Abstract
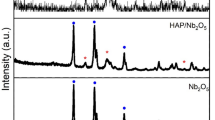
Hydroxyapatite (HAP), vanadium pentoxide (V2O5), and graphene oxide (GO) are three biocompatible materials. The antibacterial is the main characteristics of the produced TNC. Furthermore, the structural investigation was done by x-ray diffraction patterns (XRD). The investigation showed inhibition in the growth of V2O5 and in contrast with HAP, where high growth was noticed. According to XRD data, the crystallite size of HAP was grown from 6 to 8.2 nm starting with the HAP composition to TNC. The nanorod shapes of HAP reached an average of 141 and 30 nm for length and diameter. In addition, the roughness average parameter (Ra) reached 47.5 nm. The microhardness has been evaluated. It started from 3.3 ± 0.2 GPa for pure HAP and decreased to 2.9 ± 0.3 GPa for the dual composite of HAP/V2O5, and improved to 4.1 ± 0.2 GPa for the ternary combination of HAP/V2O5/GO. The biological response was represented in the cell viability measurements which increased from 96.5 ± 4 to 99.7 ± 5% at the final NC (TNC). In addition, the measurements were done toward the human osteoblast cell line in vitro. Furthermore, the antibacterial activity was measured against negative gram and positive gram strains. The inhibition zone reached 17.1 ± 1.5 and 16.3 ± 1.4 mm against E. coli and S. aureus. The results refer to the ability to be suggested TNC for biomedical applications.












Similar content being viewed by others
References
Zou, Z., Wang, L., Zhou, Z., Sun, Q., Liu, D., Chen, Y., et al.: Simultaneous incorporation of PTH(1–34) and nano-hydroxyapatite into chitosan/alginate hydrogels for efficient bone regeneration. Bioactive materials 6, 1839–1851 (2021)
Ho-Shui-Ling, A., Bolander, J., Rustom, L.E., Johnson, A.W., Luyten, F.P., Picart, C.: Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 180, 143–162 (2018)
Holmes D.: Non-union bone fracture: a quicker fix. Nature 550:S193-S (2017)
Holmes, D.: Closing the gap. Nature 550, S194–S195 (2017)
Sadowska JM, Genoud KJ, Kelly DJ, O'Brien FJ.: Bone biomaterials for overcoming antimicrobial resistance: advances in non-antibiotic antimicrobial approaches for regeneration of infected osseous tissue. Mater Today (2021)
Kurella, A., Dahotre, N.B.: Surface modification for bioimplants: the role of laser surface engineering. J. Biomater. Appl. 20, 5–50 (2005)
Aronov D, Molotskii M, Rosenman G.: Charge-induced wettability modification. Appl. Phys. Lett. 90:104104 (2007)
Dias, M., Fernandes, P., Guedes, J., Hollister, S.: Permeability analysis of scaffolds for bone tissue engineering. J. Biomech. 45, 938–944 (2012)
Prasadh, S., Wong, R.C.W.: Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Science International 15, 48–55 (2018)
Kang, Y., Chang, J.: Channels in a porous scaffold: a new player for vascularization. Regen. Med. 13, 705–715 (2018)
Zhu, J., Thompson, C.B.: Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 20, 436–450 (2019)
Senthilkumar, K., Saba, N., Rajini, N., Chandrasekar, M., Jawaid, M., Siengchin, S., et al.: Mechanical properties evaluation of sisal fibre reinforced polymer composites: a review. Constr. Build. Mater. 174, 713–729 (2018)
Wang, L., Wang, C., Wu, S., Fan, Y., Li, X.: Influence of the mechanical properties of biomaterials on degradability, cell behaviors and signaling pathways: current progress and challenges. Biomaterials science 8, 2714–2733 (2020)
