Abstract
This study focuses on how titanium oxide (TiO2) in concentrations ranging from 0.5 to 4% by weight added the hydroxyapatite (CHA) made from chicken femur bones’, affects sinterability, microstructural, mechanical, and in vitro bioactivity properties. According to the results of the experiments, it was determined that CHA decomposed into whitlockite, alpha tricalcium phosphate (α-TCP), tetracalcium phosphate (TTCP), and calcium oxide (CaO) phases at different temperatures. Rutile and perovskite (CaTiO3) phases were also found in TiO2 added CHAs in addition to these phases. With increasing sintering temperature of CHA, the diameters and the heights of the samples decreased. Density increased up to 1250 °C and decreased at 1300 °C respectively. while the partial density value showed similar behavior with density and hardness, At 1200 °C, the maximum values of fracture toughness (1.071 MPam1/2) and compressive strength (145.417 MPa) were attained; however, as sintering temperatures increased, these values shifted downward to 0.882 MPam1/2 and 111.096 MPa, respectively. It has been determined that grain growth and decomposition are the underlying factors in obtaining the highest density, hardness, fracture toughness and compressive strength values for CHA at different temperatures. Among the TiO2 added CHAs, the best properties are obtained for CHA-0.5TiO2 sintered at 1300 °C (Density: 3.0057 g/cm3, Hardness: 3.973 GPa, Fracture toughness: 1.583 MPam1/2 and Compressive strength: 170.045 MPa) and the properties of the CHA-TiO2 composite decreased with increasing TiO2 ratio. This is due to the fact that increasing TiO2 has a detrimental impact on CHA’s sinterability behavior and causes it to become more porous and degrade more quickly. It was discovered through in vitro bioactivity and cell culture assays that the addition of TiO2 had a detrimental impact on the proliferation of bone tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Development of biomaterials for the repair/replacement of hard tissue has earned much interest in the past few years, although the conventional method of using autogenous or allogenic bone was most preferred for the treatment of bone defects, disadvantages including, the limited quantity and the risk of transmission of infection (e.g., HIV, hepatitis). Therefore, the development of bone restorative materials with both higher biocompatibility and sufficient mechanical strength is strongly desired. Hydroxyapatite (HA) is one of the most used material in bone regeneration because mainly of its affinity to bone tissues and has a similar chemical and crystallographic structure to the bone mineral [1]. It does not cause any stimulating and repulsive effects when added into the human body. Moreover, HA can combine with the original bone tissue to form a solid bone. It can be synthesized chemically or extracted from biological sources. Biological HA contains different kinds of cationic and anionic impurities. These ionic impurities in biological HAs could be a reason for its better biocompatibility than synthetic HAs [2]. Therefore, many works have been devoted to the development of HA from biological sources like turkey bones [3], goat bones [4], sheep bones [5], and bovine bones [6]. To meet the world’s food demands, thousands of tons of chickens are slaughtered each year, and the majority of the leftover bones are dumped in the natural environment. The strongest issues could, however, arise from the release of chicken bones into the environment. It is crucial that this adverse effect appears. Recycling used chicken bones is one method for doing this. The conversion of leftover chicken bones into hydroxyapatites serves this goal [7, 8]. There are two methods for generating hydroxyapatite from chicken bones. The first of these involves washing the precipitated hydroxyapatite particles with distilled water three times after 24 h and drying them in a vacuum oven at 60 °C for 24 h. Waste chicken femur bones are dissolved in ChCl-urea at 100 °C for four hours, then this process is cooled to about room temperature. It was found that the hydroxyapatite (CHA) obtained from chicken bones with this method was in rod-like form, and the pertinent method was unable to produce CHA at a high efficiency [9]. Production through calcination is the second technique. Preventing animal-related health issues and adjusting these inputs with other techniques can both lead to higher efficiency. It has been determined that CHA can be produced at different calcination temperatures and durations as follows in the literature: 1000 °C for ½ hour [10], 950 °C for 2 h [11], 900 °C for 6 h [12], 700 °C for 2 h [13], 600 °C for 2 h [14], 600 °C for 4 h, 12 h, and 20 h [15], and 500 °C for 24 h [16]. However, as can also be seen in Table 1, its low mechanical properties make it unsuitable for use in the human body, and there is only one study in the literature aimed at improving the mechanical properties of CHA. In this study [17], glass addition was employed to enhance the mechanical properties of CHA. However, it was not possible to achieve compressive strength (100–230 MPa), hardness (4.87 GPa), and fracture toughness (2–12 MPam1/2) values suitable for use in the human body [18].
It has been determined that the addition of TiO2 contributes to the cell proliferation of the HA-Ag binary system [19]. Furthermore, the presence of Ti–OH groups on the TiO2 surface is associated with a high bioactive response, making it a potential biomaterial[19]. These groups induce protein binding, facilitating cellular adhesion, and are also associated with the nucleation of calcium phosphate when immersed in a solution with a composition similar to blood plasma (SBF). The addition of 11 mol% titanium to a bioactive glass has been found to enhance bioactivity and accelerate apatite formation. A study involving pigs reported that titanium implants coated with titanium nanotubes showed increased bone formation compared to unmodified surface implants, and they exhibited greater gene expression related to bone remodeling and formation [20, 21]. Due to this effect, the impact of synthetic HA [22] and HA obtained from biological sources such as bovine HA [23] and sheep HA [24] on their physical, mechanical, and/or bioactivity properties has been investigated. However, there is no study in the literature regarding its addition as an additive material to CHA.
