Abstract
Background and Objectives
In France, meningococcal serogroup B (MenB) is the most common serogroup causing invasive meningococcal disease (IMD) in infants and young children. Our objective was to illustrate the impact of model choices on health outcomes and the cost-effectiveness of infant vaccination with the multicomponent meningococcal serogroup B vaccine (4CMenB) versus no vaccine in France.
Methods
A previously published dynamic transmission-based cost-effectiveness model was adapted for the French context using updated, French-specific demographic, epidemiological, and cost data. IMD incidence and long-term sequelae were derived through analysis of French healthcare and surveillance databases. A collective perspective over a 100-year time horizon was adopted, with a discount rate of 2.5%, reduced to 1.5% after the first 30 years. Deterministic and probabilistic sensitivity and scenario analyses were performed.
Results
In the base case analysis, infant vaccination with 4CMenB avoided 3101 MenB IMD cases in infants aged < 1 year (− 54%) and 6845 cases in all age groups (− 21%). The estimated incremental cost-effectiveness ratio was €316,272/quality-adjusted life-year (QALY) but was highly sensitive to the types of sequelae included, MenB incidence, vaccine effectiveness parameters, and consideration of life-expectancy in IMD survivors (range: €65,272/QALY to €493,218/QALY).
Conclusions
Using economic models compliant with French methodology guidelines, 4CMenB does not seem cost-effective; however, results are sensitive to model choices and 4CMenB immunization is an effective strategy to prevent MenB IMD cases and to improve quality of life and economic burden associated with MenB IMD treatment, especially with regard to long-term sequelae.
Plain Language Summary
Invasive meningococcal disease (IMD) is rare but can lead to lifelong disabilities and death. It is caused by a type of bacteria called Neisseria meningitidis. IMD is most common in infants and young children, and in this group it is mostly caused by Neisseria serogroup B bacteria. We analyzed the number of IMD cases caused by serogroup B in France, as well as sequelae (long-time effects of the disease), using data from national healthcare databases. The most common sequelae observed were epilepsy, severe neurological disorders, and anxiety, occurring in approximately 5% of patients. We then calculated the costs and benefits of the multicomponent meningococcal serogroup B vaccine (4CMenB) vaccine for infants and young children in France. The results showed that 4CMenB vaccination can reduce the number of IMD cases due to serogroup B by 3101 cases (− 54%) in infants under 1 year and by 6845 cases (− 21%) in all age groups. Over 100 years, vaccination could prevent over 2000 cases of IMD that result in disabilities and 438 deaths. The estimated cost-effectiveness ratio was high. However, costs per health benefit gained decreased when focusing on long-term health benefits. In France, there is no threshold for the cost-effectiveness ratio and the French Health Authority has included 4CMenB in its vaccination schedule. This recommendation reflects results from our study, which highlights the considerable burden on families and patients, mostly because of IMD-related disabilities. Early vaccination is a good way to protect infants and young children against this serious disease.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Over a 100-year time horizon, an infant vaccination strategy with the multicomponent meningococcal serogroup B vaccine (4CMenB) could avoid 3101 MenB invasive meningococcal disease (IMD) cases in infants aged < 1 year (− 54%) and 6845 cases in all age groups (− 21%). |
Over a 100-year time horizon, the number of sequelae and deaths could be reduced by 2265 and 424, respectively (− 53%) in infants and children aged 0–4 years with an infant vaccination strategy. |
Vaccination with 4CMenB does not seem cost-effective under base-case assumptions. |
Cost-effectiveness results depend on model choices, such as discount rates, quality-of-life assumptions, vaccine effectiveness, and inclusion of all sequelae potentially related to IMD; model assumptions must be considered when interpreting incremental cost-effectiveness ratios. |
1 Introduction
Invasive meningococcal disease (IMD) is a rare but serious infection that can lead to pneumonia, meningitis, and septicemia; it is associated with a case-fatality rate of 8–15% [1]. Short-term sequelae are observed in approximately 6% of children who have IMD. The percentage with long-term sequelae, such as attention-deficit hyperactivity disorder, is higher and estimated to affect 9.7%–30% of IMD survivors [1,2,3]. Infants and young children are at highest risk of IMD, followed by adolescents and young adults [4]. IMD is caused by the Gram-negative bacteria Neisseria meningitidis (N. meningitidis), which colonizes the mucosal surface of the nasopharynx; between 1 and 10% of people are asymptomatic carriers. As N. meningitidis is an exclusively human pathogen, asymptomatic carriage is thought to represent the human reservoir from which hyperinvasive clones emerge, leading to IMD outbreaks [5]. There are 12 N. meningitidis serogroups with serogroups A, B, C, X, Y, and W accounting for more than 90% of IMD cases globally [6, 7]. The contribution of different serogroups to IMD epidemiology varies worldwide, depending on geographic location and vaccination strategies [8]. In Europe, serogroup B accounted for 51% of IMD cases with known serogroup in 2017 (92% of IMD cases had a known serogroup) [4]. In France, in the period 2009–2021, serogroup B IMD cases (MenB IMD) contributed between 42% and 70% of IMD cases with known serogroup [9,10,11]. In a retrospective study using data from the French national public health insurance database (SNDS database), inpatient and outpatient costs accrued during the first year following an IMD episode amounted to €4254 in the absence of sequelae, €10,799 in patients with one sequela, and €20,096 in patients with multiple sequelae [12]. Using a micro-costing approach to estimate the economic impact associated with sequelae, including purpura fulminans followed by limb amputation and meningitis with neurological complications, costs for acute management during the first year amounted to ≥ €160,000, while lifetime costs varied between €0.77 and 2.27 million, depending on the sequelae [13].
Currently, there are three quadrivalent meningococcal vaccines on the market, which protect against infections caused by serogroups A, C, W, and Y; a serogroup C vaccine is also available [14]. In 2013, a multicomponent meningococcal serogroup B vaccine (4CMenB) was approved by the European Medicines Agency for infants aged 2 months and older. Real-world data from the United Kingdom (UK), where 4CMenB was introduced in 2015, showed a two-dose vaccine effectiveness of 82.9% [15]. There is evidence of some additional protection by 4CMenB against non-vaccine-specific serogroups (non-B serogroups); cross-reactive immunogenicity is due to variable expression levels of antigen-specific genes in non-vaccine-specific strains [16]. Bactericidal efficacy against these non-serogroup-B-specific strains has been demonstrated in vitro, supported by real-world effectiveness data from the UK and Spain showing partial cross-protection conferred by 4CMenB against non-serogroup-B IMD, including serogroups C, W and Y [16,17,18].
