Abstract
BiFe1−xNixO3 (x = 0.7, 0.8 and 0.9) polycrystalline ceramics are synthesized by a solid-state reaction, and their structural, absorption, leakage current and electrical properties are investigated. The X-ray diffraction measurements show that the lattice parameter values increase with increasing the substitution of Ni2+ ions for Fe3+ ions. The optical absorption spectra indicate that the band gap energy increases with increasing Ni2+ ions. Leakage currents are much decreased by about three orders of magnitude with increasing Ni ions. The J-E hysteresis was also investigated. Both real and imaginary dielectric constants are investigated as a function of both frequency and temperature. The room temperature dielectric measurement with a wide frequency range of 1 KHz–1 MHz reveals that the real and imaginary dielectric constants are decreased with increasing frequency of BiFe1−xNixO3 (x = 0.7, 0.8, 0.9) ceramics. The real and imaginary dielectric constants are found to be increased with temperature. The temperature dependence of ε′ and ε″ exhibits an anomaly which shifted to lower temperature with increasing Ni2+. The anomaly indicates the possible existence of spin-glass states with Ni2+ ion substitution in places of Fe3+ ions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In multiferroic system, a coupling of at least two or all three of antiferroelectricity, antiferromagnetism and ferroelectricity characteristic are observed in the same phase [1,2,3]. The existence of both ferroelectric, ferromagnetism and elasticity in such a system will provide more degree of freedom in the field of new functional sensors and actuators [1, 4, 5]. BiFeO3 represents one of such set of perovskite multiferroics family which has a ferroelectricity phase with cure temperature (Tc) that is around 820–850 °C [6, 7] and has an antiferromagnetic transition (TN) around 370–380 °C [6, 8]. The leakage current represents the main problem for such material, this is due to its low resistivity which is reflected as its instability [9]. The co-existence of both vacancies and Fe2+ ions represents the main source of leakage current [10,11,12,13,14]. Several attempts were carried out to overcome such problems using doping techniques. This was done by substituting part of Bi+3 with dope ions at the side A of BiFeO3 ceramic structure [15,16,17,18] or with substituting another type of dope ions (Nb+5, Mn+4, Ti+4 or Cr+3) at site B (Fe3+) [19,20,21]. The doping process leads to an improvement of ferroelectric properties and a reduction of the leakage current of BiFeO3 ceramic to some extent.
As a photocatalytic material, BiFeO3 had been examined in a visible region of light. Its photocatalytic degradation returns to its narrow optical band gap (~ 2.2 eV) and its chemical stability [22, 23].
In this work, we report the synthesis of BiFe1−xNixO3 ceramic with a composition of x = 0.7, 0.8 and 0.9 and study the influence of aliovalent ions of Ni2+ substitution, on structural, optical absorption, leakage current and dielectric behaviour of BiFe1−xNixO3 ceramic. The doping with Ni2+ may prevent the formation of Fe2+ and then reduce the oxygen vacancies which cause an improvement of the electrical properties of such multiferroic ceramic. The reduction of oxygen vacancies after the Ni2+ doping process which will prevent Fe2+ formation was expected.
Experimental
By using the solid-state reaction, BiFe1−xNixO3 (x = 0.7, 0.8 and 0.9) were synthesized from highly pure Bi2O3, (1 − x) Fe2O3 and 2xNiO powders. The weighed powders were milled mixed for half an hour at room temperature in air atmosphere. This milled powder was pressed into 16 mm diameter and 1 mm thickness discs under 1.5 Mpa pressure on a hydraulic press and then were rapidly heated to 750 °C and sintered for 30 min at atmospheric pressure. The calcined powder was milled again for half an hour. Then, they were pressed again into pellets of 16 mm diameter by applying a pressure of 1.5 Mpa. The pellets of the sample were found to be dark grey upon Ni substitution. For measurements of electrical properties, the ceramic pellets were coated on both surfaces with silver paint for good contacts.
The structure of the samples is characterized by X-ray diffractometer (X’Pert-Pro MPD, Philips) using CuKα radiation with λ = 1.541874 Å. The patterns were recorded in a 2θ interval of 20–60 with increments of 0.025 (2θ). The photo-absorption of BiFe1−xNixO3 was measured by UV-visible diffuse reflectance spectrophotometer ShimadzuUV-3600. The electric properties were measured using the semiconductor characterization system SCS4200 (Keithly). The electric data were carried out as a function of temperature and frequency during heating of the samples from room temperature up to 280 °C and frequency range from 5 kHz to 5 MHz.
