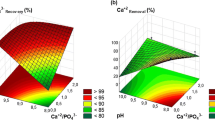
Abstract
Implementing nutrient recycling in wastewater treatment plants is essential for sustainable agriculture. In this study, we investigated a biphasic treatment system for anaerobic liquid digestate, which involved natural and K-enriched zeolite for NH4+ recovery (phase 1), followed by struvite crystallization under two conditions: NH4+ excess and Mg2+ excess (phase 2). The adsorption of NH4+ by natural zeolite enabled saving Mg and P reagents, used to achieve target Mg:NH4:PO4 ratios. The reagent use efficiency of struvite precipitation was highest with natural zeolite under NH4+ excess conditions (96%), whereas the other treatments exhibited lower yields. In this condition, the digestate enriched in Ca2+ released by zeolite; however, no P interferences occurred (Ca2+/Mg2+ < 0.5). Fractions of Ca2+ precipitated as CaCO3. Both the isomorphic NH4- and K-struvite occurred, distinguished by calibrating XRPD data (total struvite) with N contents (indicative of NH4+-struvite). The precipitates comprised NH4- and K-struvite at 60% and 30% (calcite at 9%) in the treatment that involved natural zeolite, 65% and 35% with the K-exchanged zeolite, due to higher presence of K+. Concerning the chemical evolution of the treated digestate, fewer alterations occurred for inorganic ions in the treatment that involved natural zeolite (phase 1) with NH4+ excess condition (phase 2), besides for unreacted SO42– derived from the Mg reagent. The recovered zeolite was enriched in N at 0.5%. Struvite precipitates met the EU regulations regarding permissible levels of organic C, P content, and heavy metal impurities, thereby potentially enabling its use as a fertilizer.
Highlights
-
Zeolites allowed less reagents for reaching optimum struvite precipitation.
-
N loads were reduced with zeolite–struvite treatment up to 86%.
-
Struvite from digestate is usable in agriculture and complied with EU regulations.
-
The precipitation of K-struvite limited the recovery of N from digestate.
-
To discern (K) and (NH4)-struvite, XRD must be combined with chemical information.




Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- NZT :
-
Natural chabazite zeolitic tuff
- KZT :
-
K-enriched chabazite zeolitic tuff
- NZT-S:
-
Treatment using NZT in combination with struvite
- KZT-S:
-
Treatment using KZT in combination with struvite
- CNTR:
-
Conventional struvite precipitation without using zeolitic tuff
- MR1:
-
Molar ratio 1 (Mg:NH4:PO4 = 1:1.5:1)
- MR2:
-
Molar ratio 2 (Mg:NH4:PO4 = 2:1:1)
- EA-IRMS:
-
Elemental analyzer coupled with an isotopic ratio mass spectrometer
- EC:
-
Electrical conductivity
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- SEM-EDS:
-
Scanning electron microscopy coupled with energy dispersive X-ray spectroscopy
- XRPD:
-
X-ray powder diffraction
- C e.tg :
-
NH4+-N equilibrium target concentration (target concentration for a single zeolitic tuff adsorption batch)
- T r :
-
Theoretical NH4+-N recovery
- A r :
-
Actual NH4+-N recovery
- R % :
-
NH4+-N recovery efficiency—parameter used to evaluate the efficiency of reagents use
References
Acelas NY, Flórez E, López D (2015) Phosphorus recovery through struvite precipitation from wastewater: effect of the competitive ions. Desalin Water Treat 54(9):2468–2479. https://doi.org/10.1080/19443994.2014.902337
Achilleos P, Roberts KR, Williams ID (2022) Struvite precipitation within wastewater treatment: a problem or a circular economy opportunity? Heliyon 8(7):e09862. https://doi.org/10.1016/j.heliyon.2022.e09862
Aguilar-Pozo VB, Chimenos JM, Elduayen-Echave B, Olaciregui-Arizmendi K, López A, Gómez J et al (2023) Struvite precipitation in wastewater treatment plants anaerobic digestion supernatants using a magnesium oxide by-product. Sci Total Environ 890:164084. https://doi.org/10.1016/j.scitotenv.2023.164084
