Abstract
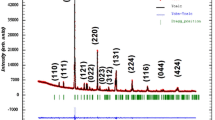
The perovskite Ce1-xKxMoO3, where x = 0.0, 0.2, and 0.4, was prepared using sol–gel technique. Samples were characterized by X-ray diffraction (XRD), differential scanning calorimetry DSC, X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, impedance spectroscopy UV–VIS, diffuse reflectance spectroscopic, and photoluminescence (PL). Besides, the XRD and Raman spectroscopy revealed an orthorhombic phase with a Pnma space group for Ce1-xKxMoO3 samples. XPS analysis proved the existence of Mo3+ and Mo4+ ions. On the other hand, Raman spectroscopy has particularly shown the existence of the B1g mode associated to the MoO6 octahedron. And the DSC curves mark the absence of inflections which qualitatively shows the thermal stability of Ce1-xKxMoO3. Moreover, impedance spectroscopy confirmed that DC conductivity can be justified by the Arrhenius law at 475–600 K temperature range; the activation energy (≈0.314 eV) decreased with the potassium amount and by Mott’s VRH model for T < 445 K. In addition, the density’s greatest value of Fermi states, N(EF) values 1.07 1023 eV−1 cm−3, and a low relaxation time τrel≈0.5 μs were obtained with CKMO04 sample. In the end, the Tauc curves revealed that the bandgap decreased from 3.10 to 2.77 eV with K+ amount; the PL measurements exhibited intense emission of visible and near-infrared light under UV light excitation. In conclusion, all results found allow Ce1-xKxMoO3 to be too useful in the field of optoelectronics.























Similar content being viewed by others
Data Availability
I declare that all data are available.
References
Dyakonov, V., Bukhanko, F., Kamenev, V., Zubov, E., Baran, S., Jaworska-Gołąb, T., Szytuła, A., Wawrzyńska, E., Penc, B., Duraj, R., Stüsser, N., Arciszewska, M., Dobrowolski, W., Dyakonov, K., Pientosa, J., Manus, O., Nabialek, A., Aleshkevych, P., Puzniak, R., Wisniewski, A., Zuberek, R., Szymczak, H.: Structural and magnetic properties of La1−xPrxMnO3+δ 0≤x≤10. Phys. REV. B. 74(2), 024418 (2006)
Li, Z., Yang, M., Park, J.-S., Wei, S.-H., Berry, J.J., Zhu, K.: Stabilizing hybrid perovskite structures by tuning tolerance factor: formation of formamidinium and cesium lead iodide solid-state alloys Chem. Mater. 28, 284–292 (2015)
Liu, C., Sun, J., Tan, W.L., Lu, J., Gengenbach, T.R., McNeill, C.R., Ge, Z., Cheng, Y., Bach, U.: Alkali cation doping for improving the structural stability of 2D perovskite in 3D/2D PSCs. Nano Lett. 20, 1240–1251 (2020)
Li, C., Wang, A., Xie, L., Deng, X., Liao, K., Yang, J.-a, Li, T., Hao, F.: Emerging alkali metal ion (Li+, Na+, K+ and Rb+) doped perovskite films for efficient solar cells: recent advances and prospects. J. Mater. Chem. 7, 24150 (2019)
Abdi-Jalebi, M., Andaji-Garmaroudi, Z., Cacovich, S., Stavrakas, C., Philippe, B., Richter, J.M., Alsari, M., Booker, E.P., Hutter, E.M., Pearson, A.J., Lilliu, S., Savenije, T.J., Rensmo, H., Divitini, G., Ducati, C., Friend, R.H., Stranks, S.D.: Maximizing and stabilizing luminescence from halide perovskites with potassium passivation. Nat. 555, 497–501 (2018)
