Abstract
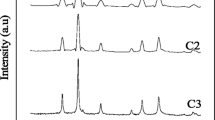
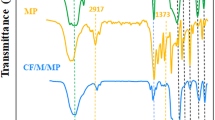
The CoFe2O4 with various morphologies has been successfully synthesized via ionic liquid-assisted hydrothermal synthetic process and was used as a catalyst to form imidazo[1,2-a]pyridines. The structural and magnetic properties of the catalyst were characterized by X-ray diffraction (XRD), field emission scanning electron microscope (FE-SEM), nitrogen sorption techniques (BET), and vibrating sample magnetometer (VSM). The results indicate that the samples are nanoparticles, nanorods, and nanosheets which clearly shows the effect of octyl-4-aza-1-azoniabicyclo[2.2.2]octane bromide ([C8dabco]Br) ionic liquid on the morphology of final products. According to the SEM and magnetic hysteresis measurements, different ratios of [C8dabco]Br resulted in different morphologies and crystalline sizes and hence different magnetic properties in the synthesized materials. Textural analysis by N2 sorption revealed that the total BET surface areas of CoFe2O4 nano powders were 23.5–48.6 m2/g. It was found that the values of remanent magnetization (Mr) and saturation magnetization (Ms) increase with increment of IL ratio. Moreover, the reaction of aldehydes, 2-aminopyridines, and isocyanide was carried out under solvent-free conditions by cobalt ferrite as a suitable catalyst, and all desired products were formed in good yields. The ultraviolet (UV) spectrum has been used to calculate the absorption coefficient (α) as a function of photon energy (hυ), and the obtained results indicate that the optical band gaps are estimated to be 1.23–1.38 eV.









Similar content being viewed by others
References
Tho, N.T., Thi, C.M., Van Hieu, L., et al.: Visible-light-driven photocatalysis for methylene blue degradation and hydrogen evolution reaction: a case of black TiO2 nanotube arrays. J. Aust. Ceram. Soc. 56, 849–857 (2020). https://doi.org/10.1007/s41779-019-00405-8
Arul, E., Sivaji, K., Manohar, P.: Effect of bismuth on copper zinc ferrites for photocatalytic applications. J. Aust. Ceram. Soc. 56, 811–817 (2020). https://doi.org/10.1007/s41779-019-00400-z
Yavaş, A., Güler, S., Erol, M.: Growth of ZnO nanoflowers: effects of anodization time and substrate roughness on structural, morphological, and wetting properties. J. Aust. Ceram. Soc. 56, 995–1003 (2020). https://doi.org/10.1007/s41779-019-00440-5
A, S.P., Devi, K.R.S., NJ, V.: Role of Sm3+ and Ce4+ on mesoporous rice husk silica for selective oxidation of benzyl alcohol to benzaldehyde. J. Aust. Ceram. Soc. 56, 217–225 (2020). https://doi.org/10.1007/s41779-019-00342-6
Prozorov, T., Bazylinski, D.A., Mallapragada, S.K., Prozorov, R.: Novel magnetic nanomaterials inspired by magnetotactic bacteria: topical review. Mater. Sci. Eng. 74, 133–172 (2013)
Li, X., Zhang, F., Zhao, D.: Lab on upconversion nanoparticles: optical properties and applications engineering via designed nanostructure. Chem. Soc. Rev. 44, 1346–1378 (2015)
Zaera, F.: Nanostructured materials for applications in heterogeneous catalysis. Chem. Soc. Rev. 42, 2746–2762 (2013)
Hayes, R., Warr, G.G., Atkin, R.: Structure and nanostructure in ionic liquids. Chem. Rev. 115, 6357–6426 (2015)