In Europe, most countries have implemented infant and adolescent immunization programs using meningococcal C vaccine only, or a combination of meningococcal C and meningococcal ACWY vaccine [19]. Introduction of 4CMenB in the national immunization schedule is heterogeneous across European countries [19, 20]. In France, a cost-effectiveness model was developed and a study using this model concluded that none of the investigated vaccination strategies was cost-effective [1]. Based on this model, the French Public Health Council (Haut Conseil de la Santé Publique-HCSP) initially recommended 4CMenB only for individuals at increased risk of IMD [21]. However, since the publication of the initial results, new real-world effectiveness and safety data from other countries have become available, and the French High Authority of Health (HAS) modified the recommendation to include 4CMenB in the vaccination schedule for all infants [15, 22, 23].
The main goal of the analyses presented here was to assess the impact of an accurate description of the disease burden in patients and caregivers on public health and the cost-effectiveness of 4CMenB, using the most recent demographic, epidemiological, and cost data for France. Another goal was to illustrate the effect of model choices and recommendations from technical guidelines on cost-effectiveness.
2 Methods
2.1 Model Structure
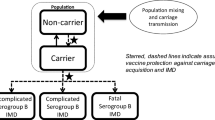
The cost-effectiveness of the 4CMenB vaccine was evaluated by adapting a validated, published, dynamic transmission-based cost-effectiveness (DyCE) model to the French context [24, 25]. In this model, the number of IMD cases over time with and without vaccination is described using a ‘susceptible–infected–susceptible’ model. The model distinguishes between three categories of serogroups, that is, serogroup B, serogroups ACWY (considered as one group), and ‘other’ (Fig. 1). Furthermore, the model allows the impact of vaccination on cross-protection and carriage to be evaluated. The contact matrix was derived from French-specific data [26]. Carriage prevalence across age groups for different serogroups was derived from a meta-analysis, which included data from 89 cross-sectional and longitudinal studies reporting on nasopharyngeal meningococcal carriage by age group [5]. The model was calibrated against French-specific IMD incidence by serogroup between 2009 and 2019, by adjusting the force of infection and the probability of infection for individuals being carriers of a specific serogroup. Model assumptions were validated by independent experts, as recommended by the French health technology assessment body [27] (Online Resource 1, electronic supplementary material [ESM] Table 1).

Adapted from Beck et al., 2020 [25]. Ψvi Proportion of people effectively protected, calculated by multiplying vaccination coverage per vaccine efficacy, λi, x infection force at age i for IMD strain x (x = B, ACWY, other); Ri, x clearance rate at age I for IMD strain x (x = B, ACWY, other). IMD invasive meningococcal disease, IMD ACWY serogroup A, C, W or Y invasive meningococcal disease, IMD B serogroup B invasive meningococcal disease
Schematic of the dynamic transmission model/decision-tree model.
Output from the dynamic transmission model served as input for a decision-tree analysis, which evaluated the health and cost outcomes for each vaccination strategy [24]. Additional input parameters for the decision tree included the proportion of patients with IMD who developed sequelae, case-fatality rate, quality of life (QoL), and cost data (Table 1).
The base case analysis adopted a collective perspective, while one scenario analysis was carried out from a societal perspective (including productivity losses and other types of informal care costs). The collective perspective covers all people or institutions affected by the production of an intervention in terms of health effects or costs. This covers informal care, home healthcare, inpatient and outpatient care, as well as personal care services associated with the health issue. Health and cost outcomes were calculated over a life-time horizon (i.e., 100-year time horizon), in line with other cost-effectiveness modeling studies [28,29,30]. The first year considered was 2020; for each subsequent year, a new birth cohort entered the model and was followed for 100−x years, where x was the number of years after 2020. A discount rate of 2.5% for costs and health outcomes was applied for the first 30 years, followed by a discount rate of 1.5% thereafter, as recommended in the 2020 health economics guidelines issued by HAS [27].
The statistical software R was used for the dynamic transition model [31]. The model was implemented in Excel 365 via BERT, which allows for seamless linkage between the two software packages [32].
2.2 Population
The model considered the entire French population stratified by age as of January 2020 [33]. The population eligible for vaccination was the birth cohort of each year, with infants receiving three doses of 4CMenB, at 3, 5, and 13 months.
2.3 Intervention and Comparator
The 4CMenB vaccination strategy was compared with no vaccination strategy, as there is no alternative serotype B-specific vaccine currently approved for use in France.
2.4 Outcomes
MenB IMD incidence and related deaths, MenB survivors who developed sequelae, costs, and quality-adjusted life-years (QALYs) were evaluated for each vaccination strategy; incremental cost-effectiveness ratios (ICERs) were calculated for the base case and for each scenario analysis, considering benefits and costs in the overall population over a 100-year time horizon.
2.5 Model Input Parameters
2.5.1 Demographics and IMD Epidemiology
The French population count by age group as of January 2020 was extracted from the National Institute of Statistics and Economic Studies (INSEE) database [33]. Mortality rates for 2019 were obtained from the National Institute for Demographic Studies (INED) [34]. The birth cohort for 2020 (infants aged < 1 year) was estimated to be 686,900 in 2020, based on 2018 data from INSEE and annual growth projections [35].
IMD is a notifiable disease in France, and incidence rates are published each year by Public Health France (Santé Publique France–SPF) [10]. Age-specific IMD incidence rates were calculated by serogroup, based on cases notified between 2009 and 2019 and corrected for incomplete notification (Table 1). A notification rate of 92% was reported in a French study evaluating completeness of IMD notification in France; this rate was confirmed by SPF in 2017 [36, 37]. IMD incidence varies in an unpredictable fashion over time; therefore, a smoothing function was applied to the incidence rate, in line with a previously developed health economic model for 4CMenB [1]. In 2019, IMD incidence (all serotypes) was estimated to be 0.76 cases per 100,000 inhabitants, while IMD B incidence was estimated to be 0.36 per 100,000 individuals [37]. Age-adjusted incidence and the relative proportion of MenB IMD to all IMD cases are provided in Table 1 and Online Resource 1, ESM Table 2). Serogroup-specific mortality rates for IMD cases were derived from data from SPF (Table 1). The proportion of patients who developed sequelae was obtained through a retrospective analysis of the national healthcare insurance database (Système National d’Information Interrégimes de l’Assurance Maladie–SNIIRAM) (Table 1) (Online Resource 1, ESM Table 3) [38]. The types of sequelae were validated through a systematic literature search of observational and medico-economic studies and independent assessment of their clinical relevance by three independent experts [3].
2.5.2 4CMenB Efficacy and Waning
A vaccination coverage of 95% for the first and second doses and 90% for the third dose of 4CMenB was assumed. Vaccination coverage was assumed to be high and similar to that reported for other infant vaccines recommended by the French Health Authority [22, 43]. Vaccine effectiveness was derived from real-world data, including the latest updated report from Public Health England; in England, 4CMenB vaccination was included in the national immunization schedule in 2015 (Table 1) [41]. The immunogenicity conferred by 4CMenB vaccination decreases over time. The model assumed an exponential decrease in vaccine efficacy, in line with other meningitis vaccination models [29, 44,45,46], and the duration of protection was estimated to be 33 months after the first and second doses and 38 months after the third dose (Table 1).
