Abstract
Nowadays, many sites are considered waste, due to high potentially toxic metal (PTM) concentration. Recycling of globally critical metals requires development of environmentally friendly processes for metal recovery. To study plants response to elevated Zn and Cu concentration in soil, a greenhouse experiment was designed using hyperaccumulator Brassica juncea. Plants were irrigated daily with PTM solutions, with final mass of both Zn and Cu added to the soil reaching 104.5, 209, 313.5, and 330 mg. After 8 weeks, samples were harvested, dried, weighed, and elemental analysis was conducted using atomic emission spectrometry (Agilent Technologies 4210 MP-AES). Phytotoxicity was determined based on visual observation, biomass, and chlorophyll measurements. The highest accumulation of Zn and Cu was found in the stem and leaf material, with observed concentrations of Zn in the leaf being 16.750 mg kg−1 and 7.170 mg kg−1 of Cu in the stem. The highest allocated in the biomass mass of Zn and Cu was in T4 treatment reaching 25.8 mg and 9.5 mg, respectively. Treatment with 330 mg Zn and Cu application displayed a 62.3% decrease in stem mass, a 25% decrease in average root mass (LD30 reached), and a 59% decrease in leaf mass when compared with the control. With increasing PTM concentration, root, biomass (from about 0.4 to 0.1 g; from about 3.8 to 2.0 g, respectively) and chlorophyll “a” (from about 24 to 19 μg/cm2) decline was observed, which correlates with observed chlorosis. This study reaffirmed the capabilities of B. juncea to bioaccumulate Zn and Cu from an enriched soil and provided further understanding as to how Zn and Cu translocate within plant tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil potentially toxic metal (PTM) pollution can result from a number of anthropogenic activities such as industry, mining, and agricultural practices including land application of contaminated sewage sludges. Therefore, due to their non-biodegradable character, PTM concentration in soil progressively increases causing irreversible damages to ecosystem (Amen et al. 2020). Copper and zinc are two of the PTMs, but they are, at the same time, essential nutrient elements, so that for the assessment of possible environmental impacts, their contents and mobility in the soil are of particular interest and must be monitored as thoroughly as possible.
Cu is needed for plant growth in limited quantities and plays a key role in plant biochemical reactions and physiological development (Lepp 1981; Shabbir et al. 2020). Background soil levels of Cu in nature can generally range from 14 to 109 mg kg−1 and the mean content, according to Kabata-Pendias (2011), is 38.9 mg kg−1 globally. When in excess within plant tissues, Cu can lead to an overproduction of reactive oxygen species (ROS) and lipid hydroperoxide (LPO). Cu toxicity in plants reduces root length and mass, causes chlorotic symptoms of the leaf tissues, signs of necrosis, inability to uptake water from the soil matrix, P and N deficiency, and ultimately decreasing the plants’ ability to photosynthesize (Lepp 1981; Shabbir et al. 2020).
Zn is an essential nutrient for thousands of proteins in plants, ensuring healthy development and biotic mechanisms (Balafrej et al. 2020). The common geochemical occurrence of Zn is estimated in the range of 10–1000 mg kg−1 (Alloway 2013). In reference to Zn biochemical properties, it is the only metal represented in all six enzyme classes (oxidoreductases, transferases, hydrolases, lyases, isomerases, ligases) (Broadley et al. 2007).
According to Englbrecht et al. (2004), the largest class of Zn-binding proteins can regulate transcription directly through effects on DNA/RNA binding, and through site-specific modifications, regulation of chromatin structure, RNA metabolism and protein–protein interactions; chlorophyll formation and conversion of starches to sugars. However, at elevated levels, Zn can become toxic and lead to chlorosis, necrosis, and eventual death of the plant (Balafrej et al. 2020). Elevated levels of Cu in Zn in soil are an environmental hazard and can result in a reduction in soil microbial activity, yield loss, as well as direct and acute toxic effects to other living organisms (Purakayastha et al. 2008). The cleanup of sites contaminated with excess PTMs is necessary for both economic development and the health and safety of the environment.
