Abstract
Endocrine pancreas regulates glucose homeostasis and prevents diabetes. Type-1 diabetes is characterized by destruction of the insulin secreting β-cells within the endocrine pancreatic islets, resulting in lower insulin release. People with type-1 diabetes can be transplanted with pancreatic islets obtained from deceased donors which restores the β-cell function. There are around 70 human islet isolation centers around the world which mostly collect endocrine pancreas from deceased donors. They assess the islet yield, functionality, viability, secretory capacity, and purity for transplantation and distribute this to donors. They also distribute a part of the pancreatic tissue for research, so that the cellular mechanisms in the human pancreatic tissue can be understood. This is crucial since human islet tissue has a unique cytoarchitecture compared to murine counterparts and therefore islet research with murine islets does not give complete picture of diabetes in humans. India is poised to take the mantle of the diabetes capital of the world in the near future. Despite this, there are no human islet isolation centers which can facilitate islet transplantation and diabetes research in India. This article highlights the glaring gap in the current infrastructure for diabetes care and provides critical insights into the role and potential of setting up islet tissue banks in the most populous country of the world.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The pancreas is a dual-function organ, accountable for exocrine as well as endocrine roles. The exocrine function includes producing pancreatic enzymes that include amylase, lipase, and protease which help in food digestion. The endocrine function of pancreas involves production of hormones, i.e., insulin, glucagon, and somatostatin, which primarily maintain blood glucose homeostasis.
Uncontrolled blood glucose levels in conjunction with various metabolic pathophysiologic pathways can cause diabetes which is responsible for considerable morbidity and mortality.8 Diabetes can be categorized into type-1 and type-2 diabetes (T1D, T2D) based on pathology, clinical manifestations, and prognosis. T1D (also called as insulin-dependent diabetes) is characterized by destruction of the insulin secreting β-cells due to endoplasmic reticulum stress within the pancreatic islets, leading to low insulin release.1 T2D, on the other hand, is mostly due to insulin resistance where blood glucose is not metabolized, which in turn has profound effects on human β-cell functioning within the pancreas.2,3 The end result, however, in both types of diabetes, is altered blood glucose levels in the fasting and fed states. Recent discoveries have shown modifications in the human pancreatic α-cells4,5 and δ-cells6 leading to loss of paracrine control within islets, thereby altering glucose homeostasis during fasting state too. Studies on human islets which have a very different cyto-architecture compared to the murine islets7 have led to better understanding of diabetes in humans. The organized arrangement of β-cells in the core and other cells at the periphery of murine islets is different from the human islets where these cells are interspersed (Fig. 1). This cyto-architectural difference necessitates human islet-focused research to understand the harsh effects of diabetes (both T1D and T2D) in humans.
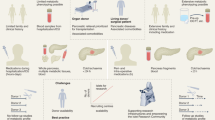
Pancreatic islet transplantation: a possibility - It is imminently conceivable that human islets are an indispensable resource for diabetes treatment and research. T1D population can be treated by transplantation of islets which restores the β-cell function.9 Transplantation is performed by isolation of pancreatic islets from the deceased donor, purified, to transplant by percutaneous catheterization of the portal vein of the T1D patient.10 This allows the transplanted β-cells within the islet to produce insulin which is then released into the bloodstream, thus restoring glucose homeostasis.11 This process is followed by insulin treatment until the patient becomes insulin independent. There are enough data to show successful treatment wherein patients no longer depend on exogenous insulin for up to 5 years after transplantation. In addition, improvement in metabolic control and significant decrease in diabetic complications have also been demonstrated in various studies.10,12,13
Distribution of transplantation facilities across the world - There are more than 70 human islet isolation centers around the world, apart from multiple small, coordinated distribution setups facilitating distribution of islets for transplantation and research. They are distributed mainly across North America and Europe. Such facilities also exist in Australia, Argentina, Brazil, China, Iran, Japan, Singapore, and South Korea.14 There is a need for more such centers to facilitate better research and treatment strategies for diabetes. India being the most populous country has a huge potential of developing such islet transplantation facilities. Such large facilities can provide islets of better yield, functionality, viability, and purity14 for transplantation in T1D patients and for diabetes research in India (Fig. 2).
However, setting up islet banks come with their own challenges. Apart from obvious ethical and logistical concerns, a centralized governance for such a facility is an absolute essential. A centralized facility may keep track of the following details:
-
(a)
Monitor quality by assessing islet structure, cell composition, cell arrangement, gene expression, islet secretory function, islet cell electrical activity, islet response to injury, stress, islet cell proliferation, identity, vasculature, and innervation.15
-
(b)
Maintain patient’s medical data (anonymized), isolation procedure, streamline protocols, maintain infrastructure for islet isolation, islet acquisition, develop islet patient derived cell lines, monitor islet distribution and shipment where there is requirement within the limits of globally accepted ethical standards.9
For a large country like India, a hub-and-spoke model can be adopted with the setting up of several sub-centers which can help with islet acquisition and protocol driven distribution across the country. Central monitoring of such sub-centers would mean a sustained workflow across the country, maintain funding and accomplishment of the objectives listed in (a) and (b). This will lead to better treatment strategies for diabetics and better opportunities for diabetes research. It is crucial to incorporate hospitals as partners and major sub-centers as they provide avenues for collection and preservation of pancreatic islets from deceased donors. This could be the starting point to overcome the limitations of availability of islets for both research and transplantation. For research purposes, initially, the tissues in the biobank can be paraffin embedded for longer storage and easy shipment. One of the more important functions of the tissue bank will be to integrate critical clinical data into the workflow for tissue acquisition and additionally, maintain secure databases which can inform clinical research and in turn health policy in the future.
