Abstract
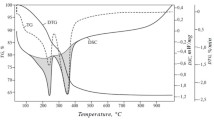
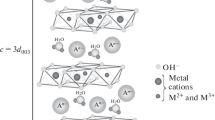
Nickel-aluminum layered double hydroxide with aluminum ions partially substituted by samarium ones was successfully synthesized via coprecipitation followed by hydrothermal treatment. X-ray diffraction data showed that the obtained sample is single-phase material with hydrotalcite-like structure. The presence of samarium in the sample was confirmed by elemental analysis. Electron microscopy demonstrated that the compound consists of very small plate-like particles with a shape similar to hexagonal. The study of thermal transformations of the material revealed that it decomposed upon heating above 300 °C with the formation of mixed oxide, and spinel-type oxide was formed while the heating temperature was increased up to 1000 °C. The rehydration ability of the sample was rather limited: no reconstruction of layered structure took place after mixed oxide was formed. The “memory effect” was observed only after heating the hydroxide at a temperature not higher than 300 °C. The thermal properties of samarium-containing samples resemble closely those of nickel-containing hydrotalcites.







Similar content being viewed by others
References
Cavani, F., Trifirò, F., Vaccari, A.: Hydrotalcite-type anionic clays: preparation, properties and applications. Catal. Today 11, 173–301 (1991). https://doi.org/10.1016/0920-5861(91)80068-K
Xu, Z.P., Zhang, J., Adebajo, M.O., Zhang, H., Zhou, C.: Catalytic applications of layered double hydroxides and derivatives. Appl. Clay Sci. 53, 139–150 (2011). https://doi.org/10.1016/j.clay.2011.02.007
Sarfraz, M., Shakir, I.: Recent advances in layered double hydroxides as electrode materials for high-performance electrochemical energy storage devices. J. Energy Storage 13, 103–122 (2017). https://doi.org/10.1016/j.est.2017.06.011
Mishra, G., Dash, B., Pandey, S.: Layered double hydroxides: a brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 153, 172–186 (2018). https://doi.org/10.1016/j.clay.2017.12.021
Zümreoglu-Karan, B., Ay, A.N.: Layered double hydroxides—multifunctional nanomaterials. Chem. Pap. 66, 1–10 (2012). https://doi.org/10.2478/s11696-011-0100-8
Wei, M., Xu, X., Wang, X., Li, F., Zhang, H., Lu, Y., Pu, M., Evans, D.G., Duan, X.: Study on the photochromism of Ni–Al layered double hydroxides containing nitrate anions. Eur. J. Inorg. Chem. 2006, 2831–2838 (2006). https://doi.org/10.1002/ejic.200600058
Takei, T., Miura, A., Kumada, N.: Soft-chemical synthesis and catalytic activity of Ni-Al and Co-Al layered double hydroxides (LDHs) intercalated with anions with different charge density. J. Asian. Ceram. Soc. 2, 289–296 (2014). https://doi.org/10.1016/j.jascer.2014.06.002
Deng, X., Huang, J., Wan, H., Chen, F., Lin, Y., Xu, X., Ma, R., Sasaki, T.: Recent progress in functionalized layered double hydroxides and their application in efficient electrocatalytic water oxidation. J. Energy Chem. 32, 93–104 (2019). https://doi.org/10.1016/j.jechem.2018.07.007
Pérez-Ramírez, J., Ribera, A., Kapteijn, F., Coronado, E., Gómez-García, C.J.: Magnetic properties of Co–Al, Ni–Al, and Mg–Al hydrotalcites and the oxides formed upon their thermal decomposition. J. Mater. Chem. 12, 2370–2375 (2002). https://doi.org/10.1039/B110314H
Abellán, G., Coronado, E., Martí-Gastaldo, C., Waerenborgh, J., Ribera, A.: Interplay between chemical composition and cation ordering in the magnetism of Ni/Fe layered double hydroxides. Inorg. Chem. 52, 10147–10157 (2013). https://doi.org/10.1021/ic401576q
Coronado, E., Galán-Mascarós, J.R., Martí-Gastaldo, C., Ribera, A., Palacios, E., Castro, M., Burreil, M.: Spontaneous magnetization in Ni-Al and Ni-Fe layered double hydroxides. Inorg. Chem. 47, 9103–9110 (2008). https://doi.org/10.1021/ic801123v