2.5.3 Utility Data
French utility values by age group in the general population were used as reference value; utility values were obtained via the EQ-5D-3L, a validated tool for QoL measures [47]. Utility decrements were applied to these reference values, distinguishing between the acute phase with and without sequelae and the long-term phase governed by sequelae (Table 2). Utility decrements for the acute phase were sourced from a study reporting on utility values in an English IMD cohort and decrements due to sequelae were applied as reported in a previous model for England [28, 48]. Utility values for specific sequelae were identified through a literature search, giving preference to studies using the EQ-5D tool, as recommended by the HAS health economic guidelines (Online Resource 1, ESM Table 3) [27]. A reduction in QoL was also assumed for family networks of IMD survivors, corresponding to 48% of the utility decrement experienced by survivors (range: 17–79%), both during the acute and the long-term phases [49]. For parents losing a child to IMD, the model assumed a QALY loss proportional to the QALY loss induced by the premature death of the child, corresponding to 9% of QALYs lost by the patient (range: 0–9.6%) [50].
2.5.4 Cost Data
The following cost items were considered in the model: vaccination costs; costs for fever management following vaccination; cost of acute IMD care; cost of sequelae (acute and long-term); outbreak management costs, including logistics for the identification of contact persons and deployment of preventive treatment; and costs associated with disability, such as allowance, special education, and institutionalization (Table 2, Online Resource 1, ESM Table 4). The most recent costs available (2016–2018 costs, except for the cost of public education, which was available for 2014 only) were used in the model. According to The Directorate of Research, Studies, Evaluation, and Statistics (DREES), costs related to goods and services have undergone minimal changes between 2014 and 2018 (< 2%) [59]. Furthermore, the index might not systematically include items related to allowances perceived by people needing special education and institutionalization. Therefore, the expected error introduced due to different reference years is considered marginal.
It was assumed that 4CMenB would be administered during routine infant health check-ups, hence no additional costs for a medical consultation were considered. The proportions of infants requiring medical attention due to fever after the first (0.96%), second (1.68%), and third dose (1.65%) were derived from clinical studies [60,61,62,64]. Costs for outpatient visits were taken from official French tariffs [64]. The proportion of individuals requiring hospitalization (0.17%) due to 4CMenB-related fever was derived from a study conducted in the UK [65].
Costs associated with IMD during the acute and long-term phases (including costs related to sequelae) were estimated by means of a retrospective database analysis, extracting data from the SNIIRAM database for the period January 2012 to December 2017. IMD cases were identified using the tenth revision of the International Classification of Diseases (ICD) codes (v.10), and the index date was set to the first diagnosis with a meningitis-related ICD-10 code. For the acute phase, transfers occurring immediately prior and/or subsequent to the index hospitalization were grouped together to yield a combined overall length of hospitalization and costs related to the acute phase. The mean length of stay was 14.8 days (95% confidence interval [CI] 14.0–15.6), and the mean cost was €11,269 (95% CI 10,869–11,643) [12]; costs by age group are provided in Table 2.
IMD sequelae were identified by searching for several indicators that occurred within a given timeframe from the index date. ICD-10 codes for primary, associated, and secondary diagnosis; reimbursement codes for prescription drugs and medical equipment specific for the treatment of sequelae (e.g., implants for hearing loss); and medical procedures or hospitalizations related to sequelae were identified by a pre-specified algorithm that has been validated by independent experts (Online Resource 1, ESM Table 3) [12]. Costs were estimated separately for the first year after IMD diagnosis and the following years. Costs due to sequelae were adjusted using a multivariable regression model to estimate incremental costs due to sequelae compared with observed costs in subjects without sequelae (Online Resource 1 ESM Table 3).
Families of children with IMD sequelae receive allocations to cover educational expenses and care (Allocation d’Education de l’Enfant - Handicapé – AEEH) [66]. These allocations are proportional to a child’s disability and are financed by the national social security system [67]; therefore, these costs form part of the collective perspective.
People with a disability are eligible for compensation services due to their disability (Prestation Compensation du Handicap—PCH), which comprises financial aid personalized for each individual who has limited autonomy due to a disability [68, 69]. For both AEEH and PCH, mean cost was calculated, considering the total number of beneficiaries in France and total aid received annually. The mean annual costs estimated for AEEH and PCH were €3667 and €6433, respectively (Online Resource 1 ESM Table 4).
Parents who stop working to care for their child during the acute phase of IMD are eligible for an additional allowance (Allocation Journalière de Présence Parentale – AJPP). An average time of 6 months off work was assumed, based on expert opinion which considered results from a survey of affected families and published literature [13]. A single cost of €5905 was estimated for AJPP.
Children with IMD sequelae can be schooled in specialized or public facilities, provided that appropriate support and logistics are available. The incremental costs related to specialized facilities, human resources, and specific materials are detailed in Online Resource 1 ESM Table 3. Similarly, adults with a disability are also eligible for various types of financial and material support (Online Resource 1 ESM Table 4).
For local outbreaks comprising two or more IMD cases in the same place and at the same time, a limited vaccination campaign is recommended, to provide protection to any exposed groups (e.g., high-school students if cases occurred in this setting) [70]. In France, two local MenB IMD outbreaks were recently reported [71, 72]. Costs for outbreak management in the present modeling study were estimated based on information from these cluster outbreaks, as well as another outbreak that involved MenW [73]. For isolated cases, contagion management is limited to antibiotic prophylaxis and immunization of contacts. Mean costs due to local outbreaks and isolated cases were estimated at €1,078,688 and €132, respectively, leading to a weighted mean of €21,703.55 for outbreak management (Online Resource 1 ESM Table 4).
2.6 Sensitivity and Scenario Analyses
Deterministic and probabilistic sensitivity analyses (DSA and PSA) were carried out for predefined parameter ranges (Tables 1 and 2). For PSA, 1000 simulations were carried out; at each iteration, input variables were sampled within the pre-specified limits of uncertainty and using the distribution shown in Online Resource 1 ESM Table 5. Twelve scenario analyses were conducted to investigate the impact of model structure parameters, vaccine effectiveness, and epidemiological parameters on ICERs. Parameter assumptions that were modified included discount rates, the QoL adjustment factor, residual cross-protection of 4CMenB against serotypes W and Y, carriage, the number and proportion of sequelae considered, and the impact of MenB IMD on life expectancy. Further information is provided in Online Resource 1 ESM Tables 3 and 6.
3 Results
In this section, the focus is on MenB IMD-related outcomes, assuming a vaccination schedule in infants at 3, 5, and 13 months of age.
