Abstract
Background and Objective
This study aimed to assess the cost-effectiveness of two regimens regarded as the standard of care for the treatment of newly diagnosed, transplant-ineligible multiple myeloma in Singapore: (1) daratumumab, lenalidomide, and dexamethasone and (2) bortezomib, lenalidomide, and dexamethasone. Additionally, it aimed to explore potential strategies to manage decision uncertainty and mitigate financial risk.
Methods
A cost-effectiveness analysis from the healthcare system perspective was conducted using a partitioned survival model to estimate lifetime costs and quality-adjusted life years (QALYs) associated with daratumumab-based treatment and the bortezomib-based regimen. The analysis used data from the MAIA and SWOG S0777 trials and incorporated local real-world data where available. Sensitivity analyses were performed to evaluate the robustness of the findings, and a risk analysis was conducted to analyze various payer strategies in terms of their payer strategy and uncertainty burden (P-SUB), which account for the decision uncertainty and the additional cost of choosing a suboptimal intervention.
Results
The incremental cost-effectiveness ratio (ICER) for daratumumab, lenalidomide, and dexamethasone (DRd) compared with bortezomib, lenalidomide, and dexamethasone (VRd) was US $90,364 per QALY gained. The results were sensitive to variations in survival for DRd, postprogression treatment costs, cost of hospice care, and hazard ratio for progression-free survival. The scenarios explored indicated that structural assumptions, such as the time horizon of the analysis, significantly influenced the results due to uncertainties arising from immature trial data and treatment efficacy over time. Among the various payer strategies compared, an upfront price discount for daratumumab emerged as the best approach with the lowest P-SUB at US $14,708.
Conclusion
In conclusion, this study finds that daratumumab as a first-line treatment for myeloma exceeds the cost-effectiveness threshold considered in this evaluation. An upfront price reduction is the recommended strategy to manage uncertainties and mitigate financial risks. These findings highlight the importance of targeted payer strategies to address specific types and sources of uncertainty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Daratumumab-based treatment (DRd) as a first-line therapy for multiple myeloma currently exceeds the cost-effectiveness threshold used in this study, considering current evidence and prices. |
It is recommended to implement an upfront price reduction for daratumumab, as this was the optimal strategy due to its lowest payer strategy and uncertainty burden across various scenarios modeled. These scenarios include waiting for complete evidence from the trial, conducting a head-to-head comparison, and conditional treatment continuation. |
Decision-makers should consider various sources of uncertainty before adopting a specific payment strategy, as a one-size-fits-all approach is not suitable and should be tailored to the type of uncertainty observed in the evaluation. |
1 Introduction
Multiple myeloma (MM) represents a costly form of blood cancer that requires the administration of multiple drugs and necessitates new treatment regimens as the disease progresses [1, 2]. Current practice guidelines in Singapore and overseas advocate for the initiation of triplet therapy regimens rather than two-drug combinations as the primary treatment approach [3,4,5]. The selection of specific agents within these regimens is contingent upon factors such as the patient’s risk status, presence of comorbidities, and financial circumstances.
In the context of first-line therapy, the established standard of care regimen is bortezomib, lenalidomide, and dexamethasone (VRd), which has exhibited a survival advantage over the administration of lenalidomide and dexamethasone alone (Rd), as demonstrated in the SWOG S0777 trial [6, 7]. However, the emergence of daratumumab, an anti-CD38 monoclonal antibody, has introduced a prominent alternative to bortezomib. This newer drug has shown clinical efficacy for both newly diagnosed and relapsed/refractory patients. Ongoing evidence from the MAIA trial reveals improvements in progression-free survival (PFS) and overall survival (OS) for patients receiving daratumumab in combination with Rd (DRd) compared with Rd alone [8, 9]. Currently, in Singapore, patients receive a subsidy for bortezomib since it is listed in the Standard Drug List. However, patients requiring daratumumab face high out-of-pocket expenses due to the absence of a subsidy [10].
In the past, daratumumab was typically reserved for the treatment of relapsed/refractory multiple myeloma (RRMM), due to the clear evidence of efficacy in this population, where it was first evaluated [11]. Given the emerging evidence of its use in newly diagnosed patients, there has been interest in shifting its use to the first-line setting, especially since the attrition rates in later lines are high [12]. Additionally, the highest response rate was observed in the first-line use of daratumumab, compared with the later lines [13]. Therefore, it is recommended to use treatments with substantial benefits as initial therapies rather than reserving them for instances of disease relapse [14].
The increasing availability of high-cost therapies necessitates payers to expand their financing options [15]. When faced with substantial uncertainty surrounding the cost-effectiveness of these therapies, payers have employed various strategies, such as negotiating lower prices, requesting additional data, or entering into risk-sharing agreements [16,17,18]. These strategies, commonly employed in the field of oncology and hematology, aim to distribute the risk between payers who must make funding decisions amidst considerable uncertainty and pharmaceutical companies seeking to optimize their return on investment in research and development [19, 20]. However, there is a notable lack of quantitative assessment regarding the impact and suitability of these payer strategies in the face of economic evaluation uncertainty. This evidentiary gap necessitates further investigation, particularly in the context of myeloma treatments, to generate robust evidence and address the need for comprehensive understanding in this field.