An environmentally friendly and relatively inexpensive technique to remove PTM from the soil is phytoremediation through the use of hyperaccumulator plant species. This technique utilizes the necessity of plants to absorb water, soluble mineral nutrients, and contaminants through the root structure and bioaccumulate into both belowground and aboveground tissues in the plant. Usually, when plants are exposed to high levels of PTMs, a physiological response can be observed in the aboveground constituents, including chlorosis, growth inhibition, discoloration of the roots, and eventual death of the host plant, caused by reduction in photosynthesis, water, and nutrient uptake (Kutrowska et al. 2017). According to Cui et al. (2023), hyperaccumulators are plants that can accumulate trace elements of a magnitude 2–3 times higher than normal. Further studies and application of such plant species contribute to the recycling of economically critical heavy metals and support the development of environmentally friendly processes for metal recovery, including phytomining.
Brassica juncea has appeared in numerous scientific studies involving the extraction and bioaccumulation of PTMs from the soil matrix (Ebbs and Kochian (1997); Mendoza-Hernández et al. 2019; Purakayastha et al. 2008; Rathore et al. 2019; Rosenkranz et al. 2016; Wang et al. 2008; Singh et al. 2022). According to a study conducted by Ebbs and Kochian (1997), B. juncea proved to be the most effective in removing Zn from the contaminated soil and proved superior to other plants undergoing the same experiment due to its high biomass and resistance to negative effects brought upon by the excess PTMs. On the contrary, in a study performed by Wang et al. (2008), B. juncea was the lowest accumulator of Zn, but the highest for Cu. Of the five hyperaccumulator species grown on soil containing excess PTMs, Rosenkranz et al. 2016 observed that B. juncea showed the highest resistance to negative effects of excess heavy metals (HMs) among all treatments and remained healthy until the end of the experiment. Brassica juncea has proven to have great potential as hyperaccumulating plant, due to natural resistance to PTMs toxicity, affinity for HM accumulation, rapid growth and high biomass production. However, with ongoing and constant release of PTMs into environment, growing interest of the application of remote sensing to study HM pollution based on data extracted from aerial photos, results is a growing need for further studies, involving hyperaccumulating species, that combine plant phytotoxicity responses related to fluorescence. To our knowledge, no study has been published relating the performance of B. juncea photosynthetic apparatus to increasing Zn and Cu concentrations.
Thus, we propose a novel study where the feasibility of B. juncea for Zn and Cu accumulation in a greenhouse experimental setup under comparatively high temperatures, sandy substrate, and relatively high treatment levels has not been extensively studied before. Further goals include determining the phytotoxicity based on biomass, visual observations, and chlorophyll “a” analysis; defining the differences in accumulation and translocation of studied PTMs within the plant tissue; evaluating the bioconcentration and translocation factors.
Materials and Methods
Greenhouse Experiment
For each pot (45 in total), 4.5 kg of sandy soil substrate (2.6% of hygroscopic moisture) was used and saturated with water (about 500 ml). Twenty seeds from an U.S. Food and Drug Administration (FDA) approved source (Rareseeds) were initially sown in and after 2 weeks of initial growth were thinned to ten plants per pot. The light and temperature conditions were controlled at a 12–12 day to night cycle, and a temperature of 24 °C on average for the first 4 weeks of growth. Solaris 100 (watt) grow lights with a beam angle of 120° were suspended at 93 cm above soil level. Pot rotations were performed weekly to provide asymmetric conditions among the treatments. An all-purpose fertilizer (b1 Universal-Flüssigdünger) was used at 3 weeks of growth after the secondary leaves were established among all treatments. In total, 45 pots were established to apply 4 various Zn and Cu concentrations (9 replications per treatment) and control plants (9 replications). After 6 weeks of plant development, the Zn (ZnSO4*7H2O; Roth) and Cu (CuSO4*5H2O; Roth) application (100 ml per day) began (Table 1). Due to poor health conditions, the application of Zn and Cu for treatment 4 were halted after 15 days. The final concentrations and application rates can be seen in Table 1.
At the end of 8 weeks, composite sampling was performed. The leaf, stem, and root tissues were collected, dried (40 °C; 24 h), and weighed. The soil was homogenized by hand and oven dried (40 °C; 24 h).
Sample Analysis
10 g of dried and sieved (< 2 mm) soil were used for pH analysis (0.01 M CaCl2, 1:2.5 ratio). Plant and soil material was ground using a Retsch RM 200 grinding machine for 20 min and then sieved using a 0.25 mm screen. Subsequently, about 0.150 g of soil and plant material were digested using 8 ml of 65% HNO3 via Mars G microwave system (20 min at 180 °C). Cu and Zn analysis was performed by microwave plasma atomic emission spectrometry (Agilent 4100 MP-AES) (Sut-Lohmann et al. 2021). All measurements were conducted using two replicates and certified reference materials Merck Certipur™ were used to control soil analysis quality and instruments calibration. Following detection limits for the analyzed elements (ppm) using MP-AES were obtained: Zn (0.7); Cu (0.1).