Kumar S, Roy DN, Dey V. A.: comprehensive review on techniques to create the anti-microbial surface of biomaterials to intervene in biofouling. Colloid Interface Sci. Commun. 43:100464 (2021)
Ghanavati, Z., Neisi, N., Bayati, V., Makvandi, M.: The influence of substrate topography and biomaterial substance on skin wound healing. Anat. Cell. Biol. 48, 251–257 (2015)
Sharma, P., Pandey, P.M.: Morphological and mechanical characterization of topologically ordered open cell porous iron foam fabricated using 3D printing and pressureless microwave sintering. Mater. Des. 160, 442–454 (2018)
Fathi, A.M., Ahmed, M.K., Afifi, M., Menazea, A.A., Uskoković, V.: Taking hydroxyapatite-coated titanium implants two steps forward: surface modification using graphene mesolayers and a hydroxyapatite-reinforced polymeric scaffold. ACS Biomater. Sci. Eng. 7, 360–372 (2021)
Ahmed, M., Al-Wafi, R., Mansour, S., El-Dek, S., Uskoković, V.: Physical and biological changes associated with the doping of carbonated hydroxyapatite/polycaprolactone core-shell nanofibers dually, with rubidium and selenite. J. Market. Res. 9, 3710–3723 (2020)
Afifi M, Ahmed MK, Fathi AM, Uskoković V.: Physical, electrochemical and biological evaluations of spin-coated ε-polycaprolactone thin films containing alumina/graphene/carbonated hydroxyapatite/titania for tissue engineering applications. Int. J. Pharm. 585 (2020)
Riaz, M., Zia, R., Ijaz, A., Hussain, T., Mohsin, M., Malik, A.: Synthesis of monophasic Ag doped hydroxyapatite and evaluation of antibacterial activity. Mater. Sci. Eng., C 90, 308–313 (2018)
Bhowmick, A., Jana, P., Pramanik, N., Mitra, T., Banerjee, S.L., Gnanamani, A., et al.: Multifunctional zirconium oxide doped chitosan based hybrid nanocomposites as bone tissue engineering materials. Carbohyd. Polym. 151, 879–888 (2016)
Balagangadharan, K., Chandran, S.V., Arumugam, B., Saravanan, S., Venkatasubbu, G.D., Selvamurugan, N.: Chitosan/nano-hydroxyapatite/nano-zirconium dioxide scaffolds with miR-590-5p for bone regeneration. Int. J. Biol. Macromol. 111, 953–958 (2018)
SM Z, S A S, T Ebrahimi S, S A S. A study on mechanical properties of PMMA/hydroxyapatite nanocomposite. Engineering 2011(2011)
Díaz M, Barba F, Miranda M, Guitián F, Torrecillas R, Moya JS.: Synthesis and antimicrobial activity of a silver-hydroxyapatite nanocomposite. J. Nanomater. 2009 (2009)
Ashraf, S., Ahmed, M., Ibrahium, H.A., Awwad, N.S., Abdel-Fattah, E., Ghoniem, M.: Nanofibers of polycaprolactone containing hydroxyapatite doped with aluminum/vanadate ions for wound healing applications. New J. Chem. 45, 22610–22620 (2021)
Sarkar, N., Bose, S.: Controlled delivery of curcumin and vitamin K2 from hydroxyapatite-coated titanium implant for enhanced in vitro chemoprevention, osteogenesis, and in vivo osseointegration. ACS Appl. Mater. Interfaces. 12, 13644–13656 (2020)
Samadian, H., Salehi, M., Farzamfar, S., Vaez, A., Ehterami, A., Sahrapeyma, H., et al.: In vitro and in vivo evaluation of electrospun cellulose acetate/gelatin/hydroxyapatite nanocomposite mats for wound dressing applications. Artif. Cells Nanomed Biotechnol. 46, 964–974 (2018)
Namasivayam, S., Venkatachalam, G., Bharani, R.: Noteworthy enhancement of wound-healing activity of triphala biomass metabolite-loaded hydroxyapatite nanocomposite. Appl. Nanosci. 11, 1511–1530 (2021)
Larcher, D., Tarascon, J.-M.: Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 7, 19–29 (2015)