Bi, C., Zheng, X., Chen, B., Wei, H., Huang, J.: Spontanoues passivation of hybride perovskite by sodium ion from glass substrates, mysterious enhancement of devices efficiency revealed. ACS. Energy. Lett. 2, 1400–1406 (2017)
Dastidar, S., Hawley, C.J., Dillon, A.D., Gutierrez-Perez, A.D., Spanier, J.E., Fafarman, A.T.: Quantitative phase-change thermodynamics and metastability of perovskite-phase cesium lead iodide. J. Phys. Chem. Lett. 8, 1278–1282 (2017)
Lee, Y.N., El-Fadli, Z., SapinÄab, F., Martinez-Tamayob, E., CorberaÂn, V.C.: Synthesis and surface characterization of nanometric La1-xKxMnO3+δ particles. Catal. Today. 52, 45–52 (1999)
Kim, H., Zheng, H., Park, J., Kim, D., Kang, C., Jang, J., Kim, D., Yoon, T.: Artificial synaptic characteristics with strong analog memristive switching in a Pt/CeO2/Pt structure. Nanotechnol. 28, 285203 (2017)
Chen, L., Zheng, G., Yao, G., Zhang, P., Dai, S., Jiang, Y., Li, H., Binbin, Yu., Ni, H., Wei, S.: Lead-free perovskite narrow-bandgap oxide semiconductors of rare-earth manganates. ACS. Omega. 5, 8766–8776 (2020)
Ansari, A.A., Adil, S.F., Alam, M., Ahmad, N., Assal, M.E., Labis, J.P., Alwarthan, A.: Catalytic performance of the Ce-doped LaCoO3 perovskite nanoparticles. Sci. Rep. 10, 15012 (2020)
Konstantinou, K., Sushko, P.V., Duffy, D.M.: Modelling the local atomic structure of molybdenum in nuclear waste glasses with ab initio molecular dynamics simulations. Phys. Chem. Chem. Phys. 18, 26125–26132 (2016)
Emery, A.A., Saal, J.E., Kirklin, S., Hegde, V.I., Wolverton, C.: High-throughput computational screening of perovskites for thermochemical water splitting applications. Chem. Mater. 28, 5621–6563 (2016)
Domenichini, B., Aymes, D., Perriat, P., Gillot, B.: Evidence of a hopping mechanism between Mo3+ and Mo4+ octahedral cations in molybdenum spine1 ferrites. Mater. Chem. Phys. 39, 80–84 (1994)
Dou, B., Lv, G., Wang, C., Hao, Q., Hui, KwanSan: Cerium doped copper/ZSM-5 catalysts used for the selective catalytic reduction of nitrogen oxide with ammonia. Chem. Eng. J. 270, 549–556 (2015)
Thuy, N.T., Minh, D.L.: Size effect on the structural and magnetic properties of nanosized perovskite LaFeO3 prepared by different methods. Adv. Mater. Sci. Eng. 2012, 1–6 (2012)
Momma, K., Izumi, F.: VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011)
Shannon, R.D.: Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. 32, 751 (1976)
Bartel, C.J., Sutton, C., Goldsmith, B.R., Ouyang, R., Musgrave, C.B., Ghiringhelli, L.M., Scheffler, M.: New tolerance factor to predict the stability of perovskite oxides and halides. Sci. Adv. 5(2), 0693 (2019)
Huang, Y., Yan, C., Wang, Z., Liao, C., Xu, G.: Variation of structural, magnetic and transport properties in RE07Sr03MnO3 granular perovskites. Solid. State. Commun. 118, 541–546 (2001)
Moure, A., et al.: Mechanosynthesis of the orthorhombic perovskites ErMn1-xNixO3 (x =0, 0.1) processing and characterization of nanostructured ceramics. Chem. Mater. 22, 2908–2915 (2010)
Stranger, R., Moran, G., Krausz, E.: Octahedral monomeric molybdenum A magneto-optical study Mo3+ doped in Cs2NaYCl6. Mol. Phys. 60, 11–31 (1990)
Klug, H.P., Alexander, L.E.: Basic aspects of X-ray absorption in quantitative diffraction analysis of powder mixture. Anal. Chem. 20, 886–889 (1948)