In this study, hydroxyapatite was produced from chicken femur bones, and the effects of titanium oxide addition at weight ratios of 0.5%, 1%, 2%, and 4% on the properties of chicken hydroxyapatite were investigated.
Materials and methods
As the matrix material, hydroxyapatite (CHA) was created using chicken femur bones from the Erpiliç Company in Istanbul, Turkey, and was produced as follows: The heads of chicken femur bones were cut off to remove visible tissues and substances on the surface, and the marrow in the shafts of all bones was removed by boiling them in water in a pressure cooker for 4 h. After boiling, the remaining shafts were deprotected with sodium hydroxide (NaOH) for 1 h, followed by washing with distilled water, and then calcined at 800 °C for 4 h at heating rates of 5 °C/min. To compare the calcined chicken bones with other bioderived HAs mentioned in the literature, their grain size was increased to -63 by pounding them in a ceramic mortar. Following this stage, titanium oxide (TiO2; Nanolab, spherical form, average 5 µ) additions were made at weight ratios of 0.5%, 1%, 2%, and 4% and the prepared mixtures were homogenized using a Retsch PM 100 planetary ball mill with 15 zirconia balls and a sufficient amount of ethyl alcohol at a speed of 180 revolutions per minute for 2 h. After the homogenization process, the prepared mixtures were first dried and then pelletized using a uniaxial pressing method under a pressure of 350 MPa to conform to the British 7253 standard. Subsequently, they were sintered at temperatures of 1100, 1150, 1200, 1250, and 1300 °C for 4 h each. Phase investigations of the sintered samples were performed using a Panalytical Expert Pro Series XRD, with Cu-Kα radiation (λ = 0.154 nm, 45 kV and 40 mA), 2θ range of 20–60◦, step size of 0.02◦ and rate of 2◦/min. A Zeiss-Evo | MA10 SEM was used to measure microstructural changes associated to the sintering temperatures and additives made. Measurements of the density, porosity, and partial density were taken, along with measurements of the diameter, length, and volume shortening rates and the physical properties. To calculate the diameter, length, and volume shortening rates, formulas 1, 2, and 3 were employed.
Sd: Diameter shrink (%), Do: Diameter before sintering (mm), D1: Diameter after sintering (mm)
Sl: Shortening in height (%), Lo: Length before sintering (mm), L1: Length after sintering (mm)
Sv: Volumetric shortening (%), Vo: Volume before sintering (mm3), V1: Volume after sintering (mm3).
The Archimedes method was used to determine density, porosity and partial density values and calculations were made according to Formulas 4, 5 and 6. Theoretical densities were determined according to the mixing rule.
d: Density (g/cm3), p: Porosity (%), Rd: Partial density (%), dt: Theoretical density (g/cm3), WD: Dry weight (gr), Wa: Suspended weight (gr), Ws: Weight in water (gr).
Hardness, fracture toughness and compressive strength tests were performed to determine the mechanical properties. Future Tech FM300 brand hardness device was used to find the hardness and fracture toughness values, and the samples were sanded with 600, 800, 1000, 1200, 2400, 4000 and 5000 mesh sandpapers, respectively, before the relevant measurements. In order to determine the fracture toughness values, polishing processes were carried out using 5 µ, 3µ, 1µ and 0.5µ diamond pastes after sanding processes. The hardness values were calculated using Formulas 7 and 8, respectively, with loads of 200 g (20 s) and 300 g (10 s), respectively. Compressive strength test 2 mm/min. It was carried out on a Devotrans 50kN brand universal testing device at speed and determined using Formula 9. The fragility index was calculated from the HV/Kıc ratio [25].
HV: Hardness value (GPa), P: Applied force (N), d: Average track diameter (mm2)
Kıc: Fracture toughness (MPam1/2), c = Distance of the crack formed from the center of the microhardness mark (m), HV: Hardness (MPa), a: Half of the mark formed in microhardness measurement (m)
σc: Compressive strength (MPa), F: Maximum force (N), Ao: Cross-sectional area (mm2).
For the in vitro test, totally, two samples were sintered to the temperatures in which they attained the highest compressive strength value with the size of ∅11 and 4 mm2. After this stage, the samples were ground by 400, 600, 800, 1000, and 1200 mesh SiC paper and then they were dried in an oven at 105 °C for 1 day. The samples were immersed in sealed test tubes containing 20 mL of SBF for 14 and 21 days. The 20-mL SBF solutions used in the in vitro tests were refreshed every day until the specified times were completed. When the specified times were completed, the samples were taken from SBF solutions without damaging the apatite layers and then cleaned by distilled water and finally dried at 60 °C for 12 h. Apatite layers occurring on the surface of the samples immersed in SBF solutions were investigated by SEM [26]. For cell culture tests, 3T3 mouse embryonic fibroblast cell line was provided by American Type Culture Collection and was grown in monolayer culture in Dulbecco’s Modified Eagle’s Medium supplemented with 10% heat-inactivated fetal bovine serum and 1%penicilin–streptomycine antibiotic mixtures at 37 °C with 5% CO2 and 95% humidity. Trypsin/EDTA0.25% solution was used to remove cells from the culture flask. Cells were reseeded at a density of 1 × 105 cells into the 25 T flasks. For SEM analysis, samples and glass cover slip were placed into the 6-wells culture plates containing of 5 ml completed DMEM medium and then 3T3 cells were seeded with the concentration of 5 × 104 cells/well for 7 days. The glass cover slip and samples were critical-point dried and mounted on appropriate stubs with carbon tape and sputter-coated with gold. Images were collected using a SEM [27].