Sarraf M, Nasiri-Tabrizi B, Yeong CH, Hosseini HRM, Saber-Samandari S, Basirun WJ. Mixed oxide nanotubes in nanomedicine: a dead-end or a bridge to the future? Ceram. Int. (2020)
Kim, Y.S., Song, M.Y., Park, E.S., Chin, S., Bae, G.-N., Jurng, J.: Visible-light-induced bactericidal activity of vanadium-pentoxide (V 2 O 5)-loaded TiO 2 nanoparticles. Appl. Biochem. Biotechnol. 168, 1143–1152 (2012)
Sun, H., Yang, Z., Pu, Y., Dou, W., Wang, C., Wang, W., et al.: Zinc oxide/vanadium pentoxide heterostructures with enhanced day-night antibacterial activities. J. Colloid Interface Sci. 547, 40–49 (2019)
Bergerud AJ.: Phase Stability and Transformations in Vanadium Oxide Nanocrystals: University of California, Berkeley (2016)
Anicic, N., Vukomanovic, M., Suvorov, D.: Design of a multifunctional vanadium pentoxide/polymer biocomposite for implant-coating applications. RSC Adv. 7, 38647–38658 (2017)
Yuvakkumar, R., Hong, S.: Structural and toxic effect investigation of vanadium pentoxide. Mater. Sci. Eng., C 65, 419–424 (2016)
Das, S., Roy, A., Barui, A.K., Alabbasi, M.M.A., Kuncha, M., Sistla, R., et al.: Anti-angiogenic vanadium pentoxide nanoparticles for the treatment of melanoma and their in vivo toxicity study. Nanoscale 12, 7604–7621 (2020)
Knöller, A., Lampa, C.P., von Cube, F., Zeng, T.H., Bell, D.C., Dresselhaus, M.S., et al.: Strengthening of ceramic-based artificial nacre via synergistic interactions of 1D vanadium pentoxide and 2D graphene oxide building blocks. Sci. Rep. 7, 1–9 (2017)
Zaaba, N., Foo, K., Hashim, U., Tan, S., Liu, W.-W., Voon, C.: Synthesis of graphene oxide using modified hummers method: solvent influence. Procedia Eng. 184, 469–477 (2017)
El-Kader MFHA, Ahmed MK, Elabbasy MT, Afifi M, Menazea AA.: Morphological, ultrasonic mechanical and biological properties of hydroxyapatite layers deposited by pulsed laser deposition on alumina substrates. Surf. Coat. Technol. 409 (2021)
Phiri, J., Johansson, L.-S., Gane, P., Maloney, T.: A comparative study of mechanical, thermal and electrical properties of graphene-, graphene oxide-and reduced graphene oxide-doped microfibrillated cellulose nanocomposites. Compos. B Eng. 147, 104–113 (2018)
Zeng, H., Qu, S., Qin, Y.: Microstructure and transport properties of cement-based material enhanced by graphene oxide. Mag. Concr. Res. 73, 1011–1024 (2021)
Chowdhury, I., Duch, M.C., Mansukhani, N.D., Hersam, M.C., Bouchard, D.: Deposition and release of graphene oxide nanomaterials using a quartz crystal microbalance. Environ. Sci. Technol. 48, 961–969 (2014)
Agarwalla, S.V., Ellepola, K., da Costa, M.C.F., Fechine, G.J.M., Morin, J.L.P., Neto, A.C., et al.: Hydrophobicity of graphene as a driving force for inhibiting biofilm formation of pathogenic bacteria and fungi. Dent. Mater. 35, 403–413 (2019)
Plachá, D., Jampilek, J.: Graphenic materials for biomedical applications. Nanomaterials 9, 1758 (2019)
Szunerits, S., Boukherroub, R.: Antibacterial activity of graphene-based materials. J. Mater. Chem. B 4, 6892–6912 (2016)
Li, J., Wang, G., Zhu, H., Zhang, M., Zheng, X., Di, Z., et al.: Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci. Rep. 4, 1–8 (2014)
Mustapha S, Ndamitso M, Abdulkareem A, Tijani J, Shuaib D, Mohammed A, et al.: Comparative study of crystallite size using Williamson-Hall and Debye-Scherrer plots for ZnO nanoparticles. Adv. Nat. Sci.: Nanosci. Nanotechnol. 10, 045013 (2019)