3.1 Public Health Impact
In the absence of vaccination, an estimated 32,120 MenB IMD cases would occur over a 100-year time horizon. In the base case analysis, 6845 IMD cases would be prevented, corresponding to a 21% reduction compared with no vaccination. The impact was highest in the youngest age groups, with 3101 out of 5786 cases (a 54% reduction) prevented in infants aged < 1 year and 3440 out of 6625 cases (a 52% reduction) in children aged 1–4 years (Table 3). Overall, 2372 MenB IMD-related sequelae and 438 deaths would be avoided. To prevent one case of MenB IMD, 9084 infants would need to be vaccinated. There is no predicted health impact in adolescents and adults (Online Resource 1 ESM Fig. 1).
3.2 Health Economic Outcomes
3.2.1 QALYs
In the absence of vaccination, MenB IMD would lead to a loss of 92,387 QALYs over a 100-year time horizon (397,961 undiscounted QALYs). Vaccination with 4CMenB would decrease QALY loss by 22.4%, to 71,712 QALYs lost (Table 3). When no discounting was applied, QALYs lost would be markedly higher in both scenarios, that is, 397,961 QALYs lost without vaccination and 297,785 QALYs lost with vaccination. The difference can be explained by the fact that long-term sequelae were the main contributors to total QALYs lost, accounting for 55.5% and 57.2% with and without vaccination, respectively (discounted values). Considering the number of MenB IMD cases over a 100-year time horizon and QALYs lost in the absence of vaccination, one case of MenB IMD would lead to 2.9 or 12.4 discounted or undiscounted QALYs lost, respectively.
3.2.2 Costs
Immunization of infants with a three-dose vaccination schedule with 4CMenB leads to vaccination costs of approximately €7 billion (discounted) (Online Resource 1 ESM Table 7). In the absence of vaccination, direct costs due to MenB IMD would amount to €1.9 billion, and costs due to long-term sequelae would contribute 47% of total direct costs (Fig. 2A). Allowances and public healthcare management costs due to outbreaks contributed 20% and 15% to total direct costs, respectively. In the case of the 4CMenB vaccination strategy, vaccination costs account for 82% of total costs and are responsible for an increase of 77% of total direct costs in the 4CMenB vaccination strategy (Fig. 2B). On the other hand, MenB IMD-related costs are reduced to €1.48 billion, leading to IMD-related direct cost savings of approximately €0.44 billion. Cost savings are mostly driven by costs related to the medical treatment of long-term sequelae and costs for special education needs and allowances (Table 3).
Contributors to direct costs and direct cost-savings with 4CMenB vaccination: 100-year time horizon. (A) Relative contribution of cost items to total direct costs with no vaccination. (B) Relative contribution of cost items to total direct costs with vaccination. 4CMenB multicomponent 4CMenB: meningococcal serogroup B vaccine
3.2.3 Cost-Effectiveness Analysis
Over a 100-year time horizon, the incremental costs (discounted) with 4CMenB vaccination were estimated to be €6.5 billion, while 20,674 QALYs were gained with the infant vaccination strategy compared with no vaccination. The resulting ICER was €316,272/QALY (Table 4). The cost-effectiveness analysis results per life-year saved are presented in Online Resource 1 ESM Table 8.
3.2.4 Scenario Analyses
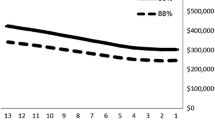
In the scenario analyses, discount rates had the largest impact on estimated ICERs, with lower discount rates leading to more favorable ICER values. When discounting only costs, but not outcomes, to reflect the long-term benefits of vaccination, the ICER was €65,272/QALY, corresponding to a reduction of 79% compared with the base case analysis (Table 4). Including the impact of 4CMenB immunization on cross-protection and carriage led to a lower ICER of €165,539/QALY, a reduction of 48%. On the other hand, assuming slightly lower vaccine effectiveness values for 4CMenB, based on a case–control study carried out in Portugal, the ICER was higher (€380,516/QALY; +20%). Finally, several alternative epidemiological assumptions were explored. Assuming lower life-expectancy in MenB IMD survivors would reduce the ICER (€215,754/QALY; −32%). On the other hand, applying the same sequelae and the same proportions of occurrence as in the Lecocq model from the HCSP evaluation [1], the resulting ICER was higher (€470,029/QALY; +49%). In the HCSP model, several sequelae linked to IMD were absent, such as kidney disease, language and speech problems, motor deficits, depression, and anxiety. When increasing the QoL adjustment factor from 1 to 3, which accounts for society’s preference to prevent severe disease, the estimated ICER was €143,182/QALY, that is, a reduction of 55% compared with the base case analysis. Vaccine effectiveness parameters also had a marked impact on the estimated ICERs. Additional scenario analyses are summarized in Fig. 3 and Table 4.
3.2.5 Deterministic Sensitivity Analyses (DSAs)
A total of 124 parameters were included in the model that could be varied according to pre-specified lower and upper bound estimates or confidence intervals. Some of these parameters were grouped together into categories for DSA, leading to 35 categories that were analyzed separately. Parameters with the largest impact on ICER were MenB IMD incidence (+147% to −30% with respect to the base case ICER), 4CMenB effectiveness (+61% to −6% with respect to the base case ICER), and impact of IMD on the QoL of parents (+15% to −12% with respect to the base case ICER) (Online Resource 1 ESM Fig. 2, ESM Table 9). Other parameters in the DSA led to relatively modest changes of ≤ 10% (Online Resource 1 ESM Table 9).
3.2.6 Probabilistic Sensitivity Analyses (PSA)
In the PSA, 1000 iterations were performed that resulted in an average ICER of €363,571/QALY (+15% compared with the base case analysis). Overall, 90% of simulations yielded an ICER ≤ €520,000/QALY (Online Resource 1 ESM Fig. 3).
3.2.7 Model Validation
The model was validated internally, by independent analysts, to ensure that it performed correctly and that results were consistent with expected outcomes for the selected input parameters. Additional validation was carried out by independent experts, who assessed the model structure and assumptions regarding model parameters.
4 Discussion
The French health authority has revised its recommendation regarding 4CMenB vaccination in 2021 to extend vaccination to all infants, despite a relatively high estimated cost-effectiveness ratio. This decision was based on several considerations, including the severity of disease and emerging real-world data from other countries.
In this study, we evaluated the effect of input parameters and model choices on public health impact and cost-effectiveness of 4CMenB, emphasizing the need for a holistic approach in assessing the benefit of vaccination. There is no official cost-effectiveness threshold being used by the French Health Agency for reimbursement purposes, but ICER thresholds between $50,000 and $200,000/QALY are commonly used in other legislations [77]. Using updated, French-specific epidemiological and cost data, and applying model recommendations issued by HAS, the estimated ICER was €316,272/QALY for infant vaccination with 4CMenB. Scenario analyses showed that ICERs were most sensitive to discount rates, QoL adjustment factor, inclusion of cross-protection against non-serogroup B strains and impact on carriage, life-expectancy of MenB IMD survivors, as well as the comprehensiveness of the long-term sequelae considered. Our DSA identified MenB IMD incidence as having the largest impact on ICER, with lower incidence rates leading to higher ICERs. ICERs ranged between €65,272/QALY and €493,218/QALY in scenario and sensitivity analyses.