Result and discussion
Structural
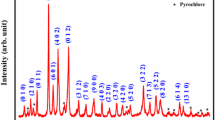
Figure 1a demonstrates XRD patterns of sintered BiFe1−xNixO3 ceramics, x = 0.7, 0.8 and 0.9. The patterns indicate that all samples have secondary phases (Bi2FeO9, Bi25FeO40, and NiFeO4) in a perovskite structure. The secondary phase peaks Bi2FeO9 and Bi25FeO40 are usually observed in BiFeO3 and its substituted samples because of the kinetic of their formation [24]. The new peak NiFe2O4 is observed corresponding to the phase associated with Ni ions which indicate that Ni ions are incorporated into the BiFeO3 structure [25]. In addition, as a result of the highest Ni content in the samples, the reflection peaks of bismuth ferrite have disappeared which is in a good agreement with the previous results reported by Betancourt-Cantera et al. [25]. The lattice parameters are deduced from the XRD patterns at room temperature. The crystal structure of BiFeO3 displays rhombohedral, while for high Ni2+ ions doping BiFe1−xNixO3 (x = 0.7, 0.8 and 0.9), the structure is found to be a triclinic unit cell (as reported before [26]). On the other hand, the data of cell parameters (Fig. 1b) were increased with the increasing of Ni2+ ion content because of the difference in ionic radius between the Ni2+ (ionic radius 0.69 Å) and the Fe3+ (0.64 Å).
Optical properties
The optical properties of BiFe1−xNixO3 samples with x = 0.7, 0.8 and 0.9 powder were investigated. Figure 2 illustrates the diffuse reflectance spectra (DRS) of BiFe1−xNixO3 samples in the wavelength range of 250 to 700 nm. Kubelka-Munk function [27, 28] is used to estimate the absorption coefficient from the DRS data. Kubelka-Munk function is defined as:
where R∞ = Rsample/Rpref.
Here, “α is the absorption coefficient, S is the scattering coefficient and F(R∞) is the KM function”. Near the absorption edge, the relation between photon energy (E = hν), absorption coefficient and band gap energy (Eg) for insulating substance is represented as:
K is a constant, and n is the exponent which depends on the type of transition [29].
The KM function can be used instead of the absorption coefficient a for the estimation of the optical energy gap (Eg) [21]. Figure 3 shows the method of calculating the optical gap in the case of a direct and an indirect transition of x = 0.9 sample as an example. It was noted that the calculation based on the direct transition shows more extended linear region as compared with the calculations based on indirect transition. For this reason, we consider the direct transition for all samples.
The direct band gap for BiFe1−xNixO3 can be predicted from the slope and intercept of the (αhν)2 vs (hν) linear portion [30]. Figure 4 illustrates this relation for samples with different Ni content (x = 0.7, 0.8, 0.9). For samples with doping concentrations of x = 0.8 and 0.9, the band gaps were suddenly reduced to about 2.5 eV. Such a sudden decrease in optical band gap due to high doping concentration can be attributed to the Mott semiconductor-metal transition [31]. The value of the optical band gap energy of the samples with x = 0.8 and 0.9 is consistent with previous report [22, 32, 33]. However, the optical band gap exhibits an increase at x = 0.7 (Eg = 3.04 eV). This can be interpreted as the results of the molecular orbital rearrangements [34].
Electric properties
The leakage current represents one of the problems which hinder the ferroelectric and transport properties of multifarious ceramics. This leakage current is produced due to the existence of defects and nonstoichiometric in the bulk samples. The improvement of multiferroic properties requires a reduction of its leakage current. Hence, the study of the leakage current represents one of the important issues in this research. For this purpose, the J-E curve was measured for the present samples (BiFe1−xNixO3 ceramics) at room temperature. During the voltage change in positive and negative directions, the mean value of the leakage current (for five consecutive readings) was recorded automatically. We did not notice any significant differences between the two directions of applied voltage which supports our previous results [35]. As shown in Fig. 5a, the leakage current density of BiFe1−xNixO3 samples has a tendency to decrease with increasing of x (Ni contents), and the value is dramatically decreased when x = 0.9 (see Fig. 5b). This result shows a significant improvement in the properties of the prepared samples which represented as the lowering in the leakage current values with Ni co-doping content. As we know, the main source of the leakage current is the charge defects such as V3−Bi (bismuth vacancies) and Vo2+ (oxygen vacancies). The oxygen vacancies are mostly generated from Bi volatility besides the changes from Fe3+ to Fe2+. The reduction in Fe3+ species to Fe2+ is possibly compensated by the substituted Ni2+ ion, and thus, it decreases the probability of oxygen vacancies formation, which may cause the initial reduction in leakage current as shown in the lattice parameters shown in Fig. 1.
The electrical hysteresis (IV loop) for sample x = 0.9 is represented in Fig. 5c. It is clearly noted that the area of the hysteresis decreases with decreasing Ni content, the symmetry of the curve behaviour (− 20 ➔ 0 ➔ + 20) increase with decreasing Ni content, and the shift between the two minima (m1 and m2 in Fig. 5c of the cycle becomes close to each other with decreasing Ni content. This behaviour can be attributed to the existence of trapping and detrapping process beside the cle bipolar resistive element which decreases the effect of the space charge carrier.