In light of these considerations, this study aims to achieve two objectives: first, to estimate the costs and benefits associated with a daratumumab-based regimen as the initial treatment compared with the standard of care from the perspective of the Singapore healthcare system and, second, to quantify the value of alternative payer strategies or schemes in terms of reducing decision risk.
2 Methods
2.1 Economic Evaluation Framework
The target population of the study was adults with newly diagnosed multiple myeloma who were not eligible for stem cell transplantation. The starting age of the simulated cohort was 66 years, which was based on the median age of newly diagnosed patients in Singapore [21]. The main intervention under evaluation was a triplet therapy consisting of daratumumab, lenalidomide, and dexamethasone (referred to as DRd). The comparator was also a bortezomib-based regimen with lenalidomide and dexamethasone (referred to as VRd), which is the subsidized standard of care regimen in Singapore for first-line treatment of multiple myeloma. The dosing and administration schedule of the regimens followed those used in the SWOG S0777 trials and MAIA, with assumptions made regarding the postprogression treatment. (See Table S1 and S2 in the Supplementary Material)
The economic evaluation was conducted from the perspective of the Singapore healthcare system using a lifetime horizon. A time horizon of 30 years was chosen to ensure that it captures the expected lifespan of all newly diagnosed patients, considering the life expectancy in Singapore [22]. To ensure granularity in costs and outcomes and to align with the 21- or 28-day treatment cycles used in the trials, a weekly cycle length was adopted. Discounting was applied to both costs and outcomes at a rate of 3%. This choice of discount rate was based on the pharmacoeconomic guidelines published by the Agency for Care Effectiveness, which is the health technology assessment (HTA) agency under the Ministry of Health in Singapore [23, 24].
2.2 Model Description
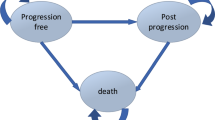
We adopted a partitioned survival model to estimate the costs and outcomes associated with the regimens, which used modeled progression-free survival and overall survival data from the clinical trials [25]. The model considered the time spent on-treatment with VRd or DRd, which is the progression-free state. Subsequently, it considered the transition to the postprogression state or the initiation of a new treatment line, and finally, the absorbing state represented death. To determine the costs of first-line therapy with daratumumab, we used the time spent on treatment based on time-to-discontinuation (TTD) estimates from the MAIA trial (Figure S1 on the Supplementary Material) [8]. For bortezomib, given the absence of TTD data, we assumed that patients would complete the initial therapy, and remain on maintenance Rd treatment until disease progression based on the estimated PFS. For subsequent treatment lines, we replicated the regimens used in Singapore using real-world data rather than relying on trial data. We assumed that patients would receive palliative care 1 month prior to their death, regardless of whether they were in the progression-free or postprogression state [26].
2.3 Input Parameters
2.3.1 Clinical Effectiveness
Progression in this study was defined according to the International Myeloma Working Group Criteria [27], which was consistently applied across the trials. We fitted individual patient-level data (IPD) from the intervention arm of the MAIA trial to estimate progression-free survival (PFS) and overall survival (OS) of patients receiving DRd. Survival outcomes for the comparator, VRd, were derived from two sources. For PFS, we conducted an indirect comparison using subgroup results from SWOG S0777 [6]. This trial provided subgroup analysis results for patients without intent to undergo a transplant, which was used in an anchored indirect comparison with DRd, given a common comparator of lenalidomide and dexamethasone (Rd) in both trials. Details of the indirect comparison are provided in the Supplementary Material. Briefly, the indirect comparison of PFS yielded a hazard ratio of 0.72 [95% confidence interval (CI) 0.50–1.03]. For OS, subgroup results for patients over 65 years old were reported in the SWOG S0777 trial and used for the VRd arm. We digitized the reported Kaplan‒Meier curves and applied the Guyot algorithm to generate pseudo-IPD [28].
At the time of the analysis, a median follow-up duration of 56.2 months was available from the MAIA trial. For SWOG S0777, the results for up to 84 months of median follow-up were available. We extrapolated survival curves over a model horizon of 30 years using parametric functions. The selection of the best-fitting curves was based on statistical fit measures [Akaike information criterion (AIC) and Bayesian information criterion (BIC); Table S3 in the Supplementary Material], visual inspection, and clinical validation [29]. For DRd, an exponential function provided the best fit for both progression-free survival and overall survival. For VRd, a piecewise exponential model was fitted to overall survival, with cutoff points at months 3, 37, and 88 (Figs. 1, 2).