In the base case analysis, discount rates were set to 2.5% for costs and outcomes during the first 30 years and 1.5% thereafter, according to French guidelines. In the previous HCSP model, higher discount rates (4% during the first 30 years and 2% thereafter) were used, based on guideline recommendations which were in place at the time. Discount rates had a major impact on ICERs, as was seen with other cost-effectiveness models for 4CMenB [28, 29, 78]. The influential nature of discount rates stems from the long time horizon that must be considered for vaccines, the full health benefits of which only appear over time, such as reducing long-term costs due to sequelae. The appropriate choice of discount rates is still under discussion [79]. While most health economic guidelines recommend that the same discount rate for costs and health outcomes should be applied, some argue that benefits should be discounted at a lower rate compared with costs [79,80,81,82]. Differential discounting has been applied in cost-effectiveness analyses of MenC vaccination in France and 4CMenB vaccination in Belgium [78, 83]. Therefore, scenario analyses with different choices for discounting were carried out and showed a large impact on ICERs, which varied between €65,272/QALY and €493,218/QALY. Applying lower discount rates to benefits than to costs resulted in lower ICERs (€65,272/QALY to €176,407/QALY). In another scenario analysis, the QoL adjustment factor was increased from 1 to 3, to account for society’s preference to prevent severe diseases; this resulted in a markedly lower ICER compared with the base case analysis (€143,182/QALY). Such an approach was endorsed by the UK’s Joint Committee on Vaccination and Immunization in the context of IMD and has been applied in other cost-effectiveness analyses [28, 84].
Several scenario analyses were carried out to explore the impact of vaccine characteristics. Inclusion of cross-protection and carriage had a favorable effect on ICERs, leading to an estimated ICER of €165,539/QALY (− 48%). The assumption of cross-reactivity is based on studies evaluating bactericidal activity of sera from infants immunized with 4CMenB against serogroups C, W, and Y [16]. Using lower estimates for serogroup B vaccine effectiveness led to an increase in ICER of approximately 20% (€380,516/QALY). In the base case analysis, vaccine effectiveness parameters were derived from the UK progress report on 4CMenB coverage and effectiveness, comprising data for the period 2015–2018 [41]. Slightly lower vaccine effectiveness values were reported for a case–control study conducted in Portugal [85]. This highlights the sensitivity of ICER estimates to changes in model assumptions.
Finally, the impact of several epidemiological parameters was evaluated, including the occurrence of sequelae and the impact of reduced life-expectancy in MenB IMD survivors. Sequelae are the main contributing factor to QALYs lost and account for 47% of direct costs due to MenB IMD in the absence of vaccination. Therefore, adequate modeling of sequelae is paramount in estimating health-economic outcomes related to MenB IMD [12]. An analysis of a French health database was carried out to obtain an accurate description and understanding of the number of MenB IMD-related sequelae and the proportion of patients developing sequelae in France [3]. To illustrate the impact of sequelae input parameters on ICERs, a scenario analysis using the sequelae considered in the HCSP model was carried out. In this model, several sequelae, such as kidney disease, severe neurological disorders, depression, and anxiety were not included; the resulting ICER was €470,029/QALY, corresponding to an increase of 49% compared with the base case analysis. The base case analysis used the conservative assumption that MenB IMD had no impact on life-expectancy in survivors. In a scenario analysis, life-expectancy was adjusted to account for a reduction in MenB IMD survivors, which led to a lower ICER (€215,754/QALY; −32%) [76].
The impact of MenB IMD incidence was only assessed via a DSA. The base case analysis used updated MenB IMD incidence data derived from the SPF database (Online resource 1 ESM Table 10). Annual incidence rates were lower (0.36 MenB IMD cases/100,000 population) during the time period considered in the updated model (2009–2019) compared with the incidence rates used in the HCSP model (0.78 MenB IMD cases/100,000 population) for the time period 2003–2011 [1]. Lower incidence rates lead to higher ICERs, as there are fewer MenB IMD cases avoided over time. A trend toward lower IMD incidence rates was also observed by Christensen et al. (2014), but as noted by the authors, currently low incidence rates may increase again in the future [28]. Indeed, increasing IMD incidence rates have been observed in France since November 2022, mostly driven by serogroups B, Y, and W [86].
Other cost contributors considered in the model that affect ICERs were allowances, which are indirectly linked to sequelae, and represent another 20% of total direct costs. Outbreak management accounted for 15% of total direct costs, while costs related to the acute phase corresponded to just 8% of total direct costs. These results underline the importance of adopting a broad perspective when assessing costs incurred by MenB IMD. A fully holistic approach for valuing the costs and benefits of vaccines has been advocated in recent literature and should include the impact of IMD on caregivers and families and productivity losses [24, 81]. In a cost-effectiveness model carried out for the UK, the impact of the adopted perspective on ICERs was evaluated in a stepwise approach, starting from a narrow, payer’s perspective that only included direct costs incurred during the acute phase and thereafter adding a) long-term costs due to sequelae, b) impact on family and caregivers, c) outbreak management and productivity losses associated with the acute phase, d) a consideration of society’s preference for the prevention of uncommon but devastating diseases, and e) reducing the discount rate to 1.5% to reflect the long-term burden of the disease [24]. The ICER of 4CMenB following these modifications in model assumptions decreased from British pounds (GBP) 360,595 to GBP18,645/QALY [24]. A similar picture emerged from a health-economic analysis of 4CMenB vaccination in Germany, where ICERs decreased upon inclusion of the impact of IMD on the quality of life of caregivers and family and applying a lower discount rate for health outcomes (1%) [87].
The estimated public health impact of 4CMenB in the present study was comparable to that of other health economic studies. MenB IMD cases avoided varied between 40 and 46% in infants/children aged 0–4 years [24, 87] and between 14 and 26% when considering the entire population [1, 28, 29, 87]. The relatively low case-avoidance in the overall population might be explained by the waning of 4CMenB efficacy, which renders immunized children susceptible again to MenB infection 3 years post-vaccination [28]. There is limited information regarding antibody persistence following 4CMenB vaccination, and further studies are needed to evaluate long-term protection and the optimal timing of a potential booster dose in children and adolescents [42, 88].