Arcas-Pilz V, Rufí-Salís M, Parada F, Petit-Boix A, Gabarrell X, Villalba G (2021) Recovered phosphorus for a more resilient urban agriculture: assessment of the fertilizer potential of struvite in hydroponics. Sci Total Environ 799:149424. https://doi.org/10.1016/J.SCITOTENV.2021.149424
Casadellà A, Kuntke P, Schaetzle O, Loos K (2016) Clinoptilolite-based mixed matrix membranes for the selective recovery of potassium and ammonium. Water Res 90:62–70. https://doi.org/10.1016/J.WATRES.2015.12.017
Cheary RW, Coelho AA, Cline JP (2004) Fundamental parameters line profile fitting in laboratory diffractometers. J Res Nat Inst Stand Technol 109(1):1–25. https://doi.org/10.6028/JRES.109.002
Desmidt E, Ghyselbrecht K, Monballiu A, Rabaey K, Verstraete W, Meesschaert BD (2013) Factors influencing urease driven struvite precipitation. Sep Purif Technol 110:150–157. https://doi.org/10.1016/J.SEPPUR.2013.03.010
Doebelin N, Kleeberg R (2015) Profex: A graphical user interface for the Rietveld refinement program BGMN. J Appl Crystallogr 48:1573–1580. https://doi.org/10.1107/S1600576715014685
European Commission (2006) Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the protection of groundwater against pollution and deterioration
European Commission (2008) Regulation 1137/2008/EC of the European Parliament and of the Council of 22 October 2008 adapting a number of instruments subject to the procedure laid down in Article 251 of the Treaty to Council Decision 1999/468/EC, with regard to the regulatory procedure with scrutiny—adaptation to the regulatory procedure with scrutiny—part one. http://data.europa.eu/eli/dir/1991/676/2008-12-11
European Economic Community (1986) Council Directive 86/278/EEC of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture
European Union (2019) 2019/1009. http://data.europa.eu/eli/reg/2019/1009/oj. Accessed 9 Aug 2021
European Union (2021) 2021/2086. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32021R2086. Accessed 11 Sept 2023
Ferretti G, Galamini G, Deltedesco E, Gorfer M, Fritz J, Faccini B et al (2021) Gross ammonification and nitrification rates in soil amended with natural and NH4-enriched chabazite zeolite and nitrification inhibitor DMPP. Appl Sci 11(6):2605. https://doi.org/10.3390/app11062605
Ferretti G, Galamini G, Medoro V, Faccini B (2023) Amount and speciation of N leached from a sandy soil fertilized with urea, liquid digestate, struvite and NH4-enriched chabazite zeolite-tuff. Soil Use Manag 39(1):456–473. https://doi.org/10.1111/sum.12855
Ferretti G, Alberghini M, Galamini G, Medoro V, Faccini B, Balzan S, Coltorti M (2024) Exploring the combined effects of different nitrogen sources and chabazite zeolite-tuff on nitrogen dynamics in an acidic sandy-loam soil. Soil Systems 8(1):16. https://doi.org/10.3390/soilsystems8010016
Food and Agriculture Organization of the United Nations (2019) United Nations, World fertilizer trends and outlook to 2022. Rome. Accessed 27 Jan 2021
Galamini G, Ferretti G, Medoro V, Tescaro N, Faccini B, Coltorti M (2020) Isotherms, kinetics, and thermodynamics of NH4+ adsorption in raw liquid manure by using natural chabazite zeolite-rich tuff. Water 12(10):2944. https://doi.org/10.3390/w12102944
Galamini G, Ferretti G, Rosinger C, Huber S, Medoro V, Mentler A et al (2023) Recycling nitrogen from liquid digestate via novel reactive struvite and zeolite minerals to mitigate agricultural pollution. Chemosphere 317:137881. https://doi.org/10.1016/J.CHEMOSPHERE.2023.137881
Galli E, Passaglia E (2011) Natural zeolites in environmental engineering. In: Holzapfel H (ed) Zeolites in chemical engineering. Process Eng Engineering GmbH, Vienna, pp 392–416
Gunay A, Karadag D, Tosun I, Ozturk M (2008) Use of magnesit as a magnesium source for ammonium removal from leachate. J Hazard Mater 156(1–3):619–623. https://doi.org/10.1016/J.JHAZMAT.2007.12.067
