emissions that can negatively affect the environment and human health [3, 4].
Among these, hydrogen (H2) is receiving attention as an eco-friendly fuel with high energy density and is considered as a promising energy carrier for future applications [5]. Despite its potential as an alternative energy source, the practical challenges associated with hydrogen production, storage, and transportation are currently hindering its widespread use. Various types of hydrides, such as metal hydrides, complex metal hydrides, and chemical hydrides, have been extensively studied for hydrogen storage.
Sodium borohydride (NaBH4) is a chemical hydride that stands out for its high hydrogen capacity, controllable hydrogen generation rate, air stability, and non-flammability, making it a promising candidate for hydrogen storage and generation [6]. Hydrogen bonds in boron hydride molecules can be broken in two different ways: thermolysis and solvolysis. While thermolysis requires high temperatures and is not practical for on-board applications, solvolysis can produce hydrogen at room temperature using solvent and an efficient catalyst, making it a more feasible method for hydrogen generation [7]. The use of sodium borohydride to generate H2 in an ambient environment was first proposed by Schlesinger in the 1950s [8], and is regarded as the cornerstone of research on NaBH4 as H2 storage materials. NaBH4 hydrolysis [9,10,11,12] and NaBH4 methanolysis [13,14,15] are two practical techniques for producing hydrogen gas from NaBH4.
Recent studies have focused on the methanolysis of borohydrides as it offers advantages such as avoiding freezing issues associated with water, fast hydrogen generation potentially without the need for a catalyst, and reaction products that can be regenerated into sodium borohydride in a single step [16]. However, controlling the progression of NaBH4 self-methanolysis can be challenging. As a result, there has been a growing interest in catalytic methanolysis of sodium borohydride in the field of hydrogen energy, with the aim of enhancing the hydrogen generation rate (HGR) and improving the delay resistance, particularly for short environmental durations [17]. Recently, an innovative methodology using bio-waste-based carbon material direct catalysts has gained widespread recognition for its cost-effectiveness and environmentally friendly approach. In this regard, Kaya and colleagues. achieved excellent hydrogen production rates using highly porous catalysts derived from diverse biological waste sources, which were chemically activated with conventional heating under an inert atmosphere [18,19,20,21,22].
Sumac, scientifically identified as Rhus coriaria L., is a plant native to the Mediterranean region and falls under the Anacardiaceae family. It has a longstanding traditional use as a spice and enhancer of flavors [23]. In a recent investigation, it was found that sumac seed (SS) biomass preserved valuable compounds, such as oleic and linoleic acids, along with a range of polyphenols like gallic acid, anthocyanins, and hydrolysable tannins. SS exhibits noteworthy ecological characteristics as it is non-toxic, propagates readily through seed dispersion, and thrives with a high growth rate even in environments with limited sunlight and poor soil conditions, conditions that might be unsuitable for many other plant species [24]. Despite, SS biomass’s abundant supply and significant economic potential, not much study has been done on using it as a feedstock for biomass pyrolysis. To the best of our knowledge, no literature has been published on the topic of using SS microwave heating pyrolysis to produce biofuels. Therefore, investigating SS biomass as a superior source for pyrolysis is quite beneficial. The increasing industrial demand for chemicals and energy may be successfully met by using SS pyrolysis to harness bioproducts and bioenergy.
Microwave heating has become increasingly popular in recent years as a method for preparing catalysts due to its various advantages over conventional heating. Some of these benefits include shorter processing times, faster and more uniform heating, higher heating rates, the use of cleaner energy sources, no direct contact between heating sources and materials, reduced equipment size, rapid temperature increases (up to 26.7 K/min), and energy efficiency [25, 26].
The main objective of this paper is to demonstrate a new approach for generating activated carbon from defatted SS waste by utilizing microwave radiation and K2CO3 as a chemical activation agent. The research explores the influence of various factors, such as impregnation time, activation agent to precursor ratio, radiation power, and radiation time, on the production of hydrogen through the methanolysis of NaBH4 using the biocatalyst that was prepared.
Experimental section
Raw materials
For this study, sumac seed was procured from a local market in Siirt, Turkey. The seeds were ground and filtered to obtain a consistent size of less than 0.14 mm. To extract the oil, hexane was used with the Soxhlet extraction method. The defatted sumac seed (DSS) powder was then dried at 80 oC overnight to eliminate any remaining moisture (Fig. 1). These procedures were necessary to prepare the sumac for the next steps involved in producing biocatalyst.
Preparation of biocatalyst
Approximately 2 g of dried DSS was impregnated with K2CO3 at various impregnation ratio (IR) (0.25, 0.5, 1.0, 2.0, 3.0 and 4.0 g/g), calculated according to Eq. (1):
where WK2CO3 is the weight of K2CO3 and WSS is the weight of the sumac seed.