2.3.2 Costs and Resource Use
Given the heterogeneity of patients, we used real-world data (RWD) to capture costs and resource use accurately. We obtained costs and resource use data related to the treatment and management of adverse events from a retrospective analysis of electronic medical and billing records at two major cancer centers in Singapore. A separate study reports the details of this analysis [30]. In summary, we conducted a retrospective cohort study analyzing data from the electronic records of 605 MM patients in Singapore. Treatment cost data, cost of managing adverse events, and utilization of subsequent treatment lines were generated from this study. The unit costs of the drugs reflect the cost prices in the hospitals as of November 2023. The cost of the DRd regimen was adjusted to incorporate the Patient Access Programme currently implemented by the manufacturer and uses the subcutaneous version of the drug. Under this program, patients pay a fixed monthly cost irrespective of their treatment cycle. DRd administration costs without the Patient Access Programme are reported in the Supplementary Material (Table S4). Costs associated with palliative care were obtained from the COMPASS cohort study, which collected end-of-life cost data for patients in Singapore with various cancer types [31]. The estimate used in this study was based on their analysis of the mean monthly cost 1 month before death. All costs are reported in US dollars (US $) and were converted using prevailing exchange rates as of May 2023 (US $1 = 1.35 Singapore dollars [32]; Table 1).
2.3.3 Quality of Life
Health utilities (Table 2) were obtained from published literature and were used to calculate quality-adjusted life-years (QALYs). Progression-free and postprogression utility values were derived from Carlson et al. [35] and the MAIA trial, while disutilities were obtained from a publication by Jakubowiak et al. in 2016 [36]. These studies estimated the health-related quality of life of multiple myeloma patients from the MAIA and ASPIRE trials, using the EuroQol five dimensions questionnaire (EQ-5D). To calculate disutilities, we multiplied the expected disutility for each adverse event by its duration in days from RWD, divided by the total number of days in a year. The risk of each adverse event was sourced from the trials and converted into probabilities on the basis of the median follow-up duration.
2.4 Uncertainty Assessment and Model Validation
To ensure the robustness and validity of our model, we conducted a series of validation meetings involving senior multiple myeloma clinicians from the three public cancer centers in Singapore. The experts were presented with the survival curves, and were asked to comment on the estimated survival at key time points, i.e., 5, 10, and 20 years, and the soundness of the treatment waning assumption. They were also asked to validate the distribution of the subsequent lines of treatment post-VRd and DRd and were presented with the results for all scenarios. We also sought input from an expert from the Singapore government HTA agency who provided feedback on the methods used and potential implications of the results. Finally, we also presented the results to the drug manufacturer to ensure that the latest clinical trial data and unit prices are used in the model.
The model development process involved utilizing both RStudio and Microsoft Excel, allowing us to cross-validate the results between the two platforms. Independent checks were performed on both models to verify the accuracy of the input data, calculations, and results. Additionally, we ran the model under various scenarios, including the use of extreme values to ensure consistency and validity. We also used the most recent published data cut from the MAIA trial to validate the results of our extrapolations, given that the trial is still ongoing [37, 38].
We evaluated alternative scenarios as part of a one-way sensitivity analysis. One scenario involved considering treatment waning, where the hazard of overall survival for DRd was assumed to be equivalent to the hazard of VRd from the SWOG-S0777 trial after 10 years. Another scenario was explored by using VRd progression-free survival data from the PEGASUS study [39], which involved deidentified data sourced from the Flatiron Health database. The data were extracted from electronic health records of patients who had received treatment at community-based oncology practices in the USA. However, it is important to note that this study solely reported PFS, as the OS data were deemed immature at the time of the study. Additionally, we explored shorter time horizons (5, 10, and 20 years) to explore the impact of uncertainty in the lifetime horizon extrapolations.
We also performed a probabilistic sensitivity analysis (PSA) that subjected all inputs to uncertainty (Table S5 in the Supplementary Material). Point estimates were randomly selected on the basis of the distribution of each input, and this process was conducted once per simulation. The incremental cost-effectiveness ratio (ICER) in terms of cost per QALY was recorded for each simulation of the cost-effectiveness analysis (CEA), and a total of 1000 simulations were conducted to determine parametric uncertainty and estimate the probability that the ICER would fall below various willingness-to-pay thresholds. While the Agency for Care Effectiveness (ACE), the government HTA body in Singapore, and the decision-making committee refrain from using an explicit and singular threshold value, they examine a range of values and encourage reporting ICERs in accordance with these benchmarks. In this study, we opted to use US $80,000 as the threshold, which was previously considered the upper limit according to their 2022 guidelines [24, 25]. In September 2023, an updated version of the guidelines was released, raising the upper limit to US $270,000. Nevertheless, we have chosen to adhere to the more conservative estimate of US $80,000 because recent decisions are consistent with an implicit threshold around this value.
2.5 Risk Analysis
In this part of the study, we applied a risk analysis framework originally developed by Grimm et al. [40] The framework consists of three main steps. First, we used the results obtained from the PSA to assess uncertainty and analyze present risks. This involved calculating the payer uncertainty burden (PUB), which is equivalent to the expected value of perfect information (EVPI), and the payer strategy burden (PSB), which quantifies the risk associated with choosing a nonoptimal intervention. By combining the PUB and PSB (referred to as P-SUB), we gained a comprehensive understanding of the potential benefits of eliminating uncertainty and the specific risk to the payer associated with the chosen option (e.g., DRd or VRd).