Results & discussion
According to the sintering temperatures, Table 2 illustrates how the physical characteristics of CHAs with and without TiO2 addition change. With increasing temperature, the diameter reduction rate of CHA has increased from 9.777 to 17.591% the length reduction rate from 9.902% to 16.732% and the volume reduction rate from 26.627% to 43.447% These ratios are compatible with waste bovine HA [28] and synthetic HA [29]. The sample sintered at 1300 °C with a 0.5% TiO2 addition had the maximum diameter, length, and volume reduction rates that belonged to pure CHA. The diameter reduction rate dropped as the TiO2 ratio increased, as did the length reduction rate from 17.738% to 14.703% and the volume reduction rate from 43.523% to 36.604%. The highest density and partial density values for CHA were achieved at 1250 °C, but at 1300 °C, they decreased from 2.973 g/cm3 to 2.964 g/cm3 and from 94.202to 93.929, respectively. As expected, the porosity ratio decreased with increasing temperature. In the CHA-TiO2 composites, density values increased with increasing temperature for CHA-0.5 T and CHA-1.0 T. However, the highest densities for CHA-2.0 T and CHA-4.0 T composites were achieved during sintering at 1250 °C, with values of 2.812 g/cm3 and 2.685 g/cm3, respectively. Sintering these composites at 1300 °C caused a decrease in density values to 2.629 g/cm3 and 2.548 g/cm3, respectively. The partial density values of CHA-TiO2 composites exhibited similar behavior to their density counterparts. The highest partial density was measured in the CHA-0.5 T composite at 95.119%, while the lowest partial density was observed in the CHA-4.0 T composite at 79.945%. The porosity ratios decreased with increasing temperature for CHA-0.5 T and CHA-1.0 T composites. As for CHA-2.0 T and CHA-4.0 T composites, the lowest porosity ratios were measured at 1250 °C, at 9.012% and 14.386%, respectively. The sintering of these composites at 1300 °C resulted in an increase in the porosity. There are several reasons why CHA-2.0 T and CHA-4.0 T composites exhibit lower physical properties compared to both CHA and CHA-0.5 T and CHA-1.0 T composites: Firstly, due to the higher melting temperature of TiO2 (1840 °C [30]) compared to HA (1614 °C [31]), the increased sintering behavior with increasing TiO2 content decreases [32]. Secondly, the increased TiO2 content leads to an increased rate of decomposition in CHA-TiO2 composites. Similar behavior has been observed in synthetic HA [33] and HA derived from biological sources [34]. Thirdly, the thermal expansion coefficients of the detected Rutile (11.1 × 10–6/°C [35]) and CaTiO3 (12.9 × 10–6/°C [36]) phases in CHA-TiO2 composites are incompatible with HA (13.6 × 10–6/°C [37]). Fourthly, in HA-TiO2 composites, when the contribution of TiO2 to the physical and mechanical properties is less than 2%, it is because the presence of 2% or more TiO2 exceeds the solubility limit within HA, leading to an increase in the decomposed ratio of HA along with an increase in the proportion of CaTiO3 [38]. For this reason, as can be seen in Table 3, the mechanical properties of CHA-2.0 T and CHA-4.0 T composites are less than CHA-0.5 T and CHA-1.0 T composites.
Table 3 shows the change of mechanical properties of CHA and CHA-TiO2 composites depending on sintering temperatures. The hardness of CHA increased up to 1250 °C and increased from 0.752GPa to 4.354GPa, but; It was stretched to 4.321GPa at 1300 °C. Similar behavior was also confirmed by Ramesh et al. [39]. This is due to the fact that the rate of total decomposes increased at 1300 °C compared to 1250 °C [40]. At 1200 °C, CHA had the greatest measured fracture toughness and compressive strength, which were 1.071 ± MPam1/2 and 145.417 ± 5.000 MPa, respectively. However, both properties decreased with increasing temperature. The highest fracture toughness of CHA is compatible with HA ceramics [41,42,43]. There are two reasons for the decrease in fracture toughness and compressive strength for CHA: First; As can be seen in Fig. 1, it is the increase in grain size in CHA sintered at 1250 °C and 1300 °C. The second reason is related to the increased decomposition of CHA into secondary phases such as α-TCP, TTCP, and CaO. This increase leads to the formation of gas voids within CHA [44, 45].
Although increasing the average grain size may cause an increase in the shortening rate, density and/or hardness of HA ceramics, it also causes a decrease in their compressive strength. As can be seen in Eq. 1 and commonly observed in ceramics, the strength increases as the porosity, p, decreases. (σ: Compressive strength (MPa), σo: Compressive strength of non-porous HA (MPa), b: Material constant, p: Pore amount)
However, as can be observed from Eq. 2, once the average particle size and the rate of decompose in HA ceramics beyond a certain point, the compressive strength decreases with temperature.
Here k: material constant, d is the average grain size [46].