To determine the chlorophyll “a” concentration in the leaves, three samples per treatment were collected. Leaf samples were cut using a ring punch with an area of 6.83 cm2 and ground with 80% acetone and the aid of quartz sand. Subsequently, samples were heated and filtered (150 mm Sartorious filter paper) and analyzed using Perkin Elmer Lambda 2S UV/VIS Spectrometer (400–800 nm). Chlorophyll concentrations were calculated according to Fischer et al. (2010).
Data Analysis
Zn and Cu uptake were compared using Tukey test. Critical values of the studentized range distribution, as well as p values corresponding to an observed value were calculated using the Gleason (1999) algorithm. Further tandem analysis of subsets within these groups was performed using ANOVA at significance levels of p ≤ 0.05 and p ≤ 0.001. Data were processed and assembled using R software.
To evaluate the plant ability to accumulate and translocate the heavy metals, bioaccumulation (BCF) and translocation factors (TF) were calculated (mg kg−1). According to Tripti (2018), following equations were applied:
BCF = metal (plant part)/metal (substrate)
TF = metal (plant shoot/leaf)/ metal (plant root)
Results and Discussion
Phytotoxicity
Visual observation of the harvested root material revealed a stunting of lateral root development among T3 and T4 when compared with control. T1 and T2 showed an increase in lateral root development, but the mass showed no significant difference (Fig. 1). In Fig. 1, a general tendency can be observed, where root mass decreases with the increasing Zn and Cu concentration. An observed increase in the mean root mass between the control and T1 can be attributed to the fact that at lower concentrations, Zn and Cu can serve as fertilizer as they are both essential for healthy development of plant tissues (Feigl et al. 2014). Previous studies revealed that Cu has a greater impact on lateral root growth than Zn when compared in isolated experiments (Yang et al. 2016; Ebbs and Kochian (1997); ). An increase in an average root mass at lower levels of Zn addition is also observed by Li et al. (2009) where hyperaccumulator Sedum alfredii was cultivated in a hydroponic morphological experiment in elevated levels of Zn and Cd. For all samples that experienced biomass loss of the root systems, an increase in HM concentration in stem and leaf tissues was observed, when compared to samples within the same treatment that had their root structures intact. This displays B. juncea’s ability to adapt to morphological stress and shift concentrations of HM within the host plant itself. Yang et al. (2016) found that in an experiment involving the addition of Zn and Cu to the growing medium of wheat, the production of ROS was increased. This correlates to the reduction in root mass observed in this experiment, as it is common with plants undergoing oxidative stress. According to Balafrej et al. (2020) and Chaudhry et al. (2020), cellular and subcellular distribution of HMs in B. juncea mainly accumulate in the xylem, which is the main route for metals translocation from roots to stems and to leaves. This distribution mechanism is believed to be linked with ability to tolerate high metals concentrations (Chaudhry et al. 2020).
An elongation of stem material occurred across all treatments and can be attributed to the warm weather conditions. Among T3 and T4, however, there was an observed thinning of the stem and spiraling of plant tissues closer to the soil surface. Stem mass showed no significant difference among control, T1, and T2; however, the observed average mass across the three treatments shows a pattern of decreasing median stem mass with increasing HM concentration (Table 2). Reduction of biomass and growth inhibition belongs to main plants responses to HM toxicity, since it impairs cell elongation and division. Higher energy demand due to stress coping mechanism caused by increased metal accumulation can also result in biomass decrease.
Visual observation revealed a decrease in leaf area and signs of chlorosis in T3 and T4. Beginning with T2, a yellowing of the leaves, a symptom of chlorosis, began to appear as the addition of HMs continued. Through visual interpretations, a correlation between the increasing yellowing of the leaves, reduction in the leaf mass, and an increase in the total Zn and Cu added to the soil solution can be observed (Table 2).
The changes in chlorophyll “a” content observed in this study can be interpreted as a direct physiological response and phytotoxic stress of the plants to the PTMs addition. Chlorophyll “a” captures and utilizes light energy, and its production requires 15 key enzymes composed of specific macronutrients and micronutrients (Zhang et al. 2011). However, the excess of HM ions, including Cu and Zn, inhibit the absorption of Mg2+, a key ion in the synthesis of chlorophyll (Beale 2005).