Pijush, Ch.: Dey, Sumit Sarkar, Ratan Das, X-ray diffraction study of the elastic properties of jagged spherical CdS nanocrystals. Mater. Sci.-Pol. 38, 271–278 (2020)
Karmakar, A., Majumdar, S., Giri, S.: Orthorhombic distortion and novel magnetic phase separation in Pr0.5Eu0.5MnO3. J. Appl. Phys. 110, 063914 (2011)
Haque, F., Wright, M.: Md Arafat Mahmud, Haimang Yi, Dian Wang, Leiping Duan, Cheng Xu, Mushfika Baishakhi Upama, and Ashraf Uddin, Effects of Hydroiodic acid concentration on the properties of CsPbI 3 perovskite solar cells. ACS. Omega. 3, 11937–11944 (2018)
Modi, A., Thakur, R., Thakur, R., Gaur, N.K., Kaurav, N., Okram, G.S.: Structural properties of chromium doped gadolinium manganites. Int. J. Modern. Phys. 22, 511–516 (2013)
Wang, H., Zuo, R., Jian, Fu., Liu, Yi.: Sol–gel derived (Li, Ta, Sb) modified sodium potassium niobate ceramics: processing and piezoelectric properties. J. Alloy. Compd. 509, 936–941 (2011)
Paunovi´c, P., Popovski, O., Dimitrov, A., Slavkov, D., Lefterova, E., Hadˇzi Jordanov, S.: Study of structural and electrochemical characteristics of Co-based hypo–hyper d-electrocatalysts for hydrogen evolution. Electrochim. Acta 52(14), 4640–4648 (2007)
Beche, E., Charvin, P., Perarnau, D.: St ´ephane Abanades and Gilles Flamant, Ce3d XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz). Surf. Interface Anal. 40, 264–267 (2008)
Mannepalli, V.R.: MM Saj Mohan, R Ranjith, Tailoring the bandgap and magnetic properties by bismuth substitution in neodymium chromite. Bull. Mater. Sci. 40, 1503e1511 (2017)
Chen, D., et al.: Single-crystalline MoO3 nanoplates: topochemical synthesis and enhanced ethanol-sensing performance. J. Mater. Chem. 21(25), 9332–42 (2011)
Diaz-Droguett, D.E., El Far, R., Fuenzalida, V.M., Cabrera, A.L.: In situ-Raman studies on thermally induced structural changes of porous MoO3 prepared in vapor phase under He and H2. Mater. Chem. Phys. 134(2–3), 631–638 (2012)
Dieterle, M., Weinberg, G., Mestl, G.: Raman spectroscopy of molybdenum oxides Part I. Structural characterization of oxygen defects in MoO3-x by DR UV/VIS Raman spectroscopy and X-ray diffraction. Phys. Chem. Chem. Phys. 4, 812–821 (2002)
Hangchun, Hu., Wachs, I.E.: Surface structures of supported molybdenum oxide catalysts: characterization by Raman and Mo L3-Edge XANES. J. Phys. Chem. 99, 10897–10910 (1995)
Joya, M.R., Alfonso, J.E., Moreno, L.C.: Photoluminescence and Raman studies of α -MoO3 doped with erbium and neodymium, Curèrent. Sci. 116, 1690–1695 (2019)
Franklin, D.: Hardcastle and Israel E Wachs, Determination of molybdenum-oxygen bond distances and bond orders by Raman spectroscopy. J. Raman. Spectros. 21, 683–691 (1990)
Christiansen, T.L., Emil, T. S., Kovyakh, K.A., Morten, L.: Röderen,c Martin Høj,Tom Vosch and Kirsten M. O. Jensen, Structure analysis of supported disordered molybdenum oxides using pair distribution function analysis and automated cluster. 1,148–158 (2020)
Avisar, D., Livneh, T.: The Raman-scattering of A-type Ce2O3. Vib.. Spectrosc. 86, 14–16 (2016)
Philip, D., Aruldhas, G., Ramakrishnan, V.: Infrared and Raman spectra of aquamolybdenum VI oxide hydrate (MoO3.2H2O). J. Phys. 30, 129–133 (1988)
Jonscher, A.K.: “The interpretation of non-ideal dielectric admittance and impedance diagrams. Phys. Status. Solidi. 32, 665676 (1975)