Figure 6 a and b represent the dependence of the real and imaginary dielectric constant ε′ and ε″ on frequency at room temperature. It is evident that for all samples, the ε′ and ε″ decrease with the increasing of frequency. In the starting low frequency range both ε′ and ε″ values decrease gradually as the frequency increases and then decrease slowly and become almost constant up to 1 MHz for all samples. These results can be attributed to the well-known dipole relaxation phenomena. At high electric field frequencies, the electric dipoles become enable to switch or rotate with the applied electric field frequency [36]. Furthermore, the real and imaginary parts of the dielectric constant are decreased as the concentration of Ni ions increases. The oxygen vacancies in BiFeO3 materials, which are mostly generated during the heat treatment, are the major origin of the determination of the insulation and the formation of Fe, according to Vox + 2Fe3+ → Vto + 2Fe2+, where Vto is an oxygen vacancy and Vox is an oxygen position. Following Le Charelier principle, the concentration of Fe2+ would decrease if the content of Fe3+ is reduced. Therefore, doping at Fe site would suppress the formation of oxygen vacancies and reduce the concentration of Fe2+ [37].
The variation of the real and imaginary dielectric constants (ε′) and (ε″) of the BiFe1−xNixO3 (x = 0.7, 0.8 and 0.9) as a function of temperature measured at as elected frequencies is shown in Fig. 7. It shows a continuous increase with temperature up to the transition temperature Tc then it comes to be decreased. The exhibited anomaly has been detected in three samples around 260 °C, 255 °C and 240 °C. The existence of these anomalies in the temperature range 240–260 was reported previously by several authors [24, 38,39,40]. These anomalies seem to be related to magnetic transition (spin-glass-like behaviour) [41]. The position of the anomaly was shifted with doping of Ni in BiFeO3 towards to lower temperature side. The shift in the position of anomaly may be attributed to the increasing in the ionic radius of Ni2+ (0.69 Å) which is larger than Fe3+ (0.64 Å). These anomalies prove that the possibility of coupling between the electric and magnetic dipole moments of BiFe1−xNixO3, which attributed to the magneto-electric coupling.
Conclusion
The high nickel dopant induced changes in the structure, optical absorption and leakage current; the J-E hysteresis and dielectric properties of BiFe1−xNixO3 samples have been investigated. It is shown that the BiFe1−xNixO3 crystallizes in the triclinic crystal structure. The cell parameters increase with increasing x doping, and optical band gaps decrease with increasing x. Leakage current were much reduced with Ni+2 ion doping, it was decreased by about three orders of magnitude. The area of the hysteresis decreases with decreasing Ni doping. It is also found that the doping of Ni+2 ions can effectively reduce the leakage current density and improve the ferroelectric properties. The dielectric constants ε′ and ε″ were found to be decreased with increased frequency. The real ε′ and imaginary ε″ dielectric constants were found to be increased with temperature. The temperature dependence of ε′ and ε″ exhibited an anomaly which shifted to lower temperature with increasing Ni+2 ion doping. The anomaly indicates the possible existence of spin-glass states with Ni2+ ion substitution in the place of Fe3+ ions.
Change history
19 October 2020
The name of third author to be read as Huda Al fannakh.