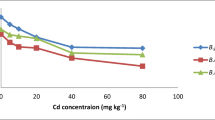
In Fig. 2, a decrease in chlorophyll “a” concentration with the increasing Cu and Zn concentration is observed, that correlates with the visually observed leaf chlorosis. Sun-induced fluorescence from chlorophyll “a” and “b” pigments provide valuable information about plant physiology and can be retrieved from remote sensing data. A pre-analysis showed a change in the reflectance spectrum within the PTM concentration in the green peaks. Those green peaks were caused by strong absorptions of chlorophyll a and b around 420, 490, and 660 nm (Zhang 2011). Cu due to redox activity and Zn due to Lewis acid strength are essential nutrients to plants (Kutrowska et al. 2017). However, as transition metals, both elements can become a potential pro-oxidants. In the presence of Fe, they participate in the Haber–Weiss and Fenton reactions, resulting in the hydroxyl radical production (Kutrowska et al. 2017). In excess of Zn and Cu, coping stress mechanism may impair (results showed in chapter 3.2) accumulation and translocation of HM to the shoots and leaves, lead to potential micronutrient deficiency for the photosynthetically active tissues.
Establishing more information about HM-induced plant phytotoxicity and its relation to chlorophyll content can profoundly minimize the cost of data collection in situ. To our knowledge, no study using B. juncea has been performed to relate the chlorophyll “a” content to Zn or Cu phytotoxicity. However, our results correlate with study conducted by Szopiński et al. (2019), where Arabidopsis arenosa and Arabidopsis halleri, in a hydroponic experiment, were exposed to elevated Zn concentrations. Analogous to our study, a decrease in chlorophyll “a” concentration with increasing PTMs amounts was observed.
PTM Uptake
The applications of HMs across the treatments was negatively correlated to soil pH, resulting in soil pH decrease from around 8.0 for control to 6.4 for T4 (Fig. 3). This can be attributed to root uptake of the cationic HMs and restoring the cation–anion balance by releasing H+ ions within the rhizosphere, thus acidifying the soil. Plant roots and associated microbiome can support metal bioavailability by rhizosphere acidification due to secretion of proton, organic acids, phytochelatins (PCs), amino acids, and enzymes. According to Kim et al. (2010), lowering of soil pH can be a result of chelating compounds released by B. juncea as an PTMs absorption mechanism, like release of histidine reported for its bonding mechanisms regarding various PTMs. B. juncea has been recorded to tolerate a range of pH values from 4.3 to 8.3, with a preference range of 5.5–8.0 (Javid et al. 2012); however, the decrease observed in Fig. 3 has an impact on the bioavailability of HM in soil. With the increasing soil acidity, mobility of Cu and Zn increases, which can result in stronger HM accumulation leading to mass reduction and chlorosis. In the system, where multiple HMs interact with the plant root zone, it is important to understand the rate at which Cu and Zn are available. Kim et al. (2010) defined the affinity for metals in the soil as Cu2+ > Zn2+, meaning Zn prior uptake by plants absorption over Cu.
In the greenhouse experiment, HMs concentrations in roots, stems, and leaves increased with the increasing HM concentration in treatments and in soil (Table 2). The highest accumulation of Zn and Cu was found in the stem and leaf material (Table 2), with highest observed concentrations of Zn in the leaf reaching T3 16.750 mg kg−1 of Zn and 7.170 mg kg−1 of Cu in the stem material. Table 3 compares the findings of the current study against previous publications involving hyperaccumulation in various plant species. To determine the effects of endophytic bacteria and their potential in promoting the growth of copper accumulation of B. juncea, Zhang et al. (2011) grew various plants on Cu-enriched quartz sand. Though an overall decrease was observed in the health of plants grown on Cu-enriched soil, there was no mention of critical limits and observed plant death within this experiment. Feigl et al. (2014), Tang et al. (2009), and Cui et al. (2023) demonstrated the absorption capabilities of hyperaccumulators grown in excess HMs. In all three experiments, while a reduction of overall root mass was observed at higher concentrations, most HMs were found to be stored in the root structure.
Table 4 displays calculated bioaccumulation (BCF) and translocation (TF) factor for both metals. BFC values greater than one demonstrate a plants species positive potential for phytoremediation (Mishra and Pandey 2019). A TF > 1 reveals tendency of accumulation in aboveground biomass (Chaudhry et al. 2020).