Ha T-H, Mahasti NNN, Lu M-C, Huang Y-H (2023) Ammonium-nitrogen recovery as struvite from swine wastewater using various magnesium sources. Sep Purif Technol 308:122870. https://doi.org/10.1016/j.seppur.2022.122870
Hermassi M, Dosta J, Valderrama C, Licon E, Moreno N, Querol X et al (2018) Simultaneous ammonium and phosphate recovery and stabilization from urban sewage sludge anaerobic digestates using reactive sorbents. Sci Total Environ 630:781–789. https://doi.org/10.1016/J.SCITOTENV.2018.02.243
Hilt K, Harrison J, Bowers K, Stevens R, Bary A, Harrison K (2016) Agronomic response of crops fertilized with struvite derived from dairy manure. Water Air Soil Pollut 227(10):1–13. https://doi.org/10.1007/S11270-016-3093-7/TABLES/8
Huang J, Hartemink AE (2020) Soil and environmental issues in sandy soils. Earth Sci Rev 208:103295. https://doi.org/10.1016/j.earscirev.2020.103295
Huchzermeier MP, Tao W (2012) Overcoming challenges to struvite recovery from anaerobically digested dairy manure. Water Environ Res 84(1):34–41. https://doi.org/10.2175/106143011X13183708018887
Kataki S, West H, Clarke M, Baruah DC (2016) Phosphorus recovery as struvite: Recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Resour Conserv Recycl 107:142–156. https://doi.org/10.1016/J.RESCONREC.2015.12.009
Kim D, Min KJ, Lee K, Yu MS, Park KY (2017) Effects of pH, molar ratios and pre-treatment on phosphorus recovery through struvite crystallization from effluent of anaerobically digested swine wastewater. Environ Eng Res 22(1):12–18. https://doi.org/10.4491/EER.2016.037
Kozik A, Hutnik N, Piotrowski K, Mazienczuk A, Matynia A (2013) Precipitation and crystallization of struvite from synthetic wastewater under stoichiometric conditions. Adv Chem Eng Sci 03(04):20–26. https://doi.org/10.4236/ACES.2013.34B004
Leng Y, Soares A (2021) The mechanisms of struvite biomineralization in municipal wastewater. Sci Total Environ. https://doi.org/10.1016/J.SCITOTENV.2021.149261
Liu X, Wang J (2019) Impact of calcium on struvite crystallization in the wastewater and its competition with magnesium. Chem Eng J 378:122121. https://doi.org/10.1016/J.CEJ.2019.122121
Liu X, Hu Z, Zhu C, Wen G, Meng X, Lu J (2013) Influence of process parameters on phosphorus recovery by struvite formation from urine. Water Sci Technol 68(11):2434–2440. https://doi.org/10.2166/WST.2013.514
Lu Z, Zhang K, Liu F, Gao X, Zhai Z, Li J, Du L (2022) Simultaneous recovery of ammonium and phosphate from aqueous solutions using Mg/Fe modified NaY zeolite: integration between adsorption and struvite precipitation. Sep Purif Technol 299:121713. https://doi.org/10.1016/J.SEPPUR.2022.121713
Mangwandi C, JiangTao L, Albadarin AB, Allen SJ, Walker GM (2013) The variability in nutrient composition of Anaerobic Digestate granules produced from high shear granulation. Waste Manage 33(1):33–42. https://doi.org/10.1016/j.wasman.2012.09.005
Margeta K, Zabukovec N, Siljeg M, Farkas A (2013) Natural zeolites in water treatment—how effective is their use. Water Treat. https://doi.org/10.5772/50738
Martí N, Pastor L, Bouzas A, Ferrer J, Seco A (2010) Phosphorus recovery by struvite crystallization in WWTPs: Influence of the sludge treatment line operation. Water Res 44(7):2371–2379. https://doi.org/10.1016/J.WATRES.2009.12.043
Medoro V, Ferretti G, Galamini G, Rotondi A, Morrone L, Faccini B, Coltorti M (2022) Reducing nitrogen fertilization in olive growing by the use of natural chabazite-zeolitite as soil improver. Land 11(9):1471. https://doi.org/10.3390/LAND11091471
Meira RCdeS, da Paz SPA, Corrêa JAM (2020) XRD-Rietveld analysis as a tool for monitoring struvite analog precipitation from wastewater: P, Mg, N and K recovery for fertilizer production. J Mater Res Technol 9(6):15202–15213. https://doi.org/10.1016/J.JMRT.2020.10.082