Abdelbar MF, El-Sheshtawy HS, Shoueir KR, El-Mehasseb I, Ebeid E-Zeiny M, El-Kemary M.: Halogen bond triggered aggregation induced emission in an iodinated cyanine dye for ultra sensitive detection of Ag nanoparticles in tap water and agricultural wastewater. RSC Adv. 8:24617–26 (2018)
Zakria MA, Menazea AA, Mostafa AM, Al-Ashkar EA.: Ultra-thin silver nanoparticles film prepared via pulsed laser deposition: synthesis, characterization, and its catalytic activity on reduction of 4-nitrophenol. Surf. Interfaces 100438 (2020)
Abdelghany AM, Menazea AA, Ismail AM.: Synthesis, characterization and antimicrobial activity of chitosan/polyvinyl alcohol blend doped with Hibiscus Sabdariffa L. extract. J. Mol. Struct. 1197:603–9 (2019)
Ismail, A.M., Menazea, A.A., Kabary, H.A., El-Sherbiny, A.E., Samy, A.: The influence of calcination temperature on structural and antimicrobial characteristics of zinc oxide nanoparticles synthesized by Sol-Gel method. J. Mol. Struct. 1196, 332–337 (2019)
Menazea AA.: Femtosecond laser ablation-assisted synthesis of silver nanoparticles in organic and inorganic liquids medium and their antibacterial efficiency. Radiat. Phys. Chem. 108616 (2019)
Lukić, M.J., Veselinović, L., Stevanović, M., Nunić, J., Dražič, G., Marković, S., et al.: Hydroxyapatite nanopowders prepared in the presence of zirconium ions. Mater. Lett. 122, 296–300 (2014)
Gopi D, Rajeswari D, Ramya S, Sekar M, R P, Dwivedi J, et al. Enhanced corrosion resistance of strontium hydroxyapatite coating on electron beam treated surgical grade stainless steel. Appl. Surf. Sci. 286, 83–90 (2013)
Hiromoto, S., Inoue, M., Taguchi, T., Yamane, M., Ohtsu, N.: In vitro and in vivo biocompatibility and corrosion behaviour of a bioabsorbable magnesium alloy coated with octacalcium phosphate and hydroxyapatite. Acta Biomater. 11, 520–530 (2015)
Mansour, S.F., El-Dek, S.I., Ahmed, M.K.: Physico-mechanical and morphological features of zirconia substituted hydroxyapatite nano crystals. Sci. Rep. 7, 43202 (2017)
Kaygili, O., Keser, S., Bulut, N., Ates, T.: Characterization of Mg-containing hydroxyapatites synthesized by combustion method. Physica B 537, 63–67 (2018)
Surekha G, Krishnaiah KV, Ravi N, Suvarna RP.: FTIR, Raman and XRD analysis of graphene oxide films prepared by modified Hummers method. Journal of Physics: Conference Series: IOP Publishing, p. 012012 (2020)
Mishra, V.K., Bhattacharjee, B.N., Parkash, O., Kumar, D., Rai, S.B.: Mg-doped hydroxyapatite nanoplates for biomedical applications: a surfactant assisted microwave synthesis and spectroscopic investigations. J. Alloy. Compd. 614, 283–288 (2014)
Kaygili, O., Tatar, C., Yakuphanoglu, F., Keser, S.: Nano-crystalline aluminum-containing hydroxyapatite based bioceramics: synthesis and characterization. J. Sol-Gel. Sci. Technol. 65, 105–111 (2012)
Janković, A., Eraković, S., Mitrić, M., Matić, I.Z., Juranić, Z.D., Tsui, G.C.P., et al.: Bioactive hydroxyapatite/graphene composite coating and its corrosion stability in simulated body fluid. J. Alloy. Compd. 624, 148–157 (2015)
Ahmed, M.K., Ramadan, R., El-dek, S.I., Uskoković, V.: Complex relationship between alumina and selenium-doped carbonated hydroxyapatite as the ceramic additives to electrospun polycaprolactone scaffolds for tissue engineering applications. J. Alloy. Compd. 801, 70–81 (2019)