Freemantle, M.: Designer solvents: ionic liquids may boost clean technology development. Chem. Eng. News. 76, 32–37 (1998)
MacFarlane, D.R., Tachikawa, N., Forsyth, M., Pringle, J.M., Howlett, P.C., Elliott, G.D., Davis, J.H., Watanabe, M., Simon, P., Angell, C.A.: Energy applications of ionic liquids. Energy Environ. Sci. 7, 232–250 (2014)
Hojniak, S.D., Silverwood, I.P., Khan, A.L., Vankelecom, H.F.J., Dehaen, W., Kazarian, S.G., Binnemans: Highly selective separation of carbon dioxide from nitrogen and methane by nitrile/glycol-difunctionalized ionic liquids in supported ionic liquid membranes. K. J Phys Chem B. 118, 7440–7449 (2014)
Dupont, J., de Souza, R.F., Suarez, P.A.Z.: Ionic liquid (molten salt) phase organometallic catalysis. Chem. Rev. 102, 3667–3692 (2002)
Sanaeishoar, H., Sabbaghan, M., Mohave, F.: Synthesis and characterization of micro-mesoporous MCM-41 using various ionic liquids as co-templates. Microporous Mesoporous Mater. 217, 219–224 (2015)
Sun, C., Lee, J.S.H., Zhang, M.: Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 60, 1252–1265 (2008)
Frey, N.A., Peng, S., Cheng, K., Sun, S.: Magnetic nanoparticles: synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 38, 2532–2542 (2009)
Rocha-Santos, T.A.P.: Sensors and biosensors based on magnetic nanoparticles. Trac-Trend Anal Chem. 62, 28–36 (2014)
Rossi, L.M., Costa, N.J.S., Silva, F.P., Wojcieszak, R.: Magnetic nanomaterials in catalysis: advanced catalysts for magnetic separation and beyond. Green Chem. 16, 2906–2933 (2014)
Takeda, E., Todoroki, N., Kitamoto, Y., Abe, M.: Faraday effect enhancement in Co-ferrite layer incorporated into one-dimensional photonic crystal working as a Fabry-Perot resonator. J. Appl. Phys. 87, 6782–6784 (2000)
Gu, B.X.: Magnetic properties and magneto-optical effect of Co0.5Fe2.5O4 nanostructured films. Appl. Phys. Lett. 82, 3707–3709 (2003)
Sun, Y., Ji, G., Zheng, M., Chang, X., Lib, S., Zhang, Y.: Synthesis and magnetic properties of crystalline mesoporous CoFe2O4 with large specific surface area. J. Mater. Chem. 20, 945–952 (2010)
Desai, S.S., Pawar, R.A., Jadhav, S.S., Shirsath, S.E., Patange, S.M.: Role of coupling divalent and tetravalent metal ions on the elastic and electric properties of CoFe2O4 ferrites prepared by sol–gel method. J. Supercond. Nov. Magn. 29, 2635–2640 (2016)
Pervaiz, E., Gul, I.H., Anwar, H.: Hydrothermal synthesis and characterization of CoFe2O4 nanoparticles and nanorods. J. Supercond. Nov. Magn. 26, 415–424 (2013)
He, G., Ding, J., Zhang, J., Hao, Q., Chen, H.: One-step ball-milling preparation of highly photocatalytic active cofe2o4–reduced graphene oxide heterojunctions for organic dye removal. Ind. Eng. Chem. Res. 54, 2862–2867 (2015)
Lu, X., Yang, L., Bian, X., Chao, D., Wang, C.: Rapid, microwave-assisted, and one-pot synthesis of magnetic palladium–CoFe2O4–graphene composite nanosheets and their applications as recyclable catalysts. Part. Part. Syst. Charact. 31, 245–251 (2014)
Gandha, K., Elkins, K., Poudyal, N., Liu, J.P.: Synthesis and characterization of CoFe2O4 nanoparticles with high coercivity. J. Appl. Phys. 117, 17A736 (2015)