Prasad, K., Lily, K., Kumari, K.P., Chandra, K.L., Yadav, S.: Sen, Electrical properties of a lead-free perovskite ceramic:(Na05Sb0.5)TiO3. Appl. Phys. A. 88, 377–383 (2007)
Ranjan, R., Kumar, N., Behera, B., Choudhary, R.N.P.: Investigations of electrical impedance and modulus properties of Pb1-xSmx (Zr0.45Ti0.55)1–x/4 O3 ceramics. Adv. Mat. Lett. 5, 138–142 (2014)
Cheng, Q., Chen, Z.: The cause analysis of the incomplete semi-circle observed in high frequency region of EIS obtained from TEL-covered pure copper. Int. J. Electrochem. Sci. 8, 8282–8290 (2013)
Debbebi, I.S., Megdiche-Borchani, S., Cheikhrouhou-Koubaa, W., Cheikhrouhou, A.: Study of complex impedance spectroscopic properties of La0.7-xDyxSr03MnO.3 perovskite oxides. R. Soc. Open. Sci. 5(11), 172201 (2018)
Balasubramani, V., Chandraleka, S., Subba Rao, T., Sasikumar, R., Kuppusamy, M.R., Sridhar, T.M.: Review—Recent advances in electrochemical impedance spectroscopy based toxic gas sensors using semiconducting metal oxides. J. Electrochem. Soc. 167(3), 037572 (2020)
Zainuddin, Z., Shaari, A.H.: Structural and electrical transport properties of La0.67Ba0.33Mn1-yTiyO3 ceramics. Adv. Mater. Res. 501, 86–90 (2012)
Bai, L., Zhang, Y., Zhang, L., Zhang, Y., Sun, Li., Ji, N., Li, X., Haochen Si, Yu., Zhang, H.H.: Jahn-Teller distortions in molybdenum oxides: an achievement in exploringhigh rate supercapacitor applications and robust photocatalytic potential. Nano. Energy. 53, 982 (2018)
Medarde, M., Mesot, J., Lacorre, P., Rosenkranz, S., Fischer, P., Gobrecht, K.: Highpressure neutron-diffraction study of the metallization process in PrNiO3. Phys. Rev. B 52, 9248–9258 (1995)
Böttger, H., Bryksin, V.V.: Hopping conductivity in ordered and disordered solids (II). Phys. Atat. Ssol. (b) 78, 415–451 (1976)
Zakhvalinskii, V.S., Laiho, R., Lisunov, K.G., Lähderanta, E., Petrenko, P.A., Stepanov, Yu.P., Stamov, V.N., Shubnikov, M.L., Khokhulin, A.V.: Variable-range hopping conduction in LaMnO3+δ. Phys. Solid. State. 49, 918–924 (2007)
Silveira, L.G.D., Dias, G.S., Cotica, L.F., Eiras, J.A., Garcia, D., Sampaio, J.A., Yokaichiya, F., Santos, I.A.: Charge carriers and small-polaron migration as the origin of intrinsic dielectric anomalies in multiferroic TbMnO3 polycrystals. J. Phys. Condens. Matter 25, 475401 (2013)
Karmakar, A., Majumdar, S., Giri, S.: Polaron relaxation and hopping conductivity in LaMn1−x FexO3. Phys. Rev. B 79, 094406 (2009)
La Macchia, G., Li Manni, G., Todorova, T.K., Brynda, M., Aquilante, F., Roos, B.O., Gagliardi, L.: G Li Manni, TK Todorova, M Brynda, F Aquilante, BO Roos, L Gagliardi, On the analysis of the Cr-Cr multiple bond in several classes of dichromium compounds. Inorg. Chem. 49, 5216–5222 (2010)
Chou, C.Y., Kaurav, N., Kuo, Y.K., Kuo, D.H.: Electrical properties of A/B –site substituted Ni-deficient La(NiFe03)O3 perovskites with A=Ag+ Pb2+ Nd+3 and B=Mn3+ Ga3+. J. Appl. Phys. 103(9), 093716 (2008)
Badapanda, T., Harichandan, R., Kumar, T.B., Mishra, S.R., Anwar, S.: Dielecric relaxation and conduction mechanism ofdysprosium doped barium bismuth titanate Aurivilliusceramics. J. Mater. Sci: Mater. Electron. 28, 2775–2787 (2017)