Second, we conducted interviews with 17 individuals representing pharmaceutical companies, the government HTA agency in Singapore, healthcare providers, academics, and patient groups to identify the most suitable schemes and payer strategies within the context of Singapore. These interviews helped us gather valuable insights and perspectives to inform our selection process. The detailed methods and results of the stakeholder interviews are reported in a separate publication by Bayani and Wee [41].
Finally, we simulated the shortlisted schemes to evaluate their potential impact on P-SUB resulting from their implementation. This analysis allowed us to assess the potential risks and benefits associated with each scheme in a systematic manner. The risks and benefits are then compared with the P-SUB in the base case scenario where no scheme is applied. To achieve this, we incorporated the payer strategies into the cost-effectiveness model and reran the PSA to derive new estimates of ICER, PUB, and PSB. A summary of the assessed strategies can be found in Table 3. The analyses were likewise carried out in RStudio, with codes adapted from the National Institute for Health and Care Excellence (NICE) Decision Support Unit Report [42] and the Sheffield Accelerated Value of Information (SAVI) platform [43] (codes are available on GitHub; see Code Availability).
3 Results
3.1 Incremental Costs, Outcomes, and ICER
In our analysis, the use of DRd for newly diagnosed, transplant-ineligible MM patients resulted in a total incremental cost of US $153,396 over a lifetime horizon when compared with VRd. The primary driver of this cost difference was the cost of daratumumab. However, it was partially offset by the lower costs of hospice care and the higher cost of postprogression treatments in the VRd group, where most patients were on a daratumumab-based triplet regimen. In terms of life years, DRd provided an additional 2.76 years, or 1.88 additional quality-adjusted life years (QALYs; see Tables 4 and 5). These findings led to a deterministic incremental cost-effectiveness ratio (ICER) of US $55,499 per life-year gained and US $81,748 per QALY gained.
3.2 Uncertainty Analyses
To address the uncertainty stemming from the immaturity of the trial and the extrapolation of survival outcomes, we explored additional scenarios as part of the deterministic sensitivity analyses. Shortening the time horizon to 5, 10, and 20 years resulted in corresponding cost per QALY estimates of US $1,746,380, USD$368,772, and US $123,208, respectively. When these time horizons are considered, daratumumab would require an 89%, 61%, and 20% price reduction, respectively, to be cost-effective.
When we considered the assumption of treatment waning from daratumumab after 10 years, the overall survival of DRd patients would expectedly shift closer to that of the VRd group, leading to a reduction in the observed benefits in terms of life years and QALYs (Fig. 3). Specifically, DRd was associated with an incremental 1.71 life years compared with VRd, resulting in 1.19 additional QALYs gained if we assume that the treatment effect wanes after 10 years. The ICER increased by 136% from the deterministic ICER, reaching US $193,056 per QALY.
Furthermore, in the scenario where the PEGASUS study was referenced for VRd PFS, the overall survival benefit of DRd compared with VRd was reduced to 2.43 life years gained and 1.68 QALYs gained. This analysis produced an ICER of US $96,130 per QALY gained.
3.3 Risk Analysis
3.3.1 Present Risk
The results of the PSA showed an average ICER of US $90,364 per QALY gained. In the analysis, 94% of the simulations fell within the northeast quadrant of the plane, indicating a higher cost and greater benefit, while 2.9% of the simulations resulted in cost savings (Fig. 4). Considering the conservative threshold of US $80,000 per QALY, VRd is expected to be the cost-effective option on the basis of the current evidence and pricing.
To better understand the influence of parameter uncertainty on the findings, we conducted an analysis of the expected value of partial perfect information (EVPPI) [44]. This allowed us to identify the parameters that have the greatest impact on the P-SUB, as well as the parameters that should be prioritized for additional data collection. Among the parameters examined, the scale coefficient for DRd overall survival showed the highest EVPPI, followed by the estimates for postprogression treatment costs for both DRd and VRd, the cost of hospice care, and the hazard ratio derived from the indirect comparison.
Figure 5 depicts a stacked bar chart illustrating the P-SUB results for the base case scenario, without any schemes applied. A significant PSB was observed for DRd, suggesting that implementing a price-based scheme could be beneficial in reducing the ICER to fall within the threshold. Conversely, both options demonstrated a substantial PUB, indicating the need for further evidence and potential data collection schemes.
3.3.2 Simulation of Schemes
Figure 6 presents the P-SUB estimate for adopting DRd under the different scenarios outlined in Table 3. Among these scenarios, the application of an upfront price discount yielded the most favorable P-SUB outcome, indicating it as the optimal strategy. If this is not achieved, then a negative recommendation for DRd, or choosing VRd instead, would be the next best option. Simulation results considering various percentages of price reduction indicated that the price of daratumumab would need to be reduced by at least 14% to achieve cost-effectiveness compared with choosing VRd.