Distilled water (10 mL) was used to dissolve K2CO3. The sample was allowed to mix at room temperature for 1 h and then transferred to an oven at 80 °C for chemical activation and dehydration. The sample was then heated in a microwave oven (MILESTONE RotoSYNTH Rotative Solid-Phase Reactor) at different radiation powers (100, 250, 500, 750, and 1000 W) and radiation times (5, 10, 15, and 20 min). N2 gas was purged through the microwave oven to ensure an inert atmosphere during the reaction. The resulting sample was subjected to a thorough washing process using 0.1 M HCl, hot water, and cold distilled water to remove residual organic matter and alkalis. The washing process was repeated until the pH of the solution became neutral. Finally, the purified sample was left to dry for 24 h at 105 °C. The prepared biocatalyst (DSS-CO3) was used for generating H2 from NaBH4.
Process of methanolysis
For each experiment using methanolysis (Eq. 2), a precise mixture was generated by combining 0.1 gram of biocatalyst, 0.25 gram of NaBH4 (98%, Sigma Aldrich). This mixture was then combined with 10 ml of methanol (> 99.9%, Sigma Aldrich). The reaction was carried out under carefully monitored conditions at a temperature of 30 °C. The hydrogen gas that was created was carefully collected in a gas collecting a device, and its volume was measured over the course of a certain amount of time (5 s) (Fig. 2). Since, amount of hydrogen released is constant, the catalyst performance in the all experiments was evaluated in terms of hydrogen production rate (mL min− 1 g.cat− 1).
Results and discussion
Effect of chemical impregnation ratio
The effect of chemical impregnation ratio on the hydrogen production was evaluated under specific conditions, including 24 h impregnation time, 500 W microwave power and 10 min irradiation time, as presented in Fig. 3. Previous research conducted by Wang et al. [27], showed that the activation agent plays a crucial role as the primary microwave absorber during the early stages of the reaction. As the pore structure of the activated carbon develops, the activated carbon itself begins to absorb microwave energy, leading to further heating and activation. The results of the current experiment, indicate that hydrogen production increased from 8277 to 10,941 mL min− 1 g.cat−1 as the impregnation ratio increased from 0.25 to 0.5. However, beyond this point, further increases in IR led to a gradual decrease in hydrogen production. The best hydrogen production rate of the biocatalyst was achieved with a K2CO3/DSS ratio of 0.5.
The observed trend can be attributed to K2CO3’s role as an activating agent that forms more pores, enhancing catalytic activity. Increasing the activating agent/carbon ratio leads to a more vigorous activation reaction, forming more pores and increasing activation capacity. The active sites on the carbon surface reach a maximum at the optimum ratio, but a ratio exceeding a certain threshold can cause pores expansion, leading to carbon burning and decreased catalytic activity [28].
Effect of impregnation time
The effect of impregnation time on hydrogen production was investigated under the conditions of an impregnation ratio of 0.5, a radiation time of 10 min, and a microwave power of 500 W. The impregnation time was varied from 12 to 48 h, and the hydrogen production rate was analyzed. The results, as can be seen in Fig. 4, indicate that the HGR on the biocatalyst first increased and then decreased with the extension of the impregnation time. The peak HGR value of 10,941 mL min− 1 g.cat−1 was observed at an impregnation time of 24 h. The initial increase in HGR can be attributed to the higher absorption of the K2CO3 solution by the biomass during longer impregnation times, resulting in the formation of more active sites for the reaction. However, excessive impregnation time can lead to the destruction of some micro-pore structures, causing a decrease in the HGR.
Effect of microwave power
The effect of microwave power on the catalytic performance of biocatalyst in the methanolysis of NaBH4 was investigated. The experimental conditions and results are shown in Fig. 5. As per the findings, the rate of hydrogen production showed a gradual increase with an increase in microwave power and peaked at 500 W. However, at higher power levels, the rate decreased. This trend is similar to the results of Deng et al. [28], who conducted a study on the microwave-assisted activation of cotton stalk using K2CO3. They found that at low microwave power, the pore structure of the activated carbon was not well-developed, but as the power increased, the pore structure improved. Nevertheless, when the radiation levels were increased beyond a certain threshold, the absorbed microwave energy surpassed the limit, resulting in the combustion of some carbon and the collapse of the pore structure.