Ben Taher, Y., Oueslati, A., Maaloul, N.K., Khirouni, K., Gargouri, M.: Conductivity study and correlated barrier hopping (CBH) conduction mechanism in diphosphate compound. Appl. Phys. Mater. Sci. Process. 120, 1537–43 (2015)
Srinivas, K., Sarah, P., Suryanarayana, S.V.: Impedancespectroscopy study of polycrystalline Bi6Fe2Ti3O18. Bull. Mater. Sci. 2, 247–53 (2003)
Aneesh Kumar, K.S., Bhowmik, R.N.: Effect of annealing temperatures on the electrical conductivity and dielectric properties of Ni15Fe15O4 spinel ferrite prepared by chemical reaction at different pH values. Mater. Res. Express. 4(12), 126105 (2017)
James, A.R., Srinivas, K.: Low temperature and impedance spectroscopy of PMNPT ceramics. Mater. Res. Bull. 34, 1301–1310 (1999)
Jin, F., Li, J., Wang, Y., Chu, X., Mingze, Xu., Zhai, Y., Zhang, Ye., Fang, W., Zou, P., He, T.: Evaluation of Fe and Mn co-doped layered perovskite PrBaCo2/3Fe2/3Mn1/2O5+δ as a novel cathode for intermediate-temperature Solid-oxide fuel cell. Ceram. Int. 44, 22489–22496 (2018)
Chithambaram, V., Das, S.J., Krishnan, S.: “ Synthesis, optical and dielectric studies on novel semi organic nonlinear optical crystal by solution growth technique. J. Alloys. Compd. 509, 4543–4546 (2011)
Ncib, W.: A Ben Jazia Kharrat, MA Wederni, N Chniba-Boudjada, K Khirouni, W Boujelben, Investigation of structural, electrical and dielectric properties of sol-gel prepared La0.67-xEuxBa033Mn0.85Fe0.15O3 (x¼0.0, 0.1) manganites. J. Alloys. Compd. 768, 249–262 (2018)
Khasa, S., Singh, P., Sanghi, S., Singh, N., Agarwal, A.: Science and technology structural analysis and dielectric characterization of Aurivillius type CaSr-Bi2Nb2O9 ceramics. J. Integr. Sci. Technol. At. J. Integr. 2, 13–21 (2014)
Chaouchi, A., Kennour, S.: Impedance spectroscopy studies on lead free (Ba0.85Ca0.15) (Ti0.9 Zr0.1)O3 ceramics. Process. Appl. Ceram. 6, 201–207 (2012)
Dutta, A., Sinha, T.P.: Dielectric relaxation in perovskite BaAl1/2Nb1/2O3. J. Phys. Chem. Solid. 67, 1484–1491 (2006)
Das, P.R., Biswal, L., Behera, B., Choudhary, R.N.P.: Structural and electrical properties of Na2Pb2Eu2W2Ti4X4O30 (X = Nb, Ta) ferroelectric ceramics. Mater. Res. Bull. 44, 1214–1218 (2009)
Li, M.-D., Tang, X.-G., Zeng, S.-M., Jiang, Y.-P., Liu, Q.-X., Zhang, T.-F., Li, W.-H.: Oxygen-vacancy-related dielectric relaxation behaviours and impedance spectroscopy of Bi(Mg1/2Ti1/2)O3 modified BaTiO3 ferroelectric ceramics. J. Materiomics. 4, 194–201 (2018)
Raut, R.R., Ambhore, N.V., Ulhe, C.S.: Synthesis, structural and dielectric properties of La doped 95BiFeO3-5BaTiO3 ceramic. IJIRSET. 7, 58–63 (2018)
Zhang, T.F., Tang, X.G., Liu, Q.X., Lu, S.G., Jiang, Y.P., Huang, X.X., Zhou, Q.F,.: Oxygen-vacancy-related relaxation and conduction behavior in (Pb1-xBax)(Zr0.95Ti0.05)O3 ceramics, AIP ADVANCES 4 107141 (2014)
Jancar, B., Suvorov, D., Valant, M., Drazic, G.: Characterization of CaTiO3-NdAlO3 dielectric ceramics. J. Eur. Ceram. Soc. 23, 1391–1400 (2003)
Hasnaouia, S., Sdiri, N.: Karima Horchani-Naifer, Mokhtar Férid, Study of the physical properties of 90 P2O5+ xV2O5(10–x) BaO(0≤x≤3%) glasses. Sensors. Actuators. A. 319, 112540 (2021)
Das, R., Choudhary, R.N.: Structural and electrical properties of double perovskite: (BaSr)FeMoO6. Physica B: Condensed. Matter. 603, 412522 (2021)