Antonel, P.S., Oliveira, C.L.P., Jorge, G.A., Perez, O.E., Leyva, A.G., Negri, R.M.: Synthesis and characterization of CoFe2O4 magnetic nanotubes, nanorods and nanowires. Formation of magnetic structured elastomers by magnetic field-induced alignment of CoFe2O4 nanorods. J. Nanopart. Res. 17, 1–14 (2015)
Yao, X., Kong, J., Tang, X., Zhou, D., Zhao, C., Zhou, R., Lu, X.: Facile synthesis of porous CoFe2O4 nanosheets for lithium-ion battery anodes with enhanced rate capability and cycling stability. RSC Adv. 4, 27488–27492 (2014)
Jing, P., Du, J., Jin, C., Wang, J., Pan, L., Li, J., Liu, Q.: Improved coercivity and considerable saturation magnetization of cobalt ferrite (CoFe2O4) nanoribbons synthesized by electrospinning. J. Mater. Sci. 51, 885–892 (2016)
Ji, G.B., Tang, S.L., Ren, S.K., Zhang, F.M., Gu, B.X., Du, Y.W.: Simplified synthesis of single-crystalline magnetic CoFe2O4 nanorods by a surfactant-assisted hydrothermal process. J. Cryst. Growth. 270, 156–161 (2004)
Menchaca-Nal, S., Londoño-Calderón, C.L., Cerrutti, P., Foresti, M.L., Pampillo, L., Bilovol, V., Candal, R., Martínez-García, R.: Facile synthesis of cobalt ferrite nanotubes using bacterial nanocellulose as template. Carbohydr. Polym. 137, 726–731 (2016)
Devi, N., Rawal, R., Singh, V.: Diversity-oriented synthesis of fused-imidazole derivatives via Groebke–Blackburn–Bienayme reaction: a review. Tetrahedron. 71, 183–232 (2015)
Shaoyong, L., Shuai, L., Zhang, J.: Harnessing allostery: a novel approach to drug discovery. Med. Res. Rev. 34, 1242–1285 (2014)
Yadav, J.S., Reddy, B.V.S., Rao, Y.G., Srinivas, M., Narsaiah, A.V.: Cu(OTf)2-catalyzed synthesis of imidazo[1,2-a]pyridines from α-diazoketones and 2-aminopyridines. Tetrahedron Lett. 48, 7717–7720 (2007)
Wu, Z., Pan, Y., Zhou, X.: Synthesis of 3-arylimidazo[1,2-a]pyridines by a catalyst-free cascade process. Synthesis. 2011, 2255–2260 (2011)
Groebke, K., Weber, L., Mehlin, F.: Synthesis of imidazo[1,2-a] annulated pyridines, pyrazines and pyrimidines by a novel three-component condensation. Synlett. 1998, 661–663 (1998)
Bienayme, H., Bouzid, K.: A new heterocyclic multicomponent reaction for the combinatorial synthesis of fused 3-aminoimidazoles. Angew. Chem. Int. Ed. 37, 2234–2237 (1998)
Blackburn, C., Guan, B., Fleming, P., Shiosaki, K., Tsai, S.: Parallel synthesis of 3-aminoimidazo[1,2-a]pyridines and pyrazines by a new three-component condensation. Tetrahedron Lett. 39, 3635–3638 (1998)
Lu, J., Li, X.T., Ma, E.Q., Mo, L.P., Zhang, Z.H.: Superparamagnetic CuFeO2 nanoparticles in deep eutectic solvent: an efficient and recyclable catalytic system for the synthesis of imidazo[1,2-a]pyridines. Chem. Cat. Chem. 6, 2854–2859 (2014)
Shaabani, A., Hooshmand, S.E.: Choline chloride/urea as a deep eutectic solvent/organocatalyst promoted three-component synthesis of 3-aminoimidazo-fused heterocycles via Groebke–Blackburn–Bienayme process. Tetrahedron Lett. 57, 310–313 (2016)
Tavakkoli, H., Yazdanbakhsh, M.: Fabrication of two perovskite-type oxide nanoparticles as the new adsorbents in efficient removal of a pesticide from aqueous solutions: kinetic, thermodynamic, and adsorption studies. Microporous Mesoporous Mater. 176, 86–94 (2013)
Tabari, T., Tavakkoli, H.: Fabrication and characterization of perovskite-type oxide LaFe0.9Co0.1O3 nanoparticles and its performance in aerobic oxidation of thiols to disulfide. Chin. J. Catal. 33, 1791–1796 (2012)