Moshoeshoe M, Silas Nadiye-Tabbiruka M, Obuseng V (2017) A review of the chemistry, structure, properties and applications of zeolites. Am J Mater Sci 2017(5):196–221. https://doi.org/10.5923/j.materials.20170705.12
Mühling KH, Läuchli E (2001) Physiological traits of sodium toxicity and salt tolerance. Plant Nutr. https://doi.org/10.1007/0-306-47624-X_182
Muryanto S, Bayuseno AP (2014) Influence of Cu2+ and Zn2+ as additives on crystallization kinetics and morphology of struvite. Powder Technol 253:602–607. https://doi.org/10.1016/J.POWTEC.2013.12.027
Muster TH, Douglas GB, Sherman N, Seeber A, Wright N, Güzükara Y (2013) Towards effective phosphorus recycling from wastewater: quantity and quality. Chemosphere 91(5):676–684. https://doi.org/10.1016/J.CHEMOSPHERE.2013.01.057
Ohlinger KN, Young TM, Schroeder ED (2000) Postdigestion struvite precipitation using a fluidized bed reactor. J Environ Eng 126(4):361–368. https://doi.org/10.1061/(ASCE)0733-9372(2000)126:4(361)
Pepè Sciarria T, Vacca G, Tambone F, Trombino L, Adani F (2019) Nutrient recovery and energy production from digestate using microbial electrochemical technologies (METs). J Clean Prod 208:1022–1029. https://doi.org/10.1016/J.JCLEPRO.2018.10.152
Petrovič A, Simonič M, Čuček L (2021) Nutrient recovery from the digestate obtained by rumen fluid enhanced anaerobic co-digestion of sewage sludge and cattail: precipitation by MgCl2 and ion exchange using zeolite. J Environ Manag 290:112593. https://doi.org/10.1016/J.JENVMAN.2021.112593
Quintana M, Sánchez E, Colmenarejo MF, Barrera J, García G, Borja R (2005) Kinetics of phosphorus removal and struvite formation by the utilization of by-product of magnesium oxide production. Chem Eng J 111(1):45–52. https://doi.org/10.1016/J.CEJ.2005.05.005
R Core Team (2019) R Studio. R Foundation for Statistical Computing, Vienna
Rautaray D, Sinha K, Shankar SS, Adyanthaya SD, Sastry M (2004) Aqueous foams as templates for the synthesis of calcite crystal assemblies of spherical morphology. Chem Mater 16(7):1356–1361. https://doi.org/10.1021/CM035182L/SUPPL_FILE/CM035182LSI20040213_123429.PDF
Saroyda JRV, Cruz RYS, Antonio RJC, Flestado CLP, Magalong JRS, Zagala KZP et al (2001) PUPAIM. https://cran.r-project.org/package=PUPAIM
Siciliano A, Limonti C, Curcio GM, Molinari R (2020) Advances in struvite precipitation technologies for nutrients removal and recovery from aqueous waste and wastewater. Sustainability 12(18):7538. https://doi.org/10.3390/su12187538
Simhayov R, Ohana-Levi N, Shenker M, Netzer Y (2023) Effect of long-term treated wastewater irrigation on soil sodium levels and table grapevines’ health. Agric Water Manag 275:108002. https://doi.org/10.1016/j.agwat.2022.108002
Stratful I, Scrimshaw MD, Lester JN (2001) Conditions influencing the precipitation of magnesium ammonium phosphate. Water Res 35(17):4191–4199. https://doi.org/10.1016/S0043-1354(01)00143-9
Stratful I, Scrimshaw MD, Lester JN (2004) Removal of struvite to prevent problems associated with its accumulation in wastewater treatment works. Water Environ Res 76(5):437–443. https://doi.org/10.2175/106143004X151491
Talboys PJ, Heppell J, Roose T, Healey JR, Jones DL, Withers PJA (2016) Struvite: a slow-release fertiliser for sustainable phosphorus management? Plant Soil 401(1–2):109–123. https://doi.org/10.1007/S11104-015-2747-3
Tambone F, Orzi V, Zilio M, Adani F (2019) Measuring the organic amendment properties of the liquid fraction of digestate. Waste Manag 88:21–27. https://doi.org/10.1016/J.WASMAN.2019.03.024
Tracy SL, Williams DA, Jennings HM (1998) The growth of calcite spherulites from solution: II. Kinetics of formation. J Cryst Growth 193(3):382–388. https://doi.org/10.1016/S0022-0248(98)00521-1
Tuszynska A, Czerwionka K, Obarska-Pempkowiak H (2021) Phosphorus concentration and availability in raw organic waste and post fermentation products. J Environ Manag 278:111468. https://doi.org/10.1016/j.jenvman.2020.111468