Effect of radiation time
In Fig. 6, the effect of radiation time on the catalytic performance of biocatalyst was investigated for a range of 5–20 min. The results demonstrate that HGR initially increases to a maximum of 10,875 mL min− 1 g.cat−1 at 10 min and then decreases as the activation time increases. This trend may be attributed to the incomplete utilization of K2CO3 at the point of maximum HGR, and further activation leading to excessive activation and possible destruction of micro-pore structures. Ji et al. [29] conducted a study comparing conventional heating to microwave heating and found that the MW heating method resulted in higher efficiency than the conventional method, as demonstrated by SBET. The MW heating method has the advantages of homogeneous heating and a rapid heating rate, allowing for the sample to have more active regions in a shorter period, leading to greater activation efficiency.
Conclusion
Recent research has been focused on the methanolysis of borohydrides due to its advantages such as avoiding the freezing problems associated with water, fast hydrogen generation potentially without the need for a catalyst, and reaction products that can be regenerated into sodium borohydride in a single step. However, it can be challenging to control the progression of NaBH4 self-methanolysis. Hence, the catalytic methanolysis of sodium borohydride has gained significant attention in the hydrogen energy field. The objective is to enhance the hydrogen generation rate (HGR) and to provide delay resistance, primarily for short environmental durations. An innovative approach using bio-waste-based carbon material direct catalysts has recently gained widespread recognition for its cost-effectiveness and environmentally friendly nature. The aim of this research is to explore the possibility of using defatted sumac seed waste as a substitute for the preparation of a biocatalyst through K2CO3 activation assisted by microwaves for hydrogen production from sodium borohydride (NaBH4). Three significant parameters contribute to the value and renewability of this study. The first is that waste is used as the primary material; the second is that the activator is less hazardous than other activators; and the third is that microwave activation is a green chemistry technique. Various experimental parameters, such as different activation agent/sample ratio (IR), impregnation time, microwave power, and irradiation time, were investigated to prepare a biocatalyst with higher catalytic activity. The results demonstrate that the biocatalyst synthesized at 0.5 IR, 24 h of impregnation time, 500 W microwave power, and 10 min of irradiation time exhibited significantly enhanced catalytic activity, with the highest hydrogen production efficiency recorded at 10,941 mL min− 1 g.cat−1. The biocatalysts synthesized in this study using waste defatted sumac seed as an alternative precursor via microwave-assisted K2CO3 activation have shown significantly enhanced catalytic activity for hydrogen production from sodium borohydride (NaBH4). These biocatalysts are environmentally friendly and have the potential to be utilized as an alternative material for H2 production via NaBH4 methanolysis, offering a cost-effective and sustainable solution for hydrogen energy.
Data availability
All data included in this study are available upon request by contact with the corresponding author.
References
Farhad, S., Saffar-Avval, M., Younessi‐Sinaki, M.: Efficient design of feedwater heaters network in steam power plants using pinch technology and exergy analysis. Int. J. Energy Res. 32(1), 1–11 (2008)
Güney, T.: Renewable energy, non-renewable energy and sustainable development. Int. J. Sustainable Dev. World Ecol. 26(5), 389–397 (2019)
Khan, K., Manir, S.M., Islam, M.S., Jahan, S., Hassan, L., Ali, M.H.: Studies on nonconventional energy sources for electricity generation. Int. J. Adv. Res. Innov. Ideas Educ. 4(4), 229–244 (2018)
Panwar, N.L., Kaushik, S.C., Kothari, S.: Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 15(3), 1513–1524 (2011)
Sharma, S., Agarwal, S., Jain, A.: Significance of hydrogen as economic and environmentally friendly fuel. Energies. 14(21), 7389 (2021)
Fakioğlu, E., Yürüm, Y., Veziroğlu, T.N.: A review of hydrogen storage systems based on boron and its compounds. Int. J. Hydrog. Energy. 29(13), 1371–1376 (2004)
Patel, N., Miotello, A.: Progress in Co–B related catalyst for hydrogen production by hydrolysis of boron-hydrides: A review and the perspectives to substitute noble metals. Int. J. Hydrog. Energy. 40(3), 1429–1464 (2015)