The strength of our model resides in the inclusion of updated, French-specific data for MenB IMD incidence and the frequency of sequelae and associated costs through analyses of SPF, SNIIRAM, and other French healthcare databases. Database analyses were preceded by systematic literature reviews, to provide a representative list of sequelae related to IMD and the impact of these sequelae on quality of life (utilities). This led to a more complete picture of the long-term consequences of MenB IMD and an improved understanding of MenB IMD in the current French context. Furthermore, extensive scenario analyses were conducted which allowed us to evaluate the influence of model choices, such as discount rates, carriage and/or cross-protection, and life-expectancy of MenB IMD survivors, on outcomes.
There are several limitations to this model. We used a variety of sources, originating from multiple countries, to populate the model’s input parameters, carrying an inherent risk of uncertainty, such as transferability of input values to the French setting. The impact of this uncertainty around input parameters was evaluated in our DSA, which showed that MenB IMD incidence had the greatest impact on ICER, consistent with other health economic models [89]. IMD incidence fluctuates over time in an erratic and unpredictable way [24, 89]. In this study, incidence data were updated using surveillance data for 2009–2019. In recent years, MenB IMD incidence has decreased, which leads to less favorable ICER estimates; therefore, ICER estimates of the updated model are based on low, conservative IMD incidence rates. The latest reported IMD incidence rates in France point toward an increasing trend [86], highlighting the need to interpret the model’s results in light of the current epidemiological situation.
5 Conclusion
Vaccination with 4CMenB is an effective strategy to decrease MenB IMD incidence and reduce costs due to long-term sequelae. These sequelae have a high impact on QoL and costs, emphasizing the need for a broad perspective when assessing the public health impact and cost-effectiveness of vaccines and for 4CMenB in particular. While 4CMenB appears not to be cost-effective under base case assumptions, results were highly sensitive to model parameters that were used to estimate the expected outcomes of an immunization program. Our study also provided insights on the impact of an enlarged scope on cost-effectiveness of vaccination strategies.
References
Lecocq H, Parent de Chatelet I, Taha MK, et al. Epidemiological impact and cost-effectiveness of introducing vaccination against serogroup B meningococcal disease in France. Vaccine. 2016;34(19):2240–50. https://doi.org/10.1016/j.vaccine.2016.03.020.
Viner RM, Booy R, Johnson H, et al. Outcomes of invasive meningococcal serogroup B disease in children and adolescents (MOSAIC): a case-control study. The Lancet Neurology. 2012;11(9):774–83. https://doi.org/10.1016/s1474-4422(12)70180-1.
Shen J, Begum N, Ruiz-Garcia Y, et al. Range of invasive meningococcal disease sequelae and health economic application—a systematic and clinical review. BMC Public Health. 2022;22(1):1078. https://doi.org/10.1186/s12889-022-13342-2.
European Centre for Disease Prevention and Control (ECDC). Invasive meningococcal disease: annual epidemiological report for 2017. Stockholm. 2019. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-invasive-meningococcal-disease.pdf. Accessed 8 Nov 2022.
Christensen H, May M, Bowen L, et al. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(12):853–61. https://doi.org/10.1016/s1473-3099(10)70251-6.
Girard MP, Preziosi MP, Aguado MT, et al. A review of vaccine research and development: meningococcal disease. Vaccine. 2006;24(22):4692–700. https://doi.org/10.1016/j.vaccine.2006.03.034.
Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369(9580):2196–210. https://doi.org/10.1016/S0140-6736(07)61016-2.
Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol. 2012;799:1–20. https://doi.org/10.1007/978-1-61779-346-2_1.
Deghmane AE, Taha MK. Changes in invasive Neisseria meningitidis and Haemophilus influenzae infections in France during the COVID-19 pandemic. Microorganisms. 2022. https://doi.org/10.3390/microorganisms10050907.
Santé Publique France (SPF). Invasive meningococcal infections in France in2017 [Les infections invasives à méningocoque en France en 2017]. 2017. Available from: http://invs.santepubliquefrance.fr/content/download/148831/541618/version/4/file/Bilan_IIM_2017.pdf. Accessed 24 Jun 2022.
Santé publique France (SPF). Invasive meningococcal infections in France in 2022 [Les infections invasives a meningocoque en France en 2022]. 2023. Available from: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-a-prevention-vaccinale/infections-invasives-a-meningocoque/documents/bulletin-national2/infections-invasives-a-meningocoque-en-france-en-2022. Accessed 14 Apr 2023.
Weil-Olivier C, Taha MK, Emery C, et al. Healthcare resource consumption and cost of invasive meningococcal disease in France: a study of the National Health Insurance Database. Infect Dis Ther. 2021;10(3):1607–23. https://doi.org/10.1007/s40121-021-00468-w.
Bénard S, Wright C, Voisine J, et al. Lifetime cost of meningococcal disease in France: scenarios of severe meningitis and septicemia with purpura fulminans. J Infect Public Health. 2016;9(3):339–47. https://doi.org/10.1016/j.jiph.2015.10.016.
Vidal. Vaccines against meningococcal meningitis [Les vaccins contre les méningites à méningocoques]. 2022. Available from: https://www.vidal.fr/medicaments/utilisation/vaccins/vaccin-meningite-meningocoque.html. Accessed 1 Dec 2022.
Parikh SR, Andrews NJ, Beebeejaun K, et al. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet. 2016;388(10061):2775–82. https://doi.org/10.1016/S0140-6736(16)31921-3.
Biolchi A, De Angelis G, Moschioni M, et al. Multicomponent meningococcal serogroup B vaccination elicits cross-reactive immunity in infants against genetically diverse serogroup C, W and Y invasive disease isolates. Vaccine. 2020;38(47):7542–50. https://doi.org/10.1016/j.vaccine.2020.09.050.
Castilla J, García Cenoz M, Abad R, et al. Effectiveness of a Meningococcal Group B Vaccine (4CMenB) in Children. N Engl J Med. 2023;388(5):427–38. https://doi.org/10.1056/NEJMoa2206433. (PMID: 36724329).
Ladhani SN, Campbell H, Andrews N, et al. First real-world evidence of meningococcal group B vaccine, 4CMenB, protection against meningococcal group W disease: prospective enhanced national surveillance, England. Clin Infect Dis. 2021;73(7):e1661–8. https://doi.org/10.1093/cid/ciaa1244.
European Centre for Disease Prevention and Control (ECDC). Vaccine Scheduler: European Centre for Disease Prevention and Control (ECDC). 2022. Available from: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=48&SelectedCountryIdByDisease=-1. Accessed 13 Nov 2022.
European Centre for Disease Prevention and Control (ECDC). Expert opinion on the introduction of the meningococcal B (4CMenB) vaccine in the EU/EEA. Stockholm. 2017. Available from: https://www.ecdc.europa.eu/en/publications-data/expert-opinion-introduction-meningococcal-b-4cmenb-vaccine-eueea. Accessed 13 Nov 2022.