Wan C, Ding S, Zhang C, Tan X, Zou W, Liu X, Yang X (2017) Simultaneous recovery of nitrogen and phosphorus from sludge fermentation liquid by zeolite adsorption: mechanism and application. Sep Purif Technol 180:1–12. https://doi.org/10.1016/j.seppur.2017.02.031
Wang J, Ye X, Zhang Z, Ye ZL, Chen S (2018) Selection of cost-effective magnesium sources for fluidized struvite crystallization. J Environ Sci 70:144–153. https://doi.org/10.1016/J.JES.2017.11.029
Wang Y, Wang X, Li J, Li Y, Xia S, Zhao J et al (2019) Coadsorption of tetracycline and copper(II) onto struvite loaded zeolite—an environmentally friendly product recovered from swine biogas slurry. Chem Eng J 371:366–377. https://doi.org/10.1016/J.CEJ.2019.04.058
Wang Z, Zhang Z, Zhang J, She L, Xiang P, Xia S (2023) Concurrent recovery of ammonia and phosphate by an electrochemical nutrients recovery system with authigenic acid and base. Chem Eng J 454:140169. https://doi.org/10.1016/j.cej.2022.140169
Wu H, Vaneeckhaute C (2022) Nutrient recovery from wastewater: a review on the integrated Physicochemical technologies of ammonia stripping, adsorption and struvite precipitation. Chem Eng J. https://doi.org/10.1016/J.CEJ.2021.133664
Wu J, Li Y, Xu B, Li M, Wang J, Shao Y et al (2022) Effects of physicochemical parameters on struvite crystallization based on kinetics. Int J Environ Res Public Health 19(12):7204. https://doi.org/10.3390/ijerph19127204
Yan H, Shih K (2016) Effects of calcium and ferric ions on struvite precipitation: a new assessment based on quantitative X-ray diffraction analysis. Water Res 95:310–318. https://doi.org/10.1016/J.WATRES.2016.03.032
Yu Z, She M, Zheng T, Diepeveen D, Islam S, Zhao Y et al (2021) Impact and mechanism of sulphur-deficiency on modern wheat farming nitrogen-related sustainability and gliadin content. Commun Biol 4(1):1–16. https://doi.org/10.1038/s42003-021-02458-7
Zhang D, Chen Y, Jilani G, Wu W, Liu W, Han Z (2012) Optimization of struvite crystallization protocol for pretreating the swine wastewater and its impact on subsequent anaerobic biodegradation of pollutants. Bioresour Technol 116:386–395. https://doi.org/10.1016/j.biortech.2012.03.107
Zilio M, Pigoli A, Rizzi B, Geromel G, Meers E, Schoumans O et al (2021) Measuring ammonia and odours emissions during full field digestate use in agriculture. Sci Total Environ 782:146882. https://doi.org/10.1016/j.scitotenv.2021.146882
Acknowledgements
The authors thank Azienda Agricola Minghini (San Biagio, Ferrara, Italy) for the furniture of liquid digestate. We also thank Dr. Renzo Tassinari for performing the ICP-MS analyses, Dr. Umberto Tessari for the particle size analyses, Dr. Nicola Tescaro for help during the preliminary studies, and Prof. Carmela Vaccaro for supporting the research.
Funding
Founded by PNRR–M4C2INV1.5, NextGenerationEU-Avviso 3277/2021 -ECS_00000033-ECOSISTER-spk1.
Author information
Authors and Affiliations
Contributions
Galamini G. and Ferretti G. contributed to conceptualization and methodology; Galamini G., Ferretti G., Medoro V., Eftekhari N., and Favero M. did formal analysis; Galamini G. was involved in investigation, data curation, and writing––original draft; Ferretti G., Faccini B., and Coltorti M. were involved in writing––review; Coltorti M. performed supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests with this work on behalf of all coauthors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Galamini, G., Ferretti, G., Medoro, V. et al. Applying Natural and K-Enriched Zeolite Before Struvite Precipitation Improved the Recovery of NH4+ from Liquid Digestate and the Reagent Use Efficiency. Int J Environ Res 18, 44 (2024). https://doi.org/10.1007/s41742-024-00595-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41742-024-00595-5