For both elements, a similar tendency is observed, where the BCF increases with the increasing concentration. Additionally, for both metals, the accumulation follows the pattern of roots > stem > leaves. For Zn, the BCF steadily increased with the increasing concentrations within the root, shoots, and leaves tissue. For the treatments C, T1, and T2, the BCF of the roots was higher than that of both the shoots and leaves. However, with T3 and T4, the BCF of the shoots exceeded that of the roots (Table 4). The leaves had the lowest BCF among all treatments when compared to the roots and shoots. For Cu, the BCF root pattern was not as clear as that of the Zn. The results were uneven among the treatments and need further analysis. BCF of the shots and leaves showed a general increase across the treatments with increasing HM concentrations (Table 4).
The Zn TF from roots to shoots steadily increased across the treatments with the exception of T2 which showed a decrease in concentration even when compared to control (Table 4). This can be attributed to an exceptionally high concentration of the metal in the root structures of the host plant (Table 2). The TF from roots to leaves bore similar results with T2 being lower than all other treatments including the control (Table 4). For Zn, the tendency can be observed, that TF is higher in shoots than in leaves.
For Cu, analogous trend is observed, where the TF from roots to shoots and the TF from roots to leaves steadily increases across all treatments except T2. However, contradictory to Zn, the TF values from roots to leaves are equal of higher than TF from roots to shoots values, revealing various translocation pattern for both elements.
For both HMs, in treatments T3 and T4, TF values > 1 were calculated, which demonstrates the potential ability of a given species for phytoremediation (Tripti 2018). Given the nature of plants and that this is a biotic process, there is a divergence of observed TF and BCF across the treatments. These observed results can be compared with the findings of Chaudhry et al. (2020) in which the TF and BCF in treatments varied greatly due to the performance of the individual specimen. Those observations showed that B. juncea can demonstrate great potential for translocation of HMs from the root structure to the aerial stem tissues under specific conditions, implying that this efficiency increases with the increasing PTM concentration in soil.
In a study measuring the effects of binary metal concentrations and their effect on B. juncea, it was observed that TF was decreased when Cu and Zn were elevated in the same treatment (Kutrowska et al. 2017). These findings led Kutrowska et al. (2017) to the conclusion that an antagonism exists between Cu and Zn uptake that do not take place at the soil–root interface, but within plant tissue during xylem loading/unloading. When comparing in Table 4 the BCF values for both metals, it can be noticed that for the roots, slightly higher bioaccumulation is observed only for Zn T4 treatment, meaning that both PTMs are comparably up taken by the roots. However, for the shoots and leaves, BFC values, especially for the T3 and T4 treatments,the superior bioaccumulation of Zn can be noticed when moving from root tissues into the xylem and further uptake within the leave structure, which correlate to Kutrowska’s et al. (2017) findings.
Hyperaccumulation and transfer of PTMs is complex in plants cells and compartments. It generally involves: (a) transport of metals across the plasma membrane of root cells; (b) xylem loading and translocation; and (c) detoxification and sequestration of metals at the whole plant and cellular levels (Lombi et al. 2002). Our results indicated ability of Brassica juncea to accumulate and translocate Zn and Cu from spiked soil. For both studied PTMs, the accumulation was greater in the underground parts (roots) than in aboveground (stems and leaves). Similar distribution pattern was observed for Hg (Cui et al. 2023) and Pb (Meyers et al. 2008) accumulation. Pb mostly accumulated in the root tip, which lacks the membrane proteins responsible for Pb influx, resulting in pre-dominantly extracellular deposition (Meyers et al. 2008). Hg was mainly distributed in the epidermis and pericycle of the root, and a large amount was also found in the central cylinder of the shoot (Cui et al. 2023). For the PTMs, it can be challenging to reach root xylem vessels, that are transporting water and nutrients to the steam and leaves. For the Zn and Cu being micronutrients, have to cross the endodermis and the suberized Casparian strips, which can strongly reduce plant’s ability to hyperaccumulate metal cations (Feng et al. 2021). Our study revealed that under applied experimental conditions, most of the accumulated Zn and Cu remained in the roots, capturing most of the bioavailable PTMs. It can be a consequence of the direct exposure of the roots to the spiked soil and avoidance mechanisms to prevent phytotoxicity in aboveground parts. Analogously to C. violifolia characterization presented by Cui et al. (2023), we can hypothesize that B. juncea root cells are more tolerant to strongly increased Cu and Zn concentrations than aerial parts.