Such islet biobanking facilities could be potential game-changers for diabetes management and research. The sheer scale of diabetics and pre-diabetics in a country like India provides for an ideal platform for tissue banks to create a palpable and meaningful impact on global health development goals and, thereby, pave the way for a healthier tomorrow.16
References
Makam AA, Biswas A, Kothegala L, Gandasi NR (2022) Setting the stage for insulin granule dysfunction during type-1-diabetes: is ER stress the culprit? Biomedicines 10:2695. https://doi.org/10.3390/biomedicines10112695
Gandasi NR, Yin P, Omar-Hmeadi M, Ottosson Laakso E, Vikman P, Barg S (2018) Glucose-dependent granule docking limits insulin secretion and is decreased in human type 2 diabetes. Cell Metab 27:470-478.e4. https://doi.org/10.1016/J.CMET.2017.12.017
Fu J, Githaka JM, Dai X, Plummer G, Suzuki K, Spigelman AF, Bautista A, Kim R, Greitzer-Antes D, Fox JEM et al (2019) A glucose-dependent spatial patterning of exocytosis in human β cells is disrupted in type 2 diabetes. JCI Insight. https://doi.org/10.1172/jci.insight.127896
Omar-Hmeadi M, Lund P-E, Gandasi NR, Tengholm A, Barg S (2020) Paracrine control of α-cell glucagon exocytosis is compromised in human type-2 diabetes. Nat Commun 11:1896. https://doi.org/10.1038/s41467-020-15717-8
Xin Y, Kim J, Okamoto H, Ni M, Wei Y, Adler C, Murphy AJ, Yancopoulos GD, Lin C, Gromada J (2016) RNA sequencing of single human islet cells reveals type 2 diabetes genes. Cell Metab 24:608–615. https://doi.org/10.1016/j.cmet.2016.08.018
Kothegala L, Miranda C, Singh M, Krieger J-P, Gandasi NR (2023) Somatostatin containing δ-cell number is reduced in type-2 diabetes. Int J Mol Sci 24:3449. https://doi.org/10.3390/ijms24043449
Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren P-O, Caicedo A (2006) The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA 103:2334–2339
Forouhi NG, Wareham NJ (2019) Epidemiology of diabetes. Medicine 47:22–27. https://doi.org/10.1016/j.mpmed.2018.10.004
Kaddis JS, Olack BJ, Sowinski J, Cravens J, Contreras JL, Niland JC (2009) Human pancreatic islets and diabetes research. JAMA 301:1580–1587. https://doi.org/10.1001/jama.2009.482
Shapiro AMJ, Pokrywczynska M, Ricordi C (2017) Clinical pancreatic islet transplantation. Nat Rev Endocrinol 13:268–277. https://doi.org/10.1038/nrendo.2016.178
Robertson RP (2004) Islet transplantation as a treatment for diabetes—a work in progress. N Engl J Med 350:694–705. https://doi.org/10.1056/NEJMra032425
Hering B, Parkey J, Kandaswamy R, Jevne R, Lervik B, Harmon J, Pakala P, Papas K, Sutherland D (2007) Long-term survival of human islet allografts in type 1 diabetes. Am J Transplant 7:224
Goto M, Johansson U, Eich TM, Lundgrem T, Engkvist M, Felldin M, Foss A, Kallen R, Salmela K, Tibell A et al (2005) Key factors for human islet isolation and clinical transplantation. Transplant Proc 37:1315–1316. https://doi.org/10.1016/j.transproceed.2004.11.042
Ng NHJ, Tan WX, Koh YX, Teo AKK (2019) Human islet isolation and distribution efforts for clinical and basic research. OBM Transplant 3:1–27. https://doi.org/10.21926/obm.transplant.1902068
Hart NJ, Powers AC (2019) Use of human islets to understand islet biology and diabetes: progress, challenges and suggestions. Diabetologia 62:212–222. https://doi.org/10.1007/s00125-018-4772-2
Pradeepa R, Mohan V (2017) Prevalence of type 2 diabetes and its complications in India and economic costs to the nation. Eur J Clin Nutr 71:816–824. https://doi.org/10.1038/ejcn.2017.40
Acknowledgements
We would like to thank Dr. Saptadipa Paul for reading the manuscript and giving comments. The work was supported by DBT Ramalingaswamy grant, ICMR-GIA scheme, IISc Startup grant, NovoNordisk Foundation grant awarded to NRG. DBT-IISc Partnership Program Phase-II is acknowledged for financial support to AR. MS is supported by Indian Institute of Science scholarships and LK is supported by University of Gothenburg.
Funding
Open access funding provided by Uppsala University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gandasi, N.R., Rangarajan, A., Rao, H. et al. Pancreatic Islet Biobanking Facilities in India: The Need of the Hour to Deal with Diabetes?. J Indian Inst Sci 103, 381–385 (2023). https://doi.org/10.1007/s41745-023-00366-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41745-023-00366-9