Liu, X.-M., Zhang, Y.-H., Zhang, X.-G., Fu, S.-Y.: Studies on Me/Al-layered double hydroxides (Me = Ni and Co) as electrode materials for electrochemical capacitors. Electrochim. Acta 49, 3137–3141 (2004). https://doi.org/10.1016/j.electacta.2004.02.028
Wang, J., Song, Y., Li, Z., Liu, Q., Zhou, J., Jing, X., Zhang, M., Jiang, Z.: In situ Ni/Al layered double hydroxide and its electrochemical capacitance performance. Energy Fuels 24, 6463–6467 (2010). https://doi.org/10.1021/ef101150b
Wang, W., Zhang, N., Shi, Z., Ye, Z., Gao, Q., Zhi, M., Hong, Z.: of Ni-Al layered double hydroxide hollow microspheres for supercapacitor electrode. Chem. Eng. J. 338, 55–61 (2018). https://doi.org/10.1016/j.cej.2018.01.024
Sanati, S., Rezvani, Z.: Co-intercalation of acid Red-27/sodium dodecyl sulfate in a Ce-containing Ni-Al-layered double hydroxide matrix and characterization of its luminescent properties. J. Mol. Liq. 249, 318–325 (2018). https://doi.org/10.1016/j.molliq.2017.10.145
Bellardita, M., Di Paola, A., Palmisano, L., Parrino, F., Buscarino, G., Amadelli, R.: Preparation and photoactivity of samarium loaded anatase, brookite and rutile catalysts. Appl. Catal. B 104, 291–299 (2011). https://doi.org/10.1016/j.apcatb.2011.03.016
Dillip, G.R., Kumar, P.M., Raju, B.D.P., Dhoble, S.J.: Synthesis and luminescence properties of a novel Na6CaP2O9:Sm3+ phosphor. J. Lumin. 134, 333–338 (2013). https://doi.org/10.1016/j.jlumin.2012.08.025
Ashwini, K., Pandurangappa, C., Avinash, K., Srinivasan, S., Stefanakos, E.: Synthesis, characterization and photoluminescence studies of samarium doped zinc sulfide nanophosphors. J. Lumin. 221, 117097 (2020). https://doi.org/10.1016/j.jlumin.2020.117097
Singh, S., Kaur, P., Kumar, V., Tikoo, K.B., Singhal, S.: Traversing the advantageous role of samarium doped spinel nanoferrites for photocatalytic removal of organic pollutants. J. Rare Earths 39, 781–789 (2021). https://doi.org/10.1016/j.jre.2020.12.008
Smalenskaite, A., Şen, S., Salak, A.N., Ferreira, M.G.S., Beganskiene, A., Kareiva, A.: Sol–gel derived lanthanide-substituted layered double hydroxides Mg3/Al1−xLnx. Acta Phys. Pol. A 133, 884–886 (2018). https://doi.org/10.12693/APhysPolA.133.884
Mitran, G., Urda, A., Tanchoux, N., Fajula, F., Marcu, I.-C.: Propane oxidative dehydrogenation over Ln-Mg-Al-O catalysts (Ln = Ce, Sm, Dy, Yb). Catal. Lett. 131, 250–257 (2009). https://doi.org/10.1007/s10562-009-0057-1
Urdă, A., Popescu, I., Cacciaguerra, T., Tanchoux, N., Tichit, D., Marcu, I.-C.: Total oxidation of methane over rare earth cation-containing missed oxides derived from LDH precursors. Appl. Catal., A. 464–465, 20–27 (2013). https://doi.org/10.1016/j.apcata.2013.05.012
Taherian, Z., Gharahshiran, V.S., Khataee, A., Orooji, Y.: Anti-coking freeze-dried NiMgAl catalysts for dry and steam reforming of methane. J. Ind. Eng. Chem. 103, 187–194 (2021). https://doi.org/10.1016/j.jiec.2021.07.032
Shen, S., Guo, W., Zhuang, W., Yang, W., Qin, L., Liu, X., Yue, Z.: Effect of Sm-doped Ni-Al layered double hydroxide on electrochemical performance for supercapacitors. J. Phys. Conf. Ser. 2009, 012008 (2021). https://doi.org/10.1088/1742-6596/2009/1/012008
Kulyukhin, S.A., Krasavina, E.P., Rumer, I.A.: Sorption of 60Co, 90Sr, 90Y and 137Cs from aqueous solutions onto Mg-Ln layered double hydroxides (Ln = Ce, Pr, Sm, Gd). Radiochemistry 55, 569–600 (2013). https://doi.org/10.1134/S1066362213060052
Golovin, S.N., Yapryntsev, M.N., Ryltsova, I.G., Veligzhanin, A.A., Lebedeva, O.E.: Novel cerium-containing layered double hydroxide. Chem. Pap. 74, 367–370 (2020). https://doi.org/10.1007/s11696-019-00877-9
Golovin, S. N., Yapryntsev, M. N., Ryl’tsova, I. G., Savilov, S. V., Maslakov, K. I., Lebedeva, O. E.: Synthesis and thermal behavior of Co AlCe layered double hydroxide. Solid State Sci. 111:106498 (2021). https://doi.org/10.1016/j.solidstatesciences.2020.106498
Bugaenko, L.T., Ryabykh, S.M., Bugaenko, A.L.: A nearly complete system of average crystallographic ionic radii and its use for determining ionization potentials. Moscow Univ. Chem. Bull. 63, 303–317 (2008). https://doi.org/10.3103/S0027131408060011