4 Discussion
When comparing the costs and benefits of daratumumab versus the standard of care in Singapore, our analysis revealed a probabilistic ICER of US $90,364 per QALY. Currently, this ICER exceeds the conservative threshold we have considered in this analysis but falls within the ranges that the Singapore government HTA agency reports. The robustness of our analysis was supported by the deterministic sensitivity analysis, which demonstrated the greatest sensitivity to variations in survival for daratumumab, postprogression treatment costs, the cost of hospice care, and the hazard ratio for PFS. The scenarios we explored revealed that the results were largely influenced by structural assumptions such as the time horizon of the analysis due to the uncertainty arising from the immature trial results and the potential waning of treatment efficacy over time. Among the various payer strategies compared, the simplest strategy, an upfront price reduction for daratumumab, proved to be the optimal approach.
Our findings align with previous studies conducted on daratumumab in the first-line setting. Patel et al. conducted a study in the USA, comparing the cost-effectiveness of DRd versus Rd using a six-state Markov model [45]. Their study reported a high ICER of US $618,018 per QALY, indicating the need for a 67% reduction in the price of daratumumab to achieve cost-effectiveness with a threshold of US $150,000. A key difference between their study and ours is that they did not compare DRd with other commonly used first-line regimens, such as VRd, due to the lack of indirect comparisons. Furthermore, their study did not include newer agents for the later lines of treatment, and they acknowledged the inability to validate long-term overall survival due to a lack of external reference data. Another published study by Narsipur et al. from the USA included VRd as a comparator using a two-state Markov model and employed the Weibull distribution for extrapolations [46]. Their results showed that neither VRd nor DRd were cost-effective compared with Rd as the standard of care, with ICERs per progression-free QALY exceeding 1 million dollars. This raises the question of the appropriate comparator, as they used Rd even though most guidelines now recommend a triplet regimen unless not tolerated by patients. Finally, a recently published technology appraisal guidance by NICE reported cost-effectiveness estimates to be within the range normally considered as acceptable use of resources, which is below £30,000 per QALY gained (approximately US $ 37,000). This evaluation compared DRd against Rd and two other bortezomib-based regimens: bortezomib plus cyclophosphamide and dexamethasone and bortezomib plus melphalan and prednisone. Although the initial draft recommendation in February 2023 was unfavorable, the final guidance recommended the use of DRd for untreated myeloma patients, contingent upon the company supplying the drug with a confidential price discount [47].
To the best of our knowledge, our study is the first in Singapore to not only report on the cost-effectiveness of daratumumab in the first-line setting but also simulate potential strategies to manage uncertainty and mitigate decision risk. However, there are several limitations that need to be acknowledged.
First, there are limitations related to the estimation of clinical effectiveness. Uncertainty remains regarding the long-term benefits of DRd, particularly in comparison to VRd, due to the lack of a head-to-head comparison in a phase III clinical trial and the limitations inherent in conducting an indirect comparison using data from the MAIA and SWOG S0777 trials. Nevertheless, two other studies that have compared DRd and VRd have concluded that DRd is associated with a lower risk of progression and death for transplant ineligible NDMM [48, 49]. A key limitation stems from the fundamental difference in the baseline characteristics of the patients recruited into the respective trials. The MAIA trial is primarily a study in transplant-ineligible patients and hence had significantly older patients (> 75% of patients were older than 70 years of age in both arms of the study) than the SWOG S0777 trial. This led to differences in subsequent treatment options, including the possibility of salvage transplantation, the spectrum of relapse treatments suitable for an older patient group, and the expected outcomes. It is acknowledged that the application of VRd in full doses in an elderly population older than 70 years is challenging. In real-world situations, this results in significant dose reductions that hamper the efficacy of this regimen, leading to inferior outcomes than what has been seen in trials. Additionally, the MAIA trial introduces a degree of uncertainty given its ongoing status and projected completion date in June 2024. Notably, its follow-up duration of 64.5 months falls significantly shorter when juxtaposed with the SWOG S0777 trial, which had a median follow-up period of 84 months. However, the MAIA trial has shown the longest progression-free survival observed to date [50], demonstrated an overall survival benefit of DRd in the latest data cutoff [37], and has been well tolerated in this setting. Furthermore, there is a question of generalizability of the SWOG S0777 and MAIA trial results to the myeloma population in Singapore, considering possible differences in clinical characteristics, risk profiles, and treatment utilization.
Second, we acknowledge limitations in the estimation of cost-effectiveness stemming from our modeling approach, the structural assumptions considered, and the input parameters used. Our use of the partitioned survival approach is subject to the inherent limitation of being unable to estimate postprogression survival, as the survival curves are derived from separate PFS and OS data reported in the trials [51]. Initially, we attempted to apply the state-transition approach described by Majer et al. [52], which independently estimates the transitions between states, leveraging patient-level data from the MAIA trial. However, given that our individual patient data (IPD) were only available for DRd and Rd, we determined that this approach was infeasible and inappropriate for our analyses, as it was crucial to compare DRd against the appropriate comparator. Further research is needed to apply this method when assessing comparative effectiveness, particularly in situations where different comparators are involved.