Tavakkoli, H., Hamedi, F.: Synthesis of Gd0.5Sr0.5FeO3 perovskite-type nanopowders for adsorptive removal of MB dye from water. Res. Chem. Intermed. 42, 3005–3027 (2016)
Sanaeishoar, H., Sabbaghan, M., Mohave, F., Nazarpour, R.: Disordered mesoporous KIT-1 synthesized by DABCO-based ionic liquid and its characterization. Microporous Mesoporous Mater. 228, 305–309 (2016)
Chiappe, C., Melai, B., Sanzone, A., Valentini, G.: Basic ionic liquids based on monoquaternized 1,4-diazobicyclo[2.2.2]octane (dabco) and dicyanamide anion: physicochemical and solvent properties. Pure Appl. Chem. 81, 2035–2043 (2009)
Bertaut, E.F.: Sur quelques progress recents dans la crystallographic des spinelles, en particulier des ferrites (Recent progress in the crystallography of spinels, in particular the ferrites). J Phys Rad. 12, 252–255 (1951)
Shirsath, S.E., Liu, X., Yasukawa, Y., Li, S., Morisako, A.: Switching of magnetic easy-axis using crystal orientation for large perpendicular coercivity in CoFe2O4 thin film. Sci. Rep. 6, 1–11 (2016)
Jia, Z., Ren, D., Zhu, R.: Synthesis, characterization and magnetic properties of CoFe2O4 nanorods. Mater. Lett. 66, 128–131 (2012)
Slonczewski, J.C.: Origin of magnetic anisotropy in cobalt-substituted magnetite. Phys. Rev. 110, 1341–1348 (1958). https://doi.org/10.1103/PhysRev.110.1341
Kumar, Y., Sharma, A., Shirage, P.M.: Impact of different morphologies of CoFe2O4 nanoparticles for tuning of structural, optical and magnetic properties. J. Alloys Compd. 778, 398–409 (2019)
Burns, R.G.: Mineralogical applications of crystal field theory. Cambridge University Press, Cambridge (1993)
Sharma, D., Khare, N.: Tuning of optical bandgap and magnetization of CoFe2O4 thin films. Appl. Phys. Lett. 105, 032404 (2014). https://doi.org/10.1063/1.4890863
Varma, R.S., Kumar, D.: Microwave-accelerated three-component condensation reaction on clay: solvent-free synthesis of imidazo[1,2-a] annulated pyridines, pyrazines and pyrimidines. Tetrahedron Lett. 40, 7665–7669 (1999)
Adib, M., Sheikhi, E., Rezaei, N.: One-pot synthesis of imidazo[1,2-a]pyridines from benzyl halides or benzyl tosylates, 2-aminopyridines and isocyanides. Tetrahedron Lett. 52, 3191–3194 (2011)
Adib, M., Mahdavi, M., Alizadeh, N.M., Mirzaei, P.: Catalyst-free three-component reaction between 2-aminopyridines (or 2-aminothiazoles), aldehydes, and isocyanides in water. Tetrahedron Lett. 48, 7263–7265 (2007)
Shaabani, A., Rezazadeh, F., Soleimani, E.: Ammonium chloride catalyzed one-pot synthesis of imidazo[1,2-a]pyridines. Monatsh. Chem. 139, 931–933 (2008)
Sanaeishoar, T., Tavakkoli, H., Mohave, F.: A facile and eco-friendly synthesis of imidazo [1, 2-a] pyridines using nano-sized LaMnO3 perovskite-type oxide as an efficient catalyst under solvent-free conditions. Appl Catal A-Gen. 470, 56–62 (2014)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghaemi, A., Mohave, F., Farhadi, A. et al. Hydrothermal synthesis of mesoporous cobalt ferrite by ionic liquid-assisted process; catalytic performance, morphology, and magnetic studies. J Aust Ceram Soc 57, 1321–1330 (2021). https://doi.org/10.1007/s41779-021-00630-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-021-00630-0