Ramesh, T.N., Jayashree, R.S., Kamath, P.V.: Disorder in layered hydroxides: DIFFaX simulation of the X-ray powder diffraction patterns of nickel hydroxide. Clays Clay Miner. 51, 570–576 (2003). https://doi.org/10.1346/CCMN.2003.0510511
Shivaramaiah, R., Navrotsky, A.: Energetics of order-disorder in layered magnesium aluminum double hydroxides with interlayer carbonate. Inorg. Chem. 54, 3253–3259 (2015). https://doi.org/10.1021/ic502820q
Sławińsky, W.A., Sjåstad, A.O., Fjellvåg, H.: Stacking faults and polytypes for layered double hydroxides: what can we learn from simulated and experimental X-ray powder diffraction data? Inorg. Chem. 55, 12881–12889 (2016). https://doi.org/10.1021/acs.inorgchem.6b02247
Wang, L., Lü, Z., Li, F., Duan, X.: Study on the mechanism and kinetics of the thermal decomposition of Ni/Al layered double hydroxide nitrate. Ind. Eng. Chem. Res. 47, 7211–7218 (2008). https://doi.org/10.1021/ie800609c
Mahjoubi, F.Z., Elhalil, A., Elmoubarki, R., Sadiq, M., Khalidi, A., Cherkaoui, O., Barka, N.: Performance of of Zn-, Mg- and Ni-Al layered double hydroxides in treating an industrial textile wastewater. J. Appl. Surf. Interfaces. 2, 1–11 (2017). https://doi.org/10.48442/IMIST.PRSM/jasi-v2i1-3.10033
Herrero, M., Benito, P., Labajos, F.M., Rives, V.: Stabilization of Co2+ in layered double hydroxides (LDHs) by microwave-assisted ageing. J. Solid State Chem. 180, 873–884 (2007). https://doi.org/10.1016/j.jssc.2006.12.011
Zhang, Y., Liu, J., Li, Y., Yu, M., Yin, X., Li, S.: Enhancement of active anticorrosion via Ce-doped Zn-Al layered double hydroxides embedded in sol-gel coatings on aluminum alloy. J. Wuhan. Univ. Technol. Mater. Sci. Ed. 32, 1199–1204 (2017). https://doi.org/10.1007/s11595-017-1731-6
Rey, F., Fornés, V., Rojo, J.M.: Thermal decomposition of hydrotalcites An infrared and nuclear magnetic resonance spectroscopic study. J Chem Soc Faraday Trans. 88, 2233–2238 (1992). https://doi.org/10.1039/FT9928802233
Prevot, V., Caperaa, N., Taviot-Guého, C., Forano, C.: Glycine-assisted hydrothermal synthesis of NiAl-layered double hydroxide nanostructures. Cryst. Growth Des. 9, 3646–3654 (2009). https://doi.org/10.1021/cg900384n
Vicente, P., Pérez-Bernal, M.E., Ruano-Casero, R.J., Duarte, A., Almeida Paz, F.A., Rocha, J., Rives, V.: Luminescence properties of lanthanide-containing layered double hydroxides. Microporous Mesoporous Mater. 226, 209–220 (2016). https://doi.org/10.1016/j.micromeso.2015.12.036
Dubey, P., Kaurav, N.: Stoichiometric and nonstoichiometric compounds. In: Tanasescu, S. (ed.) Structure Processing Properties Relationships in Stoichiometric and Nonstoichiometric Oxides. IntechOpen, London (2019). https://doi.org/10.5772/intechopen.89402
Sato, T., Fujita, H., Endo, T., Shimada, M., Tsunashima, A.: Synthesis of hydrotalcite-like compounds and their physico-chemical properties. React. Solids 5, 216–228 (1988). https://doi.org/10.1016/0168-7336(88)80089-5
Funding
The reported study was funded by Russian Foundation for Basic Research according to the research project no. 20–33-90178. The work was carried out using the equipment of the Joint Research Center of Belgorod State National Research University «Technology and Materials».
Author information
Authors and Affiliations
Contributions
Conceptualization: OEL; methodology: OEL; investigation: SNG, MNY; writing — original draft: SNG; writing — review and editing: OEL; funding acquisition: OEL; resources: MNY; supervision: OEL; visualization: SNG.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Golovin, S.N., Yapryntsev, M.N. & Lebedeva, O.E. Synthesis and thermal transformations of layered double hydroxide containing samarium. J Aust Ceram Soc 58, 1615–1622 (2022). https://doi.org/10.1007/s41779-022-00798-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-022-00798-z