Additionally, there was uncertainty in the estimation of postprogression survival and costs, which is a common issue when evaluating first-line treatments, as detailed data on subsequent lines of treatment are often not reported in trial publications [53]. Therefore, for this study, we relied on real-world data to estimate the market share of subsequent lines of treatment aligned with current financed treatments, which may not necessarily mirror the treatment patterns used in the trials.
The estimation of postprogression utility was determined using patients who had experienced progression. However, it is important to note that some patients may achieve a progression-free state after their initial relapse. This particular scenario was not accounted for in the simplified three-state model structure, potentially leading to an underestimation of the overall QALYs in the postprogression state.
Moreover, there remains uncertainty in the projections made from extrapolations, and without external data for validation, we cannot be certain about the long-term survival outcomes for patients and must rely on clinical expert opinion regarding plausibility. Although we considered a scenario involving treatment waning as a conservative approach, there was no sufficient basis for this, consistent with the appraisal of daratumumab by NICE [54], which excluded treatment waning and cited a lack of clinical evidence supporting it. Furthermore, we recognize a limitation in the method employed to apply the treatment waning assumption in the model, as it was only performed on the intervention arm and not on both arms.
Finally, there are limitations in the risk analysis conducted. The method developed by Grimm et al. was primarily designed for NICE appraisals in a context where an explicit and consistent threshold guides decision-making. In the Singapore context, decisions are not solely based on a threshold but rather on a series of criteria encompassing not only clinical need but also affordability and sustainability. This framework needs further development and contextualization in the local setting or in any country seeking to adapt it to refine its usefulness and applicability to local decision-making contexts. Moreover, the payer strategies explored in our study were highly simplified, and other options and costs may be incurred when implementing risk-sharing agreements. However, our findings were consistent with the value-based pricing strategy employed by the Singapore government HTA agency, where negotiations typically revolve around price reductions. Historically, such negotiations have been successful in reducing prices for most cancer drugs by an average of 30% [55]. Last, we acknowledge the presence of other uncertainties not captured in the probabilistic sensitivity analysis. These uncertainties require further investigation, and their potential impact should be communicated. The authors of the framework have advocated for the use of new tools, such as the Assessment of Risk Table and the Appraisal of Risk Chart, to address these uncertainties effectively [56, 57].
5 Conclusion
In summary, our study demonstrates that, based on the existing evidence and pricing, opting for daratumumab as a first-line treatment alternative to the bortezomib-based regimen surpasses the conservative cost-effectiveness threshold set forth in our analysis, yet it aligns with the ranges typically reported by the Singapore government HTA agency. Nevertheless, the most effective approach for mitigating uncertainty and associated payer risk remains an upfront price reduction.
References
Rajkumar SV. Value and cost of myeloma therapy. Am Soc Clin Oncol Educ Book. 2018;2:662–6.
Cowan AJ, Allen C, Barac A, et al. Global burden of multiple myeloma: a systematic analysis for the global burden of disease study 2016. JAMA Oncol. 2018;4:1221–7.
Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up<sup>†</sup>. Ann Oncol. 2021;32:309–22.
Kumar SK, Callander NS, Adekola K, et al. Multiple myeloma, version 3.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18:1685–717.
de Mel S, Chen Y, Gopalakrishnan SK, et al. The Singapore myeloma study group consensus guidelines for the management of patients with multiple myeloma. Singapore Med J. 2017;58:55–71.
Durie BGM, Hoering A, Sexton R, et al. Longer term follow-up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J. 2020;10:53.
Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389:519–27.
Facon T, Kumar SK, Plesner T, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:1582–96.
Facon T, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380:2104–15.
Agency for Care Effectiveness. Daratumumab-based regimens for newly diagnosed multiple myeloma. Technology Guidance from the MOH Drug Advisory Committee. Singapore, 2022.
Abdallah N, Kumar SK. Daratumumab in untreated newly diagnosed multiple myeloma. Ther Adv Hematol. 2019;10:2040620719894871.
Fonseca R, Usmani SZ, Mehra M, et al. Frontline treatment patterns and attrition rates by subsequent lines of therapy in patients with newly diagnosed multiple myeloma. BMC Cancer. 2020;20:1087.
Atrash S, Thompson-Leduc P, Tai MH, et al. Treatment patterns and effectiveness of patients with multiple myeloma initiating daratumumab across different lines of therapy: a real-world chart review study. BMC Cancer. 2021;21:1207.
Fonseca R, Facon T, Hashim M, et al. First-Line use of daratumumab, lenalidomide, and dexamethasone confers survival benefit compared with second-line use of daratumumab-based regimens in transplant-ineligible patients with multiple myeloma: analysis of different clinical scenarios. Blood. 2021;138:118.
Leighl NB, Nirmalakumar S, Ezeife DA, et al. An arm and a leg: the rising cost of cancer drugs and impact on access. Am Soc Clin Oncol Educ Book. 2021;41:1–12.