References
Hill, N.A.: J. Phys. Chem. B. 104(29), 6694–6709 (2000)
S. Kumari, D.K. Pradhan, R.S. Katiyar, A. Kumar,: Elsevier, , p. 571 (2018)
N.I. Ilic, B.D. Stojanovic: Elsevier, p. 527, (2018)
C.E. Ciomaga, L. Mitoseriu,: Elsevier, p. s,( 2018)
Delshad, M., Safaee, I., Kazazi, M., Shahraki, M.M.: Ceram. Int. 44(12), 14281–14285 (2018). https://doi.org/10.1016/j.ceramint.2018.05.032
Roginskaya, Y.E., Tomashpol’Skii, Y.Y., Venevtsev, Y.N., Petrov, V., Zhdanov, G.: Soviet. J. Exp. Theor. Phys. 23(47), (1966)
Kubel, F., Schmid, H.: Structural Science. 46, 698–702 (1990)
Kiselev, S., Ozerov, R.P., Zhudanov, G.S.: Detection of magnetic order in ferroelectric BiFeO3 by neutron diffraction. Sov Phys. 7, 742 (1963)
Ederer, C., Spaldin, N.A.: Curr. Opinion Solid State Mater. Sci. 9, 128 (2005)
Wang, Y., Zhou, L., Zhang, M., Chen, X., Liu, J.-M., Liu, Z.: Appl. Phys. Lett. 84, 1731 (2004)
Palkar, V., Kumara, K.G., Malik, S.: Appl. Phys. Lett. 84, 2856 (2004)
Sarkar, T., Elizabeth, S., Kumar, P.A.: J. Magn. Magn. Mater. 448(266), (2018)
Dai, H., Ye, F., Chen, Z., Li, T., Liu, D.: J. Alloys Compd. 734, 60 (2018)
Wen, X., Chen, Z., Liu, E., Lin, X., Chen, C.: J. Alloys Compd. 678, 511 (2016)
Yu, B., Li, M., Liu, J., Guo, D., Pei, L., Zhao, X.: J. Phys. D. Appl. Phys. 41(065003), (2008)
Kawae, T., Tsuda, H., Morimoto, A.: Appl. Phys. Express. 1, 051601 (2008)
Yau, C., Palan, R., Tran, K., Buchanan, R.: Appl. Phys. Lett. 86, 032907 (2005)
Kojima, T., Sakai, T., Watanabe, T., Funakubo, H., Saito, K., Osada, M.: Appl. Phys. Lett. 80, 2746 (2002)
Kumar, M.M., Srinivas, A., Suryanarayana, S.: J. Appl. Phys. 87, 855 (2000)
Liu, H., Liu, Z., Yao, K.: Phys. B Condens. Matter. 391, 103 (2007)
Kumar, V., Gaur, A., Kotnala, R.: J. Alloys Compd. 551, 410 (2013)
Gao, F., Yuan, Y., Wang, K., Chen, X., Chen, F., Liu, J.-M., Ren, Z.: Appl. Phys. Lett. 89, 102506 (2006)
Gao, F., Chen, X., Yin, K., Dong, S., Ren, Z., Yuan, F., Yu, T., Zou, Z., Liu, J.M.: Adv. Mater. 19, 2889 (2007)
Biswal, M.R., Nanda, J., Mishra, N.C., Anwar, S., Mishra, A.: Adv. Mater. Lett. 5, 531 (2014)
Betancourt-Cantera, L.G. ; Bolarín-Miró, A.M., Cortés-Escobedo C.A.;, Hernández-Cruz, L.E.;Sánchez-De Jesús, F.; J. Magn. Magn. Mater., 456, 381( 2018)
Ishiwata, S., Azuma, M., Hanawa, M., Moritomo, Y., Ohishi, Y., Kato, K., Takata, M., Nishibori, E., Sakata, M., Terasaki, I.: Phys. Rev. B. 72, 045104 (2005)
Yang, L., Miklavcic, S.J.: JOSA A. 22, 1866 (2005)
Kortüm, G.: Reflectance spectroscopy: principles, methods, applications. Springer Science & Business Media (2012)
Barton, D.G., Shtein, M., Wilson, R.D., Soled, S.L., Iglesia, E.: J. Phys. Chem. B. 103, 630 (1999)
Butler, M.: J. Appl. Phys. 48, 1914 (1977)
Kim, K.J., Park, Y.R.: Appl. Phys. Lett. 78, 475 (2001)
Katsura, H., Balatsky, A.V., Nagaosa, N.: Phys. Rev. Lett. 98, 027203 (2007)
Van Minh, N., Quan, N.G.: J. Alloys Compd. 509, 2663 (2011)
Bhushan, B., Basumallick, A., Vasanthacharya, N., Kumar, S., Das, D.: Solid State Sci. 12, 1063 (2010)
Arafat, S., Ibrahim, S.: Mater. Sci. Appl. 8, 716 (2017)
Uniyal, P., Yadav, K.: J. Alloys Compd. 492, 406 (2010)
Zhang, Y., Yu, S., Cheng, J.: J. Eur. Ceram. Soc. 30, 271 (2010)
Chaudhary, Y.A., Mahajan, C.M., Jagtap, P.P., Bendre, S.T.: J. Adv. Ceramics. 2(2), 135 (2013)
Karim, S., Reaney, I.M., Levin, I., Sterianous, I.: Appl. Phys. Lett. 94(112903), (2009)
Gautam, A., Singh, K., Sen, K., Kotnala, R.K., Singh, M.: J. Alloys Compd. 517, 87 (2012)
Catalan, G., Scott, J.F.: Adv. Mater. 21, 2463 (2009)
Acknowledgments
The authors would like to express their deep gratitude to King Faisal University, College of Science–Physics Department and the Deanship of Scientific Research for their kind supports. This study was supported by the Deanship of a Scientific Research (King Faisal University): proposed no. 186036.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Arafat, S.S., Ibrahim, S. & Al fannakh, H. Structural, optical and electrical properties of multiferroic BiFe1-xNixO3 ceramic. J Aust Ceram Soc 56, 867–872 (2020). https://doi.org/10.1007/s41779-019-00408-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-019-00408-5