Haut Conseil de Sante Publique (HCSP). Cost-effectiveness analysis of Bexsero® vaccine for serogroup B invasive meningococcal diseases (IMD B) [Analyse coût/efficacité de la vaccination par le vaccin Bexsero® contre les infections invasives à méningocoque de sérogroupe B (IIM B)]. 2013. Available from: https://www.hcsp.fr/Explore.cgi/Telecharger?NomFichier=hcspr20131025_vaccmeningocoqueBBexseroann1.pdf Accessed 16 Dec 2022.
Haute Autorité de Santé (HAS). Vaccination strategy for the prevention of invasive meningococcal diseases: Serogroup B and the place of BEXSERO® [Stratégie de vaccination pour la prévention des infections invasives à méningocoques : Le sérogroupe B et la place de BEXSERO®]. 2021. Available from: https://www.has-sante.fr/jcms/p_3066921/fr/strategie-de-vaccination-pour-la-prevention-des-infections-invasives-a-meningocoques-le-serogroupe-b-et-la-place-de-bexsero. Accessed 1 Dec 2022.
Rodrigues F, Marlow R, Simoes MJ, et al. Protocol summary: case-control study to evaluate the effectiveness of 4CMenB vaccine in the prevention of invasive meningococcal disease in Portugal. 2019. European Meningococcal and haemophilus Disease Society (EMGM). Lisbon, Portugal. Available from: https://emgm.eu/meetings/emgm2019/emgm2019_abstracts.pdf. Accessed 01 Dec 2022
Beck E, Klint J, Neine M, et al. Cost-effectiveness of 4CMenB infant vaccination in England: A comprehensive valuation considering the broad impact of serogroup B invasive meningococcal disease. Value Health. 2021;24(1):91–104. https://doi.org/10.1016/j.jval.2020.09.004.
Beck E, Klint J, Garcia S, et al. Modelling the impact of 4CMenB and MenACWY meningococcal combined vaccination strategies including potential 4CMenB cross-protection: an application to England. Vaccine. 2020;38(47):7558–68. https://doi.org/10.1016/j.vaccine.2020.08.007.
Béraud G, Kazmercziak S, Beutels P, et al. The French connection: the first large population-based contact survey in France relevant for the spread of infectious diseases. PloS ONE. 2015;10(7):e0133203. https://doi.org/10.1371/journal.pone.0133203.
Haute Autorité de Santé (HAS). Methodological choices for economic evaluation - HAS [Choix méthodologiques pour l'évaluation économique à la HAS]. Saint-Denis la Plaine. 2020. Available from: https://www.has-sante.fr/upload/docs/application/pdf/2020-07/guide_methodologique_evaluation_economique_has_2020_vf.pdf. Accessed 24 Jun 2022.
Christensen H, Trotter CL, Hickman M, et al. Re-evaluating cost effectiveness of universal meningitis vaccination (Bexsero) in England: modelling study. BMJ. 2014;349:g5725. https://doi.org/10.1136/bmj.g5725.
Christensen H, Irving T, Koch J, et al. Epidemiological impact and cost-effectiveness of universal vaccination with Bexsero((R)) to reduce meningococcal group B disease in Germany. Vaccine. 2016;34(29):3412–9. https://doi.org/10.1016/j.vaccine.2016.04.004.
Huels J, Clements K, McGarry L, et al. Modelled evaluation of multi-component meningococcal vaccine (Bexsero®) for the prevention of invasive meningococcal disease in infants and adolescents in the UK. Epidemiol Infect. 2014;142(9):2000–12. https://doi.org/10.1017/S095026881300294X.
R core team. R: a language and environment for statistical computing [Computer software]. 2020. Vienna, Austria. Available from: www.R-project.org.
BERT. Basic Excel R Toolkit [Computer software]. 2018. San Francisco, California, USA. Available from: https://bert-toolkit.com/.
Institut National de la Statistique et des Etudes Economiques (INSEE). Tables of the French economy [Tableau de l’économie française]. 2020 Edition, France: Institut National de la Statistique et des Etudes Economiques (INSEE). 2020. Available from: https://www.insee.fr/fr/statistiques/4277619?sommaire=4318291. Accessed 24 Jun 2022.
Institut National d'Etudes Démographiques (INED). Mortality rates by sex and age in 2020 [Taux de mortalité par sexe et âge en 2020]. France. 2022. Available from: https://www.ined.fr/fr/tout-savoir-population/chiffres/france/mortalite-cause-deces/taux-mortalite-sexe-age/. Accessed 16 Jul 2022.
Institut National de la Statistique et des Etudes Economiques (INSEE). Population projections 2013-2070 for France [Projections de population 2013-2070 pour la France]. France. 2016. Available from: https://www.insee.fr/fr/statistiques/2496793. Accessed 16 Dec 2022.
Berger F, Parent du Châtelet I, Bernillon P, et al. Surveillance of the invasive meningococcal infections in France in 2005: Quantitative evaluation by the three sources capture-recapture method [Surveillance des infections invasives à méningocoque en France métropolitaine en 2005 – Évaluation quantitative par la méthode de capture-recapture à trois sources]. Sanitaire Idv, editor. Saint-Maurice, FR. 2010. Available from: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-a-prevention-vaccinale/infections-invasives-a-meningocoque/documents/rapport-synthese/surveillance-des-infections-invasives-a-meningocoque-en-france-metropolitaine-en-2005.-evaluation-quantitative-par-la-methode-de-capture-recapture. Accessed 24 Jun 2022
Santé Publique France (SPF). Invasive meningococcal infections in France in 2019 [Les infections invasives à méningocoque en France en 2019]. 2020. Available from: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-a-prevention-vaccinale/infections-invasives-a-meningocoque/documents/bulletin-national2/les-infections-invasives-a-meningocoque-en-france-en-2019. Accessed 24 Jun 2022.
Weil-Olivier C, Taha MK, Bouee S, et al. Care pathways in invasive meningococcal disease: a retrospective analysis of the French national public health insurance database. Hum Vaccin Immunother. 2022;18(1):2021764. https://doi.org/10.1080/21645515.2021.2021764.
Cohen R, Gaudelus J, Leboucher B, et al. Impact of mandatory vaccination extension on infant vaccine coverages: Promising preliminary results. Med Mal Infect. 2019;49(1):34–7. https://doi.org/10.1016/j.medmal.2018.10.004.
Bryan P, Seabroke S, Wong J, et al. Safety of multicomponent meningococcal group B vaccine (4CMenB) in routine infant immunisation in the UK: a prospective surveillance study. Lancet Child Adolesc Health. 2018;2(6):395–403. https://doi.org/10.1016/S2352-4642(18)30103-2.
Public Health England (PHE). Progress report on 4CMenB (Bexsero) vaccine coverage and effectiveness in England. In: Third annual report 01 September 2015–31 August 2018. 2018.