The brittleness index increased with increasing temperature and increased from 1.538μ−1/2 to 4.936 μ−1/2. The brittleness index for CHA-TiO2 composites varies between 0.728 and 2.509 μ−1/2. As can be seen from these values, the lowest and highest fragility index values of CHA have been reduced by approximately 50%. This is similar to ref. [47,48,49] the rate of decrease. The mechanical properties of CHA-TiO2 composites decreased with increasing TiO2 content. It is seen that the highest mechanical properties in CHA-TiO2 composites belong to CHA-0.5 T sintered at 1300 °C. For this composite, hardness was calculated as 3.973 GPa, fracture toughness as 1.583 MPam1/2 and compressive strength as 170.045 MPa. As can be seen from these values, 47.80% increase in fracture toughness, 16.93% increase in compression strength and 49.16% decrease in brittleness index can be achieved when compared to the highest strength values of CHA. This is the result of TiO2 having higher mechanical properties to HA, as seen in Table 4.w
The decline in mechanical characteristics of CHA-TiO2 composites with increasing TiO2 ratio can be attributed to a number of factors. First, increasing the TiO2 ratio causes porous structures to emerge, as shown in Fig. 2. Latter; increasing TiO2 causes an increase in the rate of decomposition [52]. This rise is the result of a faster rate of phase production in CHA, which produces phases with lower densities than HA. (α-TCP: 2.866 g/cm3 [53], TTCP: 2.97 g/cm3 [54]). Third: Rutile and CaTiO3 have better mechanical qualities than -TCP, TTCP, and CaO phases [55, 56].
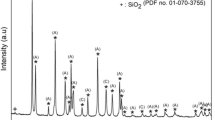
As a result of XRD analysis of pure CHA, it was determined that CHA decomposed into whitlockite (XRD card number: 98–000-0800) and α-TCP (XRD card number: 98–007-8499) phases for temperatures of 1100 and 1150 °C (Fig. 3). The sintering of pure CHA from 1200 °C resulted in its decomposition into tetracalcium phosphate (TTCP; XRD card number: 98–000-6146) and calcium oxide (CaO; 98–003-4978) phases in addition to whitlockite and α-TCP. According to the sintering temperatures, Table 5 displays the chemical composition of CHA as determined by Rietvield analysis (by using High Score Plus Version 5.2). In CHA, the HA rate dropped from 83.7% to 74.9%. Up to 1250 °C, the proportion of the whitlockite phase grew with rising temperature, but at 1300 °C, it dropped from 20.6% to 20.2%. At this temperature, the ratio of α-TCP, TCP and CaO gases was calculated as 1.9%, 2.8% and 0.2%, respectively. It was noticed that the natural hydroxyapatite started to decompose at temperatures above 700 °C, while CaO occurrence began at a temperature of approximately 800 °C. The presence of CaO in natural HAs continue to temperature of 1100 °C [57], but it is an undesirable phase because it leads to the decohesion and may also alter the rate and extent of biodegradation of sintered HA when implanted in human body [58]. TCP has three polymorphs, such as: β-TCP is stable below 1180 °C, α-TCP between 1180 °C and 1400 °C, and ά-TCP above 1470 °C [59]. The co-existence of TCP phases with HA is recently desired, due to they contribute to the formation of bony tissues on the around of HA implants at a less time compared to monolithic HA [59].
The reason why the whitlockite phase is detected in CHA is due to the Mg found in chicken femur bones (Fig. 4) [50]. A similar formation has also been confirmed in the HAs obtained from tuna [51]and pork [60] found in the literature. The presence of the whitlockite phase contributes to the condensation, biocompatibility and mechanical properties of HA. Apart from this, its solubility in the body is lower than β-TCP [61].
In addition to the phases found in pure CHA, such as HA, Whitlockite, TTCP, -TCP, and CaO, Rutile and CaTiO3 phases were also found in TiO2 added CHAs, as shown in Fig. 5. It was determined that the intensity of Whitlockite, TTCP, α-TCP and CaO phases detected in pure CHA increased depending on the increasing TiO2 ratio. In research on HA-TiO2 composites, it was determined that the decomposition rate of HA into β-TCP increased as the TiO2 ratio increased and HA decomposed as seen in Reaction 2. [62]. In this study, it was determined that the formation of the β-TCP phase did not occur due to the whitlockite phase. The formation of the CaTiO3 phase occurs due to the decomposition of THA into CaO and the reaction of CaO with TiO2 as seen in Reaction 3 [63].
While rutile and anatase are formed due to the transformation of TiO2 around 600 °C, the CaTiO3 phase begins to form in HA-TiO2 composites starting from 900 °C [64]. The presence of the rutile phase contributes to the increase in the in vitro bioactivity of HA-TiO2 composites [65].
The chemical composition of CHA-TiO2 composites is given in Table 6. Depending on the rising TiO2 ratio in CHA-TiO2 composites, the rate of HA conversion to secondary phases rises. The secondary phase ratio grew to 46% in the CHA-4.0 T composite from the CHA-0.5 T composite’s value of 30.1%. The CHA-4.0 T composite sintered at 1300 °C has the highest breakdown rate among CHA-TiO2 composites. However, this value is considerably less than the 79% decomposition rate reported by M.A. Monge et al. [66]. The main reason for this is that the secondary phase occurring in CHA is whitlockite.
The SEM microstructures of the in vitro bioactivity CHA and CHA-0.5 T composite are shown in Fig. 6. According to in vitro bioactivity experiments, CHA-0.5 T composite surfaces produce more apatite than CHA does during the course of both the 14-day and 21-day SBF periods. This is due to two factors: The first is related to the type and proportions of phases present in the CHA-0.5 T composite. The fact that the ratio of α-TCP and TTCP phases in the CHA-0.5 T composite was higher than that of CHA increased its in vitro bioactivity. Because the in vitro bioactivity of HA ceramics is increased by the presence of these phases [67]. In addition, since these phases are bioabsorbable, they trigger the formation of an apatite layer [68]. Rutile phase accelerates the formation of apatite layer in (100), (110) and (001) directions [69]. The coexistence of CaTiO3 and α-TCP phases facilitates the bone-implant interface interaction and increases the amount of apatite precipitation. The second is; CHA-0.5 T composite has a lower average grain size than CHA. The decrease in average grain size accelerates the precipitation of apatite grains [70].