Schlesinger, H., Brown, H.C., Finholt, A., Gilbreath, J.R., Hoekstra, H.R., Hyde, E.K.: Sodium borohydride, its hydrolysis and its use as a reducing agent and in the generation of hydrogen1. J. Am. Chem. Soc. 75(1), 215–219 (1953)
Baytar, O., Şahin, Ö., Ekinci, A.: Effect of environmentally friendly and efficient metal-free hydrochars as catalysts on sodium borohydride hydrolysis. Fuel. 346, 128308 (2023)
Ekinci, A., Horoz, S., Baytar, O., Şahin, Ö.: Hydrogen generation by hydrolysis of NaBH4 with efficient Co-La-WB catalyst for PEM fuel cells. J. Optoelectronic Biomedical Mater. 12(2), 25–32 (2020)
Ekinci, A., Şahin, Ö., Saka, C., Avci, T.: The effects of plasma treatment on electrochemical activity of Co–W–B catalyst for hydrogen production by hydrolysis of NaBH4. Int. J. Hydrog. Energy. 38(35), 15295–15301 (2013)
Izgi, M.S., Şahin, Ö., Baytar, O., Saka, C., Effects: 43(16), 1933–1944 (2021)
Cafer, S.: Highly active hydrogen generation from sodium borohydride methanolysis and ethylene glycolysis reactions using protonated chitosan-zeolite hybrid metal-free particles. Appl. Catal. B. 325, 122335 (2023)
Sahiner, N., Yasar, A.O.: A new application for colloidal silica particles: Natural, environmentally friendly, low-cost, and reusable catalyst material for H2 production from NaBH4 methanolysis. Ind. Eng. Chem. Res. 55(43), 11245–11252 (2016)
Sahiner, N., Demirci, S.: Natural microgranular cellulose as alternative catalyst to metal nanoparticles for H2 production from NaBH4 methanolysis. Appl. Catal. B. 202, 199–206 (2017)
Ramya, K., Dhathathreyan, K., Sreenivas, J., Kumar, S., Narasimhan, S.: Hydrogen production by alcoholysis of sodium borohydride. Int. J. Energy Res. 37(14), 1889–1895 (2013)
Wang, T., Jiang, T., Zhang, H., Zhao, Y.: Advances in catalysts for hydrogen production by methanolysis of sodium borohydride. Int. J. Hydrog. Energy. 47(32), 14589–14610 (2022)
Elma Karakaş, D., Kaya, M., Horoz, S.: Efficient hydrogen generation from the NaBH4 methanolysis by waste material: Banana peel. Carbon Lett. 32(6), 1593–1601 (2022)
Karakaş, D.E.: A novel cost-effective catalyst from orange peel waste protonated with phosphoric acid for hydrogen generation from methanolysis of NaBH4. Int. J. Hydrog. Energy. 47(24), 12231–12239 (2022)
Karakaş, D.E., Akdemir, M., Imanova, G.T., Kivrak, H.D., Horoz, S., Kaya, M.: Biomass-based metal-free catalyst as a promising supercapacitor electrode for energy storage. J. Mater. Sci.: Mater. Electron. 33(22), 18111–18123 (2022)
Karakaş, D.E., Mustafa, K., Horoz, S.: Catalytic activites of a biomaterial (sumac) catalyst in sodium borohyride methanolysis reactions. J. Mol. Struct. 1273, 134276 (2023)
Özarslan, S., Atelge, M.R., Kaya, M., Ünalan, S.: A novel tea factory waste metal-free catalyst as promising supercapacitor electrode for hydrogen production and energy storage: A dual functional material. Fuel. 305, 121578 (2021)
Rayne, S., Mazza, G.: Biological activities of extracts from sumac (Rhus spp.): A review. Nat. Precedings, 1–1 (2007)
Wang, S., Zhu, F.: Chemical composition and biological activity of staghorn sumac (Rhus typhina). Food Chem. 237, 431–443 (2017)
Ahmad, M.A., Puad, N.A.A., Bello, O.S.: Kinetic, equilibrium and thermodynamic studies of synthetic dye removal using pomegranate peel activated carbon prepared by microwave-induced KOH activation. Water Resour. Ind. 6, 18–35 (2014)
Hessien, M.: Microwave-assisted hydrothermal carbonization of pomegranate peels into hydrochar for environmental applications. Energies. 15(10), 3629 (2022)
Wang, T., Tan, S., Liang, C.: Preparation and characterization of activated carbon from wood via microwave-induced ZnCl2 activation. Carbon. 47(7), 1880–1883 (2009)
Deng, H., Li, G., Yang, H., Tang, J., Tang, J.: Preparation of activated carbons from cotton stalk by microwave assisted KOH and K2CO3 activation. Chem. Eng. J. 163(3), 373–381 (2010)
Ji, Y., Li, T., Zhu, L., Wang, X., Lin, Q.: Preparation of activated carbons by microwave heating KOH activation. Appl. Surf. Sci. 254(2), 506–512 (2007)
Acknowledgements
I express my gratitude to Dr. Mustafa Kaya and Dr. Duygu Elma Karakaş from Siirt University for their valuable support in the methanolysis studies.
Funding
This work was not financially supported by any organization.
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Canpolat, G. Microwave-assisted sumac based biocatalyst synthesis for effective hydrogen production. J Aust Ceram Soc (2024). https://doi.org/10.1007/s41779-024-01013-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41779-024-01013-x