Anwara, Z., Khan, M.A., Ali, I., Asghar, M., Sher, M., Shajir, I., Sarfraz, M.: M Farook Warsi, Investigation of dielectric behaviour of new Tb3+ doped BiFeO3 nanocrystals synthesized via va micro-emulsion route. J. Ovonic. Res. 10, 265–273 (2014)
Bobe, J.M., Reau, J.M., Senegas, J., Poulain, M.: F ion conductivity and diffusion properties in ZrF4-based fluoride glasses with various NaF concentrations (0≤ xNaF ≤ 045). Solid. State. Ionics. 82, 39–52 (1995)
Bergman, R.: General susceptibility functions for relaxations in disordered systems. J. Appl. Phys. 88, 1356 (2000)
Melnikov, P., Leon, C., Santamaria, J., Sanchez-Quesada, F.: Lanthanum–lithium–sodium double chromates as ionic conductors. J. Alloy. Compd. 250, 520–523 (1997)
Daniels, L.M., Kashtiban, R.J., Kepaptsoglou, D., Ramasse, Q.M., Sloan, J., Walton, R.I.: Local A-site layering in rare-earth orthochromite perovskites by solution synthesis. Chem. Eur J. 22, 18362–18367 (2016)
Wilson, J.N., Frost, J.M., Wallace, S.K., Walsh, A.: Dielectric and ferroic properties of metal halide perovskites. APL. Mater.. 7, 010901 (2019)
Jia, T., Zeng, Z., Lin, H.Q., Duan, Y., Ohodnicki, P.: First-principles study on the electronic, optical and thermodynamic properties of ABO3 (A = La, Sr B =Fe, Co) perovskites. RSC. Adv. 7(62), 38798–804 (2017)
Kim, K.H., Jung, J.H., Noh, T.W.: Polaron absorption in a perovskite manganite La0.7Ca0.3MnO3. Phys. Rev. Lett. 81(7), 1517 (1998)
Yakuphanoglu, F., Sekerci, M., Ozturk, O.F.: The determination of the optical constant of Cu(ll) compound having 1-chloro-2, 3- o -cyclohexylidinepropane thin film. Opt. Commun. 239, 275–280 (2004)
Kumar, S., Thakur, N.,: Effect of alkali metal (Na, K) ion ratio on structural, optical and photoluminescence properties of K0.5Na0.5NbO3 ceramics prepared by sol–gel technique. Bull. Mater. Sci. 1—4 (2021)
Im, J.H., Chung, J., Kim, S.J., Park, N.G.: Synthesis, structure, and photovoltaic property of a nanocrystalline 2H perovskite-type novel sensitizer (CH3CH2NH3) PbI3. Nanoscale. Res. Lett. 7, 353 (2012)
Arima, T., Tokura, Y., Torrance, J.B.: Variation of optical gaps in perovskite-type 3d transition-metal oxides. Phys. Rev. B 48, 17006–17009 (1993)
Marzouk, S., Abo-Naf, S.M., Hammam, M., El-Gendy, Y.A., Hassan, N.S.: FTIR spectra and optical properties of molybdenum phosphate glasses. J. Appl. Sci. Res. 7, 935–946 (2011)
Inzani, K., Nematollahi, M., Vullum-Bruer, F., Grande, T., Reenaas, T.W., Selbach, S.M.: Electronic properties of reduced molybdenum oxides. Phys. Chem. Chem. Phys. 19, 9232 (2017)
Huang, P.R., He, Y., Cao, C., Lu, Z.H.: Impact of lattice distortion and electron doping on a-MoO3 electronic structure. Sci. Rep. 4(1), 7131 (2014)
He, C.J., et al.: Orientation effects on the bandgap and dispersion behavior of0.91Pb(Zn 1/3Nb 2/3)O3–009PbTiO3 single crystals. Chin. Phys. B 21(5), 054207 (2012)
Haron, W., Wisitsoraat, A., Sirimahachai, U., Wongnawa, S.: A simple synthesis and characterization of LaMO3 (M=Al Co, Fe, Gd) perovskites via chemical co-precipitation method, Songklanakarin. J. Sci. Technol. 40, 484–491 (2018)