Conclusion
In this study, feasibility of B. juncea as a bioaccumulator could be proven in extreme and unusual conditions, like sandy substrates, under comparatively high temperatures, with increasing treatment contents and decreasing vitality. B. juncea demonstrated the ability to adapt to morphological stress and shift concentrations of HM within the host plant itself. Plants that experienced biomass loss of the root systems, had an observed increase in HM concentration in stem and leaf tissues. BCF and TF analysis revealed superior translocation of the Zn from root tissues into the xylem and leave structure. However, both elements, especially for the lower concentration treatments, were remaining in the root systems. With increasing Zn and Cu concentration, both metals followed the same accumulation pattern of roots > stem > leaves, but various translocation order. For Zn, the TF was higher in shoots than leaves, whereas for Cu, higher TF was observed for shoots.
In this study, an increase of Zn and Cu concentrations within root, stem, and leaf’s tissues with the increasing treatments concentrations has been observed. Consequently, increasing soil acidity, enhanced the mobility and accumulation of Cu and Zn, leading to mass reduction, thinning of the stem, spiraling of plant tissues, yellowing of the leaves, and chlorosis.
Visual signs of B. juncea phytotoxicity, especially noticeable in the highest treatments, correlated with the decreasing chlorophyll “a” concentration. Further studies are in preparation to directly relate chlorophyll fluorescence to examine HM accumulation in vegetation using remote sensing data.
Data availability
The data used to support the findings of this study are included within the article.
References
Alloway BJ (2013) Introduction. [Environmental pollution] Heavy metals in soils, vol 22. Springer
Amen R, Bashir H, Bibi I, Shaheen SM, Niazi NK, Shahid M, Hussain MM, Antoniadis V, Shakoor MB, Al-Solaimani SG, Wang H, Bundschuh J, Rinklebe J (2020) A critical review on arsenic removal from water using biochar-based sorbents: the significance of modification and redox reactions. Chem Eng J 396:125195. https://doi.org/10.1016/j.cej.2020.125195
Bani A, Ehevarria G (2010) Nickel hyperaccumulation by the species of Alyssum and Thlaspi (Brassicaceae) from the ultramafic soils of the Balkans, Botanica Serbica 34(3)
Balafrej H, Bogusz D, Triqui ZA, Guedira A, Bendaou N, Smouni A, Fahr M (2020) Zinc hyperaccumulation in plants: a review. Plants 9(5):562. https://doi.org/10.3390/plants9050562
Beale SI (2005) Green genes gleaned. Trends Plant Sci 10(7):309–312. https://doi.org/10.1016/j.tplants.2005.05.005
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173:677–702. https://doi.org/10.1111/j.1469-8137.2007.01996.x
Chaudhry H, Nisar N, Mehmood S, Iqbal M, Nazir A, Yasir M (2020) Indian mustard Brassica juncea efficiency for the accumulation, tolerance, and translocation of zinc from metal contaminated soil. Biocatal Agric Biotechnol 23:101489. https://doi.org/10.1016/j.bcab.2019.101489
Cui L, Tian X, Xie H, Cong X, Cui L, Wu H, Wang J, Li B, Zhao J, Cui Y, Feng X, Li YF (2023) Cardamine violifolia as a potential Hg hyperaccumulator and the cellular responses. Sci Total Environ 863:160940. https://doi.org/10.1016/j.scitotenv.2022.160940
Ebbs S, Kochian L (1997) Toxicity of zinc and copper to Brassica species: implications for phytoremediation. J Environ Qual. https://doi.org/10.2134/jeq1997.00472425002600030026x
Englbrecht CC, Schoof H, Böhm S (2004) Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genom 5:39. https://doi.org/10.1186/1471-2164-5-39
Feigl G, Lehotai N, Molnár Á, Ördög A, Rodríguez-Ruiz M, Palma JM, Corpas FJ, Erdei L, Kolbert Z (2014) Zinc induces distinct changes in the metabolism of reactive oxygen and nitrogen species (ROS and RNS) in the roots of two Brassica species with different sensitivity to zinc stress. Ann Bot 116(4):613–625. https://doi.org/10.1093/aob/mcu246