Lin, K., Zhou, Y., Zhou, Y., Qu, H., Chen, F., Zhu, Y., et al.: Biomimetic hydroxyapatite porous microspheres with co-substituted essential trace elements: surfactant-free hydrothermal synthesis, enhanced degradation and drug release. J. Mater. Chem. 21, 16558 (2011)
Kato, A., Kowada, H., Deguchi, M., Hotehama, C., Hayashi, A., Tatsumisago, M.: XPS and SEM analysis between Li/Li3PS4 interface with Au thin film for all-solid-state lithium batteries. Solid State Ionics 322, 1–4 (2018)
Chen, X., Wang, X., Fang, D.: A review on C1s XPS-spectra for some kinds of carbon materials. Fullerenes, Nanotubes, Carbon Nanostruct. 28, 1048–1058 (2020)
Reddy, B.M., Ganesh, I., Reddy, E.P.: Study of dispersion and thermal stability of V2O5/TiO2− SiO2 catalysts by XPS and other techniques. J. Phys. Chem. B 101, 1769–1774 (1997)
Doveren Hv, Verhoeven JT.: XPS spectra of Ca, Sr, Ba and their oxides. J. Electron Spectros. Relat. Phenom. 21, 265–73 (1980)
Donya H, Darwesh R, Ahmed M.: Morphological features and mechanical properties of nanofibers scaffolds of polylactic acid modified with hydroxyapatite/CdSe for wound healing applications. Int. J. Biol. Macromol (2021)
Negrila CC, Predoi MV, Iconaru SL, Predoi D.: Development of zinc-doped hydroxyapatite by sol-gel method for medical applications. Molecules 23 (2018)
Zavala-Sanchez, L.A., Hirata, G.A., Novitskaya, E., Karandikar, K., Herrera, M., Graeve, O.A.: Distribution of Eu(2+) and Eu(3+) ions in hydroxyapatite: a cathodoluminescence and raman study. ACS Biomater. Sci. Eng. 1, 1306–1313 (2015)
Wang, M., Liu, Y., Ren, G., Wang, W., Wu, S., Shen, J.: Bioinspired carbon quantum dots for sensitive fluorescent detection of vitamin B12 in cell system. Anal. Chim. Acta 1032, 154–162 (2018)
Zhen, Q., Li, L., Li, R., Lu, F., Li, Z., Wang, Y.: Morphology controllable preparation and infrared emissivity of vanadium pentoxide. Infrared Phys. Technol. 71, 303–306 (2015)
El-Ghannam, A.R.: Advanced bioceramic composite for bone tissue engineering: design principles and structure-bioactivity relationship. J. Biomed. Mater. Res., Part A 69, 490–501 (2004)
Ahmed MK, Afifi M, Mansour SF, Ibrahium HA, Aldulmani SAA, Awwad NS.: Morphological behaviors of brushite/vivianite nanocomposites and their potency for Se(IV) and Cd(II) removal from aqueous solutions. Mater. Chem. Phys. 259 (2021)
Lutzweiler, G., Ndreu Halili, A., Engin, V.N.: The overview of porous, bioactive scaffolds as instructive biomaterials for tissue regeneration and their clinical translation. Pharmaceutics 12, 602 (2020)
Ahmed MK, Afifi M, Awwad NS, Ibrahium HA.: Pb(II) and Cd(II) removal, mechanical and morphological features of nanofibrous membranes of cellulose acetate containing fillers of hydroxyapatite, graphene oxide, and magnetite. Appl. Phys. A: Mater. Sci. Process. 126 (2020)
Gholami-Shabani M, Sotoodehnejadnematalahi F, Shams-Ghahfarokhi M, Eslamifar A, Razzaghi-Abyaneh M.: Mycosynthesis and physicochemical characterization of vanadium oxide nanoparticles using the cell-free filtrate of fusarium oxysporum and evaluation of their cytotoxic and antifungal activities. J. Nanomater. 2021 (2021)
Srivastava, S., Kumar, N., Roy, P.: Role of ERK/NFκB in vanadium (IV) oxide mediated osteoblast differentiation in C3H10t1/2 cells. Biochimie 101, 132–144 (2014)
Hatamie S, Ahadian MM, Zomorod MS, Torabi S, Babaie A, Hosseinzadeh S, et al.: Antibacterial properties of nanoporous graphene oxide/cobalt metal organic framework. Mater. Sci. Eng.: C 104, 109862 (2019)