Figure 7 shows the cell ratio on the surface of CHA and CHA-0.5 T composite subjected to cell culture testing for 1, 4 and 7 days. During cell culture, proteins, mainly extracellular matrix (ECM) proteins adsorb onto material surfaces and the adsorbed proteins help in cell attachment onto material surfaces [71]. It was determined that the CHA-0.5 T composite provided a higher rate of cell formation compared to CHA in 1 and 4-day cell culture tests. In contrast to CHA, it resulted in a slower rate of cell development after 7 days. A similar situation was also confirmed by Zheng et al. [72]. Figure 8 shows the SEM images of CHA and CHA-0.5 T composite subjected to cell culture testing for 7 days. Although there was a decrease in the number of cells formed on the surface of the CHA-0.5 T composite after 7 days, the typical osteoblast phenotype is healthy cells with some filopodial and lamellipodial extensions. These characteristics indicate that the cells highly adhered and spread onto the surface and suggest the good viability of the cells on the CHA-0.5 T composite [73].
Conclusions
On the basis of experimental results of this investigation, several concluding statements can be made:
In the present study, the potential of TiO2 on the sintering performance and properties of CHA was evaluated by microstructural and mechanical analyses.
In TiO2 added CHAs, the maximum size reduction was obtained in 0.5%TiO2 added sample sintered at 1300 °C.
Depending on the rising TiO2 ratio in CHA-TiO2 composites, the rate of HA conversion to secondary phases rises.
It has been determined that grain growth and decomposition are the underlying factors in obtaining variable density, hardness, fracture toughness and compressive strength values for CHA at different and increasing sintering temperatures.
Among the TiO2 added CHAs, the best properties were obtained in CHA-0.5TiO2 samples sintered at 1300 °C, and the increased additive ratio negatively affected the properties. The increasing amount of TiO2 reduces the sintering of CHA and increases the porosity and degradation rate of the structure.
However, as a result of in vitro bioactivity and cell culture tests, it was determined that although TiO2 addition to CHA was high at first, as the time increased, it negatively affected and decreased the proliferation of bony tissues in the structure.
References
Ning, C.Q., Zhou, Y.: On the microstructure of biocomposites sintered from Ti, HA and bioactive glass. Biomaterials 25, 3379–3387 (2004)
Pazarlioğlu, S., Bakdemir, S., Gökçe, H.: Evaluation of the potential of barium zirconate on the sinterability and properties of bovine hydroxyapatite. Ceramics-Silikaty 67(1), (2004). https://doi.org/10.13168/cs.2023.0009
SerdarPazarlioglu, S., Gokce, H., Ozyegin, S., Salman, S.: Effect of sintering on the microstructural and mechanical properties of meleagris gallopova hydroxyapatite. Bio-Med. Mater. Eng. 24, 1751–1769 (2014). https://doi.org/10.3233/BME-140987
Akyurt, N., Yetmez, M., Oktar, F.N.: Studies on goat hydroxyapatite/commercial inert glass biocomposites. J. Aust. Ceram. Soc. 55, 697–702 (2019). https://doi.org/10.1007/s41779-018-0279-z
Demirkol, N., Oktar, F.N., Kayali, E.S.: Mechanical and Microstructural properties of sheep hydroxyapatite (SHA)-niobium oxide composites. Acta Phys. Pol., A 121(1), 274–276 (2012). https://doi.org/10.12693/APhysPolA.121.274
Ayatollahi, M.R., Yahya, M.Y., Shirazi, H.A., Hassan, S.A.: Mechanical and tribological properties of hydro-xyapatite nanoparticles extracted from natural bovine bone and the bone cement developed by nano-sized bovine hydroxyapatite filler. Ceram. Int. 41, 10818–10827 (2015). https://doi.org/10.1016/j.ceramint.2015.05.021
Boutinguiza, M., Pou, J., Comesaña, R., Lusquiños, F., de Carlos, A., León, B.: Biological hydroxyapatite obtained from fish bones. Mater. Sci. Eng. C 32, 478–486 (2012)
Hart, A., Ebiundu, K., Peretomode, E., Onyeaka, H., Nwabore, O.F., Obileke, K.: Value-added materials recovered from waste bone biomass: technologies and applications. RSC Adv. 12, 22302–22320 (2022)
Song, W., Yang, Y., Deng, Y.: Research of preparation conditions for regeneration of hydroxyapatite and influence on crystalline forms. Chem. Ind. 67, 11–12 (2018)
Jorge Humberto, L.D., Héctor, T.J., Heriberto, H.C., Margarita, G.H., José Aaron, M.B., Hakan, N.: Development and in vivo response of hydroxyapatite/whitlockite from chicken bones as bone substitute using a chitosan membrane for guided bone regeneration. Ceram. Int. 44(18), 22583–22591 (2018)