Feng R, Wang L, Yang J, Zhao P, Zhu Y, Yu Y, Liu H (2021) Underlying mechanisms responsible for restriction of uptake and translocation of heavy metals (metalloids) by selenium via root application in plants. J Hazard Mater 402:123570. https://doi.org/10.1016/j.jhazmat.2020.123570
Fischer T, Veste M, Wieche W, Lange P (2010) Water repellency and pore clogging at early successional stages of microbiotic crusts on inland dunes, Brandenburg NE Germany. CATENA 80(1):47–52. https://doi.org/10.1016/j.catena.2009.08.009
Ge J, Wang H, Lin J, Tian S, Zhao J, Lin X, Lu L (2020) Nickel tolerance, translocation and accumulation in a Cd/Zn co-hyperaccumulator plant Sedum alfredii. J Hazard Mater 398:123074. https://doi.org/10.1016/j.jhazmat.2020.123074
Gleason JR (1999) An accurate, non-iterative approximation for studentized range quantiles. Comput Stat Data Anal 31(2):147–158. https://doi.org/10.1016/s0167-9473(99)00002-x
Javid M, Ford R, Nicolas ME (2012) Tolerance responses of Brassica juncea to salinity, alkalinity and alkaline salinity. Funct Plant Biol 39(8):699. https://doi.org/10.1071/fp12109
Kabata-Pendias A (2011) Trace elements in soils and plants, 3rd edn. CRC Press, Boca Raton
Kim K, Owens G, Kwon S (2010) Influence of Indian mustard (Brassica juncea) on rhizosphere soil solution chemistry in long-term contaminated soils: a rhizobox study. J Environ Sci 22(1):98–105. https://doi.org/10.1016/s1001-0742(09)60080-2
Ksenija J, Bani A, Pavlova D, Konstantinou M (2020) Hyperaccumulator plant discoveries in the Balkans: Accumulation, distribution, and practical applications, Botanica Serbica 46(2). https://doi.org/10.2298/BOTSERB2202161J
Kutrowska A, Małecka A, Piechalak A, Masiakowski W, Hanć A, Barałkiewicz D, Andrzejewska B, Zbierska J, Tomaszewska B (2017) Effects of binary metal combinations on zinc, copper, cadmium and lead uptake and distribution in Brassica juncea. J Trace Elem Med Biol 44:32–39. https://doi.org/10.1016/j.jtemb.2017.05.007
Lepp NW (1981) Effect of Heavy Metal Pollution on Plants, Vol 1. Applied Science Publishers, London and New Jersey, Wiley
Li T, Yang X, Lu L, Islam E, He Z (2009) Effects of zinc and cadmium interactions on root morphology and metal translocation in a hyperaccumulating species under hydroponic conditions. J Hazard Mater 169(1–3):734–741. https://doi.org/10.1016/j.jhazmat.2009.04.004
Lombi E, Tearall KL, Howarth JR, Zhao FJ, Hawkesford MJ, McGrath SP (2002) Influence of iron status on calcium and zinc uptake by different ecotypes of the hyperaccumulator Thlaspi caerulescens. Plant Physiol 128:1359–1367. https://doi.org/10.1104/pp.010731
Mendoza-Hernández JC, Vázquez-Delgado OR, Castillo-Morales M, Varela-Caselis JL, Santamaría-Juárez JD, Olivares-Xometl O, Arriola Morales J, Pérez-Osorio G (2019) Phytoremediation of mine tailings by Brassica juncea inoculated with plant growth-promoting bacteria. Microbiol Res. https://doi.org/10.1016/j.micres.2019.126308
Meyers D, Auchterlonie GJ, Webb RI, Wood B (2008) Uptake and localisation of lead in the root system of Brassica juncea. Environ Pollut 153:323–332. https://doi.org/10.1016/j.envpol.2007.08.029
Mishra T, Pandey VC (2019) Phytoremediation of red mud deposits through natural succession. Phytomanagement Pollut Sites. https://doi.org/10.1016/b978-0-12-813912-7.00016-8
Purakayastha TJ, Viswanath T, Bhadraray S, Chhonkar PK, Adhikari PP, Suribabu K (2008) Phytoextraction of zinc, copper, nickel and lead from a contaminated soil by different species of Brassica. Int J Phytorem 10(1):61–72. https://doi.org/10.1080/15226510701827077
Rathore SS, Shekhawat K, Dass A et al (2019) Phytoremediation mechanism in Indian mustard (Brassica juncea) and Its enhancement through agronomic interventions. Proc Natl Acad Sci, India Sect B 89:419–427. https://doi.org/10.1007/s40011-017-0885-5