Kim AE, Choi DH, Chang J, et al. Performance-based risk-sharing arrangements (PBRSA): is it a solution to increase bang for the buck for pharmaceutical reimbursement strategy for our nation and around the world? Clin Drug Investig. 2020;40:1107–13.
Siddiqui M, Rajkumar SV. The high cost of cancer drugs and what we can do about it. Mayo Clin Proc. 2012;87:935–43.
Vokinger KN, Hwang TJ, Daniore P, et al. Analysis of launch and postapproval cancer drug pricing, clinical benefit, and policy implications in the US and Europe. JAMA Oncol. 2021;7:e212026–126.
Carlson JJ, Chen S, Garrison LP Jr. Performance-based risk-sharing arrangements: an updated international review. Pharmacoeconomics. 2017;35:1063–72.
Garrison LP Jr, Towse A, Briggs A, et al. Performance-based risk-sharing arrangements-good practices for design, implementation, and evaluation: report of the ISPOR good practices for performance-based risk-sharing arrangements task force. Value Health. 2013;16:703–19.
Huynh K, Aoki N, Kim K, et al. The Asia-Pacific Myeloma and Related Diseases Registry: preliminary results of real-world treatment patterns and clinical outcomes. Blood. 2020;136:30–1.
Singapore DoS. Complete life tables for Singapore resident population, 2021–2022. 2022.
Procedures and guidelines for company submissions to the Agency for Care Effectiveness for funding consideration. Singapore: Agency for Care Effectiveness, 2022.
Drug and Vaccine Evaluation Methods and Process Guide. Singapore: Agency for Care Effectiveness, 2021.
Woods B, Sideris E, Palmer S, et al. Nice DSU Technical Support Document 19: partitioned survival analysis for decision modelling in health care: a critical review. Report By The Decision Support Unit. NICE Decision Support Unit, 2017.
Wong XY, Chng WJ, Aziz MIA, et al. Cost-effectiveness of daratumumab in combination with lenalidomide and dexamethasone for relapsed and/or refractory multiple myeloma. Expert Rev Pharmacoecon Outcomes Res. 2022;22:207–15.
Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–46.
Guyot P, Ades AE, Ouwens MJNM, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9.
Latimer NR, Adler AI. Extrapolation beyond the end of trials to estimate long term survival and cost effectiveness. BMJ Med. 2022;1: e000094.
Bayani DB, Lin YC, Ooi MG, et al. Real-world utilization and healthcare costs for multiple myeloma: a retrospective analysis of patients in Singapore. eJHaem. 2023;4:2.
Doble B, Wong WHM, Finkelstein E. End-of-life cost trajectories and the trade-off between treatment costs and life-extension: findings from the cost and medical care of patients with advanced serious illness (COMPASS) cohort study. Palliat Med. 2021;35:893–903.
XE. Convert US dollars to singapore dollars. XE. 2023.
Singapore healthcare resource sheet. In: Agency for Care Effectiveness, ed. Singapore, 2023.
Durie B, Hoering A, Rajkumar SV, et al. Bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT): results of the randomized phase III trial SWOG S0777. Blood. 2015;126:25.
Carlson JJ, Guzauskas GF, Chapman RH, et al. Cost-effectiveness of drugs to treat relapsed/refractory multiple myeloma in the United States. J Manag Care Spec Pharm. 2018;24:29–38.
Jakubowiak AJ, Campioni M, Benedict Á, et al. Cost-effectiveness of adding carfilzomib to lenalidomide and dexamethasone in relapsed multiple myeloma from a US perspective. J Med Econ. 2016;19:1061–74.
Weisel K, Kumar S, Moreau P, et al. P09 Daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) alone in transplant-ineligible patients with newly diagnosed multiple myeloma (Ndmm): updated analysis of the phase 3 Maia study. Hemasphere. 2023;7:2.
Kumar SK, Moreau P, Bahlis NJ, et al. Daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) alone in transplant-ineligible patients with newly diagnosed multiple myeloma (NDMM): updated analysis of the phase 3 Maia study. Blood. 2022;140:10150–3.
Durie BGM, Kumar SK, Usmani SZ, et al. Daratumumab-lenalidomide-dexamethasone vs standard-of-care regimens: efficacy in transplant-ineligible untreated myeloma. Am J Hematol. 2020;95:1486–94.
Grimm SE, Strong M, Brennan A, et al. The HTA risk analysis chart: visualising the need for and potential value of managed entry agreements in health technology assessment. Pharmacoeconomics. 2017;35:1287–96.
Bayani DB, Wee HL. Value-based payment for high-cost treatments in Singapore: a qualitative study of stakeholders’ perspectives. Int J Technol Assess Health Care. 2024;40: e22.
Grimm S, Strong M, Brennan A, et al. Framework for analysing risk in health technology assessments and its application to managed entry agreements. Decision Support Unit, ScHARR, University of Sheffield, Regent Court, 30 Regent Street, Sheffield, S1 4DA 2016.