Prasanna, R., Gold-Parker, A., Leijtens, T., Conings, B., Babayigit, A., Boyen, H.G., Toney, M.F., McGehee, M.D.: Band gap tuning via lattice contraction and octahedral tilting in perovskite materials for photovoltaics. J. Am. Chem. Soc. 139, 11117–11124 (2017)
Wahab, H.: Effect of A – site disorder on the bonding mechanism and optical properties of Smx(Al2O3)1–x system. Phys. B Condens. Matter. 481, 24–31 (2016)
Muhammed Shafi, P., Chandra Bose, A.: Impact of crystalline defects and size on X-ray line broadening: a phenomenological approach for tetragonal SnO2 nanocrystals. AIP Adv. 5(5), 057137 (2015)
Xinyu Shen, Yu., Zhang, S.V., Kershaw, T.L., Wang, C., Zhang, X., Wang, W., Li, D., Wang, Y., Min, Lu., Zhang, L., Sun, C., Zhao, D., Qin, G., Xue, B., Yu, W.W., Rogach, A.L.: Zn-alloyed CsPbI3 nanocrystals for highly efficient perovskite light-emitting devices. Nano. Lett. 19, 1552–1559 (2019)
Feng, L., Yan, H., Zhang, R., Liu, J.: Growth of S-doped MoO2 nanosheets with a controlled bandgap by chemical vapour deposition. J. Vac. Sci. Technol., A 36, 05G507 (2018)
Kepenekian, M., Molard, Y., Costuas, K., Lemoine, P., Gautier, R., et al.: Red-NIR luminescence of Mo 6 monolayered assembly directly anchored on Au(001). Materials Horizons, cRoyal Society of Chemistry 6, 1828–1833 (2019)
Peters, T.E., Baglio, J.A.: “Luminescence and structural property of thiogallate phosphors Ce3+ and Eu2+ - activated phosphors” Part-1. J. Electrochemn. Soc. Solid. State. Sci .Techno. 119 230 (1972)
Ramanantoanina, H., Urland, W., García-Fuente, A., Cimpoesu, F., Daul, C.: Calculation of the 4f1→ 4f05d1 transitions in Ce3+-doped systems by ligand field density functional theory. Chem. Phys. Lett. 588, 260–266 (2013)
Herrmann, A., Othman, H.A., Assadi, A.A., Tiegel, M.: Stefan Kuhn1 and Christian Rüssel Spectroscopic properties of cerium-doped aluminosilicate glasses. Opt. Mater. Express. 5, 676–925 (2015)
Taheri, M., Kremer, R.K., Trudel, S., Razavi, F.S.: Exchange bias effect and glassy-like behavior of EuCrO3 and CeCrO3 nano-powders. J. Appl. Phys. 118, 124306 (2015)
Baltrusaitis, J., Mendoza-Sanchez, B., Fernandez, V., Veenstra, R., Dukstiene, N., Roberts, A., Fairley, N.: Generalized molybdenum oxide surface chemical state XPS determination via informed amorphous sample model. Appl. Surf. Sci. 326, 151–161 (2015)
Ji, W., Shen, R., Yang, R., Yu, G., Guo, X., Peng, L., Ding, W.: Partially nitrided molybdenum trioxide with promoted performance as an anode material for lithium-ion batteries. J. Mater. Chem. A 2, 699–704 (2014)
Author information
Authors and Affiliations
Contributions
We confirm that all the authors have had material input into the submission.
Corresponding author
Ethics declarations
Conflict of interest
In our paper, we have provided a new perovskite with electronic conduction, high electrical conductivity, good dielectric properties, good optics refractive index, and a good chemical and thermal stability.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghrissi-Hamrouni, W., Sdiri, N., Horchani-Naifer, K. et al. Structural and physical properties of Ce1-xKxMoO3 for x = 0.0, 0.2, and 0.4 prepared by sol–gel method. J Aust Ceram Soc 59, 685–705 (2023). https://doi.org/10.1007/s41779-023-00866-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-023-00866-y