Marzieh, R., Arvydas, P., Ahmad, M., Sohrab, N., Andrius, V., Giedrius, J.: Comparing methods for calculating nano crystal size of natural hydroxyapatite using X-ray diffraction. Nanomaterials 10(9), 1627 (2020)
Hanny, A., Islam, M.R., Sumdani, M.G., Rashidi, N.M.: The effects of sintering on the properties of epoxy composites reinforced with chicken bone-based hydroxyapatites. Polym. Test. 78, 105987 (2019)
Vijayaraghavan, P., Rathi, M.A., Khalid, S.A., Fatima, S.A., Yahya, B.E., Soon, W.C., Balasubramani, R.: Preparation and antibacterial application of hydroxyapatite doped silver nanoparticles derived from chicken bone. J. King Saud Univ.- Sci. 34(2), 101749 (2022)
Ramesh, S., Loo, Z.Z., Tan, C.Y., Kelvin Chew, W.J., Ching, Y.C., Tarlochan, F., Hari, C., Krishnasamy, S., Bang, L.T., Sarhan, A.A.D.: Characterization of biogenic hydroxyapatite derived from animal bones for biomedical applications. Ceram. Int. 44(9), 10525–10530 (2018)
Bee, S.L., Abdul Hamid, Z.A.: Characterization of chicken bone waste-derived hydroxyapatite and its functionality on chitosan membrane for guided bone regeneration. Compos. Part B 163, 562–573 (2019)
Monıka, Š, Gražyna, S.M., Zbyněk, S.: Bioapatite made from chicken femur bone. Ceramics – Silikáty 55, 3 (2011)
Demirkol, N., Oral, A.Y., Oktar, F.N., Kayali, E.S.: Effects of Commercial Inert Glass (CIG) Addition on Mechanical and Microstructural Properties of Chicken Hydroxyapatite (CHA). Key Eng. Mater. 587, 33–38 (2014)
Kokubo, T., Kim, H.M., Kawashita, M.: Novel bioactive materials with different mechanical properties. Biomaterials 24, 2161–2175 (2013)
Pratap Reddy, M., Venugopal, A., Subrahmanyam, M.: Hydroxyapatite-supported Ag-TiO2 as Escherichia coli disinfection photocatalyst. Water Res. 41, 379–386 (2007). https://doi.org/10.1016/j.watres.2006.09.018
de Paula Miranda, R.B., Miranda Junior, W.G., Ricci Lazar, D.R., Ussui, V., Marchi, J., Cesar, P.F.: Effect of titania content and biomimetic coating onthe mechanical properties of the Y-TZP/TiO2 composite. Dent. Mater. 34(238), 245 (2018). https://doi.org/10.1016/j.dental.2017.11.003
Hara, S., Jumpei Aisu, Yu., Nishizaki, H.K., Sanae, G., Kurebayashi, S., Shimizu, S., Ikake, H.: Bulk structure of poly (ethylene glycol)/Titania hybrid system and the evaluation of their influence on apatite growth using simulated body fluid (SBF). Polym. Testing 94, 106984 (2021). https://doi.org/10.1016/j.polymertesting.2020.106984
Khattab, R.M., Badr, H.A., Zawrah, M.F.: Effect of processing techniques on properties of porous TiO2 and TiO2/hydroxyapatite composites. Ceram. Int. 44(7), 8643–8649 (2018)
Oktar, F.N.: Hydroxyapatite-TiO2 composites. Mater. Lett. 60(17–18), 2207–2210 (2006)
Singh, N., Chakraborty, R., Gupta, R.K.: Mutton bone derived hydroxyapatite supported TiO2 nanoparticles for sustainable photocatalytic applications. J. Environ. Chem. Eng. 6(1), 459–467 (2018)
Pazarlioglu, S., Algan, O., Isikogullari, Ah.M., Gokce, H.: The effect of lanthanum addition on the microstructure and mechanical properties of Mg-modified hydroxyapatite ceramics. Process. Appl. Ceram. 15(3), 226–237 (2021). https://doi.org/10.2298/PAC2103226P
Pazarlioglu, S.S., Salman, S.: The effect of alumina additive and sintering temperature on the microstructural, physical, mechanical, and bioactivity properties of hydroxyapatite–alumina composites. J. Aust. Ceram. Soc. 56, 413–431 (2020). https://doi.org/10.1007/s41779-019-00345-3
Gulsoy, H.O., Pazarlioglu, S., Gulsoy, N., Gundede, B., Mutlu, O.: Effect of Zr, Nb and Ti addition on injection molded 316L stainless steel for bio-applications: Mechanical, electrochemical and biocompatibility properties. J. Mech. Behav. Biomed. Mater. 51, 215–224 (2015)
Herliansyah, M.K., Hamdi, M., Ide-Ektessabi, A., Wildan, M.W., Toque, J.A.: The influence of sintering temperature on the properties of compacted bovine hydroxyapatite. Mater. Sci. Eng., C 29(5), 1674–1680 (2009). https://doi.org/10.1016/j.msec.2009.01.007
Sofronia, A.M., Baies, R., Anghel, E.M., Marinescu, C.A., Tanasescu, S.: Thermal and structural characterization of synthetic and natural nanocrystalline hydroxyapatite. Mater. Sci. Eng., C 43, 153–163 (2014). https://doi.org/10.1016/j.msec.2014.07.023
St. Pierre, P.D.S.: A note on the melting point of titanium dioxide. J. Am. Ceram. Soc. 35(7), 188 (1952). https://doi.org/10.1111/j.1151-2916.1952.tb13097.x