Qiu, J., Wang, D., Geng, H., Guo, J., Qian, S., Liu, X.: How oxygen-containing groups on graphene influence the antibacterial behaviors. Adv. Mater. Interfaces 4, 1700228 (2017)
Slavin, Y.N., Asnis, J., Hafeli, U.O., Bach, H.: Metal nanoparticles: understanding the mechanisms behind antibacterial activity. Journal of nanobiotechnology 15, 65 (2017)
Hashemi, E., Akhavan, O., Shamsara, M., Rahighi, R., Esfandiar, A., Tayefeh, A.R.: Cyto and genotoxicities of graphene oxide and reduced graphene oxide sheets on spermatozoa. RSC Adv. 4, 27213–27223 (2014)
AlSalem HS, Keshk AA, Ghareeb RY, Ibrahim AA, Abdelsalam NR, Taher MM, et al.: Physico-chemical and biological responses for hydroxyapatite/ZnO/graphene oxide nanocomposite for biomedical utilization. Mater. Chem. Phys. 283, 125988 (2022)
Mallakpour, S., Okhovat, M.: Hydroxyapatite mineralization of chitosan-tragacanth blend/ZnO/Ag nanocomposite films with enhanced antibacterial activity. Int. J. Biol. Macromol. 175, 330–340 (2021)
Citradewi PW, Hidayat H, Purwiandono G, Fatimah I, Sagadevan S.: Clitorea ternatea-mediated silver nanoparticle-doped hydroxyapatite derived from cockle shell as antibacterial material. Chem. Phys. Lett. 769, 138412 (2021)
Awwad, N., Ahmed, M., Afifi, M., Ibrahium, H.A.: Rb (I)/Se (IV) co-dopant into hydroxyapatite; their structural, morphological, and antibacterial effectiveness for biomedical applications. Appl. Phys. A 127, 1–10 (2021)
Al Jahdaly BA, Khalil AM, Ahmed MK, Shoueir KR.: Tuning the compositional configuration of hydroxyapatite modified with vanadium ions including thermal stability and antibacterial properties. J. Mol. Struct. 1242, 130713 (2021)
Hassan AA, Radwan HA, Abdelaal SA, Al-Radadi NS, Ahmed MK, Shoueir KR, et al.: Polycaprolactone based electrospun matrices loaded with Ag/hydroxyapatite as wound dressings: morphology, cell adhesion, and antibacterial activity. Int. J. Pharmaceutics 593, 120143 (2021)
Mohd Yusoff MF, Abu Kasim NH, Himratul-Aznita WH, Saidin S, Genasan K, Kamarul T, et al.: Physicochemical, antibacterial and biocompatibility assessments of silver incorporated nano-hydroxyapatite synthesized using a novel microwave-assisted wet precipitation technique. Materials Characterization 178, 111169 (2021)
Patty DJ, Nugraheni AD, Ana ID, Yusuf Y.: In vitro bioactivity of 3D microstructure hydroxyapatite/collagen based‐egg white as an antibacterial agent. J. Biomed. Mater. Res. Part B: Appl. Biomater. (2022)
Babaei, P., Safai-Ghomi, J., Rashki, S.: Engineered dual-purpose Ta-doped ZnO/hydroxyapatite nanocomposites: antibacterial activity and robust catalyst in MW-Induced synthesis of chromopyrimidines. Ceram. Int. 48, 8359–8373 (2022)
El-Naggar, M.E., Abu Ali, O.A., Saleh, D.I., Abu-Saied, M., Ahmed, M., Abdel-Fattah, E., et al.: Microstructure, morphology and physicochemical properties of nanocomposites containing hydroxyapatite/vivianite/graphene oxide for biomedical applications. Luminescence 37, 290–301 (2022)
Funding
The authors appreciate the Deputyship for Research and Innovation, Minsitry of education in Saudi Arabia for funding this research work through the project number : IFP-KKU-2020/11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ashraf, S., El-Morsy, M.A., Awwad, N.S. et al. Physicochemical changes of hydroxyapatite, V2O5, and graphene oxide composites for medical usages. J Aust Ceram Soc 58, 1399–1413 (2022). https://doi.org/10.1007/s41779-022-00735-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-022-00735-0