Strong M, Oakley JE, Brennan A. Estimating multiparameter partial expected value of perfect information from a probabilistic sensitivity analysis sample: a nonparametric regression approach. Med Decis Making. 2014;34:311–26.
Brennan A, Kharroubi S, O’Hagan A, et al. Calculating partial expected value of perfect information via Monte Carlo sampling algorithms. Med Decis Making. 2007;27:448–70.
Patel KK, Giri S, Parker TL, et al. Cost-effectiveness of first-line versus second-line use of daratumumab in older, transplant-ineligible patients with multiple myeloma. J Clin Oncol. 2021;39:1119–28.
Narsipur N, Bulla S, Yoo C, et al. Cost-effectiveness of adding daratumumab or bortezomib to lenalidomide plus dexamethasone for newly diagnosed multiple myeloma. J Manag Care Spec Pharm. 2021;27:1691–702.
National Institute for Health and Care Excellence. Daratumumab with lenalidomide and dexamethasone for untreated multiple myeloma when a stem cell transplant is unsuitable. 2023.
Durie BG, Kumar S, Ammann EM, et al. Adjusted indirect treatment comparison of progression-free survival (PFS) associated with DRd and VRd based on MAIA and SWOG S0777 individual patient-level data. J Clin Oncol. 2023;41:8037–137.
Gordan L, Medhekar R, Fu AZ, et al. Systematic literature review and meta-analysis of comparative clinical evidence investigating daratumumab, lenalidomide, and dexamethasone (DRd) versus bortezomib, lenalidomide, and dexamethasone (VRd) as first-line treatment for transplant-ineligible newly diagnosed multiple myeloma. Blood. 2023;142:1963.
Fonseca R, Facon T, Hashim M, et al. Impact of treatment sequencing on overall survival in patients with transplant-ineligible newly diagnosed myeloma. Oncologist. 2023;28:e263–9.
Woods BS, Sideris E, Palmer S, et al. Partitioned survival and state transition models for healthcare decision making in oncology: where are we now? Value Health. 2020;23:1613–21.
Majer I, Kroep S, Maroun R, et al. Estimating and extrapolating survival using a state-transition modeling approach: a practical application in multiple myeloma. Value Health. 2022;25:595–604.
Mohyuddin GR, Koehn K, Abdallah A-O, et al. Reporting of postprotocol therapies and attrition in multiple myeloma randomized clinical trials: a systematic review. JAMA Netw Open. 2021;4:e218084–184.
National Institute for Health and Care Excellence. Daratumumab with lenalidomide and dexamethasone for untreated multiple myeloma when stem cell transplant is unsuitable [ID4014]. 2023.
Loh L. Assistance available for cancer patients who need medications not on new list. 2022.
Grimm SE, Pouwels X, Ramaekers BLT, et al. State of the ART? Two new tools for risk communication in health technology assessments. Pharmacoeconomics. 2021;39:1185–96.
Grimm SE, Pouwels X, Ramaekers BLT, et al. Development and validation of the Transparent Uncertainty Assessment (TRUST) tool for assessing uncertainties in health economic decision models. Pharmacoeconomics. 2020;38:205–16.
Acknowledgments
We would like to extend our sincere gratitude to Professor Chia Kee Seng and Ng Kwong for providing their feedback to this study as part of the Thesis Advisory Committee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
D.B. is a recipient of a Ph.D. Research Scholarship from the National University of Singapore. The rest of the authors received no support for the research, authorship, and publication of this article.
Conflicts of Interest
J.C. has been a member of the Myeloma UK Access Advisory Group since 2021 advising on HTA. This is an unpaid role for the patient charity. The rest of the authors have no conflicts to declare.
Ethics approval
The study received ethics approval from the Domain Specific Review Board (DSRB) of the National Healthcare Group in Singapore (DSRB Reference: 2021-00038).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
This study used data obtained from the Yale University Open Data Access Project, which has an agreement with Janssen Research & Development, L.L.C. The interpretation and reporting of research using this data are solely the responsibility of the authors and does not necessarily represent the official views of the Yale University Open Data Access Project or Janssen Research & Development, L.L.C.
Code availability
The R codes used in this study are available on GitHub (https://github.com/D-Bayani/CEA-Daratumumab-NDMM).
Author contributions
D.B. conceptualized the study, reviewed the literature, performed the analyses, and drafted the manuscript. C.N., A.T., M.O., and L.Y.C. assisted with data acquisition and management and contributed to the study design and manuscript preparation. J.C. and W.H.L. reviewed the analysis and findings. D.B. drafted the manuscript, and all authors reviewed, edited, and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bayani, D.B., Lin, Y.C., Nagarajan, C. et al. Modeling First-Line Daratumumab Use for Newly Diagnosed, Transplant-Ineligible, Multiple Myeloma: A Cost-Effectiveness and Risk Analysis for Healthcare Payers. PharmacoEconomics Open (2024). https://doi.org/10.1007/s41669-024-00503-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s41669-024-00503-9