Bulina, N.V., Makarova, S.V., Baev, S.G., Matvienko, A.A., Gerasimov, K.B., Logutenko, O.A., Bystrov, V.S.: A study of thermal stability of hydroxyapatite. Minerals 11, 1310 (2021). https://doi.org/10.3390/min11121310
Nie, J., Zhou, J., Huang, X., Wang, L., Liu, G., Cheng, J.: Effect of TiO2 doping on densification and mechanical properties of hydroxyapatite by microwave sintering. Ceram. Int. 45(11), 13647–13655 (2019). https://doi.org/10.1016/j.ceramint.2019.04.007
Yim, S., Park, I., Park, J.: Sintering behavior, mechanical properties, and biocompatibility of TiO2-Co-calcium phosphates composites prepared by AROS process. Mater. Chem. Phys. 252, 123255 (2020). https://doi.org/10.1016/j.matchemphys.2020.123255
Albayrak, O., El-Atwani, O., Altintas, S.: Hydroxyapatite coating on titanium substrate by electrophoretic deposition method: effects of titanium dioxide inner layer on adhesion strength and hydroxyapatite decomposition. Surf. Coat. Technol. 202(11), 2482–2487 (2008)
Sugiyama, K., Takéuchi, Y.: The crystal structure of rutile as a function of temperature up to 1600° C. Z. Kristallogr.- Cryst. Mater. 194(1–4), 305–314 (1991). https://doi.org/10.1524/zkri.1991.194.14.305
Patil, B.M., Wadhawa, G.C., Chaskar, A.C., Walke, P.S.: Low temperature synthesis and thermal expansion study of nanocrystalline CaTiO3. Mater. Lett. 333, 133627 (2023). https://doi.org/10.1016/j.matlet.2022.133627
Pazarlioglu, S.Ü.L.E.Y.M.A.N., Salman, S.E.R.D.A.R.: Effect of yttria on thermal stability, mechanical and in vitro bioactivity properties of hydroxyapatite/alumina composite. J. Ceram. Process. Res. 20(1), 99–112 (2019)
Guidara, A., Chaari, K., Fakhfakh, S., Bouaziz, J.: The effects of MgO, ZrO2 and TiO2 as additives on microstructure and mechanical properties of Al2O3-Fap composite. Mater. Chem. Phys. 202, 358–368 (2017)
Muralithran, G., Ramesh, S.: The effects of sintering temperature on the properties of hydroxyapatite. Ceram. Int. 26, 221–230 (2000)
Hoepfner, T.P., Case, E.D.: The influence of the microstructure on the hardness of sintered hydroxyapatite. Ceram. Int. 29, 699–706 (2003)
Ruys, A.J., Wei, M., Sorrell, C.C., Dickson, M.R., Brandwood, A., Milthome, B.K.: Sintering effects on the strength hydroxyapatite. Biomaterials 16, 409–415 (1995)
Ramesh, S., Tan, C.Y., Tolouei, R., Amiriyan, M., Purbolaksono, J., Sopyan, I., Teng, W.D.: Sintering behavior of hydroxyapatite prepared from different routes. Mater. Des. 34, 148–154 (2012)
Pazarlıoglu, S., Salman, S.: Sintering effect on the microstructural, mechanical, and in vitro bioactivity properties of a commercially synthetic hydroxyapatite. J. Aust. Ceram. Soc. 53, 391–401 (2017)
Rahavi, S., Monshi, A., Emadi, R., Doostmohammadi, A., Akbarian, H.: Determination of crystallite size in synthetic and natural hydroxyapatite: A comparison between XRD and TEM results. Adv. Mater. Res. 620, 28–34 (2012)
Wu, S.C., Hsu, H.C., Hsu, S.K.: Effects of calcination on synthesis of hydroxyapatite derived from oyster shell powders. J. Aust. Ceram. Soc. 55, 1051–1058 (2019)
Royer, A., Viguie, J.C., Heughebaert, M., Heughebaert, J.C.: Stoichiometry of hydroxyapatite: Influence on the flexural strength. J. Mater. Sci. - Mater. Med. 4, 76–82 (1993). https://doi.org/10.1007/BF00122982
Pazarlioglu, S.S.: The effect of alpha-lithium aluminate incorporation on the properties of bovine bone-derived hydroxyapatite. J. Aust. Ceram. Soc. 58, 1585–1601 (2022). https://doi.org/10.1007/s41779-022-00796-1
Shaly, A.A., Priya, G.H., Mahendiran, M., Linet, J.M., Mani, J.A.M.: An intrinsic analysis on the nature of alumina (Al2O3) reinforced hydroxyapatite nanocomposite. Phys. B: Condensed Matter 642(1), 414100 (2022)
Shiw, S.L., Pan, W.: Machinable Ti3SiC2/hydroxyapatite bioceramic composites by sparkplasma sintering. J. Am. Ceram. Soc. 90(10), 3331–3333 (2007)
Xiaoheng, G., Xiao, L., Huichang, G., Xuetao, S., Naru, Z., Yingjun, W.: Hydrothermal growth of whitlockite coating on β-tricalcium phosphate surfaces for enhancing bone repair potential. J. Mater. Sci. Technol. 34(6), 1054–1059 (2018)
Seo, D.S., Kim, Y.G., Hwang, K.G., Lee, J.K.: Preparation of Hydroxyapatite Powder from Tuna Bone and Its Sinterability Property. J. Korean Ceram. Soc. 45, 10 (2008)
Kutbay, I., Yilmaz, B., Evis, Z., Usta, M.: Effect of calcium fluoride on mechanical behavior and sinterability of nano-hydroxyapatite and titania composites. Ceram. Int. 40(9), 14817–14826 (2014)
Sinusaite, L., Renner, A. M., Schuetz, M. B., Antuzevics, A., Rogulis, U., Grigoraviciute-Puroniene, I., ..., Zarkov, A.: Effect of Mn doping on the low-temperature synthesis of tricalcium phosphate (TCP) polymorphs. J. Eur Ceram. Soc. 39(10), 3257–3263 (2019)