Rosenkranz T, Kisser J, Wenzel WW, Puschenreiter M (2016) Waste or substrate for metal hyperaccumulating plants —the potential of phytomining on waste incineration bottom ash. Sci Total Environ 575:910–918. https://doi.org/10.1016/j.scitotenv.2016.09.144
Seregin IV, Ivanova TV, Voronkov AS, Kozhevnikova AD, Schat H (2023) Zinc- and nickel-induced changes in fatty acid profiles in the zinc hyperaccumulator Arabidopsis halleri and non-accumulator Arabidopsis lyrata. Plant Physiol Biochem. https://doi.org/10.1016/j.plaphy.2023.107640
Shabbir Z, Sardar A, Shabbir A, Abbas G, Shamshad S, Khalid S, Natasha N, Murtaza G, Dumat C, Shahid M (2020) Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 259:127436. https://doi.org/10.1016/j.chemosphere.2020.127436
Singh S, Kumar V, Gupta P, Singh A (2022) Conjoint application of novel bacterial isolates on dynamic changes in oxidative stress responses of axenic Brassica juncea L. in Hg-stress soils. J Hazard Mater 434:128854. https://doi.org/10.1016/j.jhazmat.2022.128854
Sut-Lohmann M, Ramezany S, Kästner F, Raab T (2021) Feasibility of pXRF to evaluate chosen heavy metals in soil highly influenced by municipal waste disposal—a former sewage farm monitoring study. Land Degrad Dev. https://doi.org/10.1002/ldr.4147
Szopiński M, Sitko K, Gieroń Ż, Rusinowski S, Corso M, Hermans C, Verbruggen N, Małkowski E (2019) Toxic effects of Cd and Zn on the photosynthetic apparatus of the Arabidopsis halleri and Arabidopsis arenosa Pseudo-Metallophytes. Front Plant Sci. https://doi.org/10.3389/fpls.2019.00748
Tang Y, Qiu R, Zeng X, Ying R, Yu F, Zhou X (2009) Lead, zinc, cadmium hyperaccumulation and growth stimulation in Arabis paniculata Franch. Environ Exp Bot 66(1):126–134. https://doi.org/10.1016/j.envexpbot.2008.12.016
Tripti M (2018) Phytomanagement of polluted sites, vol 1. Elsevier
Wang Y, Li Q, Shi J, Lin Q, Chen X, Wu W, Chen Y, (2008). Assessment of microbial activity and bacterial community composition in the rhizosphere of a copper accumulator and a non-accumulator. Soil Biol and Biochem 40(5):1167–1177. doi:https://doi.org/10.1016/j.soilbio.2007.12.010
Yang Y, Ma T, Ding F, Ma H, Duan X, Gao T, Yao J (2016) Interactive zinc, iron, and copper-induced phytotoxicity in wheat roots. Environ Sci Pollut Res 24(1):395–404. https://doi.org/10.1007/s11356-016-7659-0
Zhang Y (2011) Forest leaf chlorophyll study using hyperspectral remote sensing. Hyperspectral Remote Sens Veg. https://doi.org/10.1201/b11222
Zhang Y, He L, Chen Z, Wang Q, Qian M, Sheng X (2011) Characterization of ACC deaminase-producing endophytic bacteria isolated from copper-tolerant plants and their potential in promoting the growth and copper accumulation of Brassica napus. Chemosphere 83(1):57–62. https://doi.org/10.1016/j.chemosphere.2011.01.041
Acknowledgements
This study is a contribution to the HyPhy project (“Innovative drone-based hyperspectral detection of heavy metals in plants in relation to Phytomining”) which is funded by the Federal Ministry for Economic Affairs and Technology through AiF.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by Bundesministerium für Wirtschaft und Technologie, ZF4296102BA9, Magdalena Sut-Lohmann.
Author information
Authors and Affiliations
Contributions
MS-L: conceptualization, methodology, manuscript preparation, supervision. MG: lab and field work, data visualization. FK: field work, manuscript revision. TR: manuscript revision. MH: field and lab work. TF: lab work and manuscript revision.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sut-Lohmann, M., Grimm, M., Kästner, F. et al. Brassica juncea as a Feasible Hyperaccumulator of Chosen Potentially Toxic Metals Under Extreme Environmental Conditions. Int J Environ Res 17, 38 (2023). https://doi.org/10.1007/s41742-023-00528-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41742-023-00528-8