Morejón Alonso, L., Coelho, W. T. G., Santos, L. A. D.: Dual setting α-tricalcium phosphate composite cement obtained by 3d printing. Revista CENIC ciencias químicas [recurso eletrônico]. La Habana, Cuba. 46(1), 62–65 (2015)
Takahashi, K., Fujishiro, Y., Yin, S., Sato, T.: Preparation and compressive strength of α-tricalcium phosphate based cement dispersed with ceramic particles. Ceram. Int. 30(2), 199–203 (2004)
Mimi, M.M., Haque, M.R., Hasan, M.R.: effect of addition of cao on compressive strength of high-volume fly ash concrete. J. Civ. Eng. Sci. Technol. 14(1), 64–76 (2023)
Szewczyk-Nykiel, A., Nykiel, M.: Analysıs of the sintering process of 316l–hydroxyapatite composite biomaterials. Technical Transactions Mechanics 79 (2015)
Suchanek, W., Yashima, M., Kakihana, M., Yoshimura, M.: Hydroxyapatite ceramics with selected sintering additives. Biomaterials 18(13), 923–933 (1997)
Ryu, H.S., Youn, H.J., Hong, K.S., Chang, B.S., Lee, C.K., Chung, S.S.: An improvement in sintering property of β-tricalcium phosphate by addition of calcium pyrophosphate. Biomaterials 23(3), 909–914 (2002)
Ramirez-Gutierrez, C.F., Londoño-Restrepo, S.M., Del Real, A., Mondragón, M.A., Rodriguez-García, M.E.: Effect of the temperature and sintering time on the thermal, structural, morphological, and vibrational properties of hydroxyapatite derived from pig bone. Ceram. Int. 43(10), 7552–7559 (2017)
Maroua, T., Ibrahim, A., Algarni, H., Ayed, F.B., Yousef, E.S.: Mechanical and tribological properties of the tricalcium phosphate-magnesium oxide composites. Mater. Sci. Eng. C 96, 716–729 (2019)
Nie, J., Zhou, J., Huang, X., Wang, L., Liu, G., Cheng, J.: Effect of TiO2 doping on densification and mechanical properties of hydroxyapatite by microwave sintering. Ceram. Int. 45(11), 13647–13655 (2019)
Guidara, A., Chaari, K., Fakhfakh, S., Bouaziz, J.: The effects of MgO, ZrO2 and TiO2 as additives on microstructure and mechanical properties of Al2O3-FAp composite. Mater. Chem. Phys. 202, 358–368 (2017)
Hannora, A.E., Ataya, S.: Structure and Compression Strength of Hydroxyapatite/Titania Nanocomposites Formed by High Energy Mall Milling. J. Alloys Compd. 658, 222–233 (2016)
Wu, J.M.: Low-temperature preparation of anatase and rutile layers on titanium substrates and their ability to induce in vitro apatite deposition. J. Am. Ceram. Soc. 87, 9 (2004)
Auger, M.A., Savoini, B., Muňoz, A., Leguey, T., Monge, M.A., Pareja, R., Victoria, J.: Mechanical characteristics of porous hydroxyapatite/oxide composites produced by post-sintering hot isostatic pressing. Ceram. Int. 35, 2373–2380 (2009). https://doi.org/10.1016/j.ceramint.2009.01.016
Tronco, M.C., Cassel, J.B., dos Santos, L.A.: α-TCP-based calcium phosphate cements: A critical review. Acta Biomater. 151, 70–87 (2022)
Qin, T., Li, X., Long, H., Bin, S., Xu, Y.: Bioactive tetracalcium phosphate scaffolds fabricated by selective laser sintering for bone regeneration applications. Materials 13(10), 2268 (2020)
Lindberg, F., Heinrichs, J., Ericson, F., Thomsen, P., Engqvist, H.: Hydroxylapatite growth on single-crystal rutile substrates. Biomaterials 29(23), 3317–3323 (2008)
Macon, A. L., Kim, T. B., Valliant, E. M., Goetschius, K., Brow, R. K., Day, D. E., ..., Jones, J. R.: A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci.: Mater. Med. 26, 1–10 (2015). https://doi.org/10.1007/s10856-015-5403-9
Narayanan, R., Lee, H.J., Kwon, T.Y., Kim, K.H.: Anodic TiO2 nanotubes from stirred baths: hydroxyapatite growth & osteoblast responses. Mater. Chem. Phys. 125(3), 510–517 (2011)
Zheng, X.B., Ding, C.X.: Characterization of plasma-sprayed hydroxyapatite/TiO2 composite coatings. J Therm Spray Tech 9, 520–525 (2011). https://doi.org/10.1007/BF02608556
Yajing, Y., Qiongqiong, D., Yong, H., Han, S., Pang, X.: Magnesium substituted hydroxyapatite coating on titanium with nanotublar TiO2 intermediate layer via electrochemical deposition. Appl. Surf. Sci. 305, 77–85 (2014)
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gökçe, H. Investigation of the effects of titanium oxide concentration on the sinterability, microstructural characteristics, mechanical properties, in vitro bioactivity, and cell culture behavior of chicken-derived hydroxyapatite. J Aust Ceram Soc 60, 471–484 (2024). https://doi.org/10.1007/s41779-024-01005-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-024-01005-x