Martinón-Torres F, Martinez AC, Simkó R, et al. Antibody persistence and booster responses 24–36 months after different 4CMenB vaccination schedules in infants and children: A randomised trial. J Infect. 2018;76(3):258–69. https://doi.org/10.1016/j.jinf.2017.12.005.
Ministère de la Santé et de la Prévention. Third annual review of compulsory infant vaccinations [Troisième bilan annuel des obligations vaccinales du nourrisson] Paris. 2022. Available from: https://sante.gouv.fr/IMG/pdf/bilan_3eme_annee_obligations_vaccinales.pdf. Accessed 31 Jan 2024.
Pouwels KB, Hak E, van der Ende A, et al. Cost-effectiveness of vaccination against meningococcal B among Dutch infants: crucial impact of changes in incidence. Hum Vaccin Immunother. 2013;9(5):1129–38. https://doi.org/10.4161/hv.23888
Christensen H, Hickman M, Edmunds WJ, et al. Introducing vaccination against serogroup B meningococcal disease: an economic and mathematical modelling study of potential impact. Vaccine. 2013;31(23):2638–46. https://doi.org/10.1016/j.vaccine.2013.03.034.
Ginsberg GM, Block C, Stein-Zamir C. Cost-utility analysis of a nationwide vaccination programme against serogroup B meningococcal disease in Israel. Int J Public Health. 2016;61(6):683–92. https://doi.org/10.1007/s00038-016-0821-0.
Janssen MF, Szende A, Cabases J, et al. Population norms for the EQ-5D-3L: a cross-country analysis of population surveys for 20 countries. Eur J Health Econ. 2019;20(2):205–16. https://doi.org/10.1007/s10198-018-0955-5.
Kennedy ITR, van Hoek AJ, Ribeiro S, et al. Short-term changes in the health state of children with group B meningococcal disease: a prospective, national cohort study. PLoS ONE. 2017;12(5): e0177082. https://doi.org/10.1371/journal.pone.0177082.
Al-Janabi H, Van Exel J, Brouwer W, et al. Measuring health spillovers for economic evaluation: a case study in meningitis. Health Econ. 2016;25(12):1529–44. https://doi.org/10.1002/hec.3259.
Song J, Floyd FJ, Seltzer MM, et al. Long-term effects of child death on parents’ health related quality of life: a dyadic analysis. Fam Relat. 2010;59(3):269–82. https://doi.org/10.1111/j.1741-3729.2010.00601.x.
Erickson LJ, De Wals P, McMahon J, et al. Complications of meningococcal disease in college students. Clin Infect Dis. 2001;33(5):737–9. https://doi.org/10.1086/322587.
Brown MM, Brown GC, Sharma S, et al. Utility values associated with blindness in an adult population. Br J Ophthalmol. 2001;85(3):327–31. https://doi.org/10.1136/bjo.85.3.327.
Oostenbrink R, Moll HA, Essink-Bot ML. The EQ-5D and the Health Utilities Index for permanent sequelae after meningitis: a head-to-head comparison. J Clin Epidemiol. 2002;55(8):791–9. https://doi.org/10.1016/S0895-4356(02)00448-1
Wyld M, Morton RL, Hayen A, et al. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med. 2012;9(9): e1001307. https://doi.org/10.1371/journal.pmed.1001307.
Blakeney P, Meyer W 3rd, Robert R, et al. Long-term psychosocial adaptation of children who survive burns involving 80% or greater total body surface area. J Trauma. 1998;44(4):625–32 (discussion 633-4). https://doi.org/10.1097/00005373-199804000-00011
Saarni SI, Suvisaari J, Sintonen H, et al. Impact of psychiatric disorders on health-related quality of life: general population survey. Br J Psychiatry. 2007;190(4):326–32. https://doi.org/10.1192/bjp.bp.106.025106.
Bennett JE, Sumner W 2nd, Downs SM, et al. Parents’ utilities for outcomes of occult bacteremia. Arch Pediatr Adolesc Med. 2000;154(1):43–8. https://doi.org/10-1001/pubs.Pediatr Adolesc Med.-ISSN-1072-4710-154-1-poa9043. (PMID: 10632249)
Ameli. Drug database and pricing information [Base des Médicaments et Informations Tarifaires]. 2019. Available from: http://www.codage.ext.cnamts.fr/codif/bdm_it/index_presentation.php?p_site=AMELI Accessed 15 May 2022.
Institut National de la Statistique et des Etudes Economiques (INSEE). Consumer price index - Base 2015 - All households - France - Health services - Identifier 001763845 [Indice des prix à la consommation - Base 2015 - Ensemble des ménages - France - Services de santé - Identifiant 001763845]. 2019. Available from: https://www.insee.fr/fr/statistiques/serie/001763845#Telechargement. Accessed 24 Jun 2022.
Zafack JG, Bureau A, Skowronski DM, et al. Adverse events following immunisation with four-component meningococcal serogroup B vaccine (4CMenB): interaction with co-administration of routine infant vaccines and risk of recurrence in European randomised controlled trials. BMJ Open. 2019;9(5):e026953. https://doi.org/10.1136/bmjopen-2018-026953.
Vesikari T, Esposito S, Prymula R, et al. Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet. 2013;381(9869):825–35. https://doi.org/10.1016/S0140-6736(12)61961-8. (PMID: 23324563).
Martinon-Torres F, Safadi MAP, Martinez AC, et al. Reduced schedules of 4CMenB vaccine in infants and catch-up series in children: Immunogenicity and safety results from a randomised open-label phase 3b trial. Vaccine. 2017;35(28):3548–57. https://doi.org/10.1016/j.vaccine.2017.05.023.
Gossger N, Snape M, Yu L, et al. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA. 2012;307(6):573–82. https://doi.org/10.1001/jama.2012.85.
Service Public français. Reimbursement of a medical consultation for a child under 16 years old [Remboursement d'une consultation médicale pour un enfant de moins de16 ans]. 2021. Available from: https://www.service-public.fr/particuliers/vosdroits/F10874 Accessed 24 Jun 2022.
Nainani V, Galal U, Buttery J, et al. An increase in accident and emergency presentations for adverse events following immunisation after introduction of the group B meningococcal vaccine: an observational study. Arch Dis Child. 2017;102(10):958–62. https://doi.org/10.1136/archdischild-2017-312941
Service Public Français. Education allowance for disabled children [Allocation d’éducation de l’enfant handicapé (AEEH)]. 2019. Available from: https://www.service-public.fr/particuliers/vosdroits/F14809. Accessed 15 May 2022.
Caisse Allocations Familiales (CAF). Education allowance for disabled children [Allocation d’éducation de l’enfant handicapé (AEEH)]. 2019. Available from: http://data.caf.fr/dataset/foyers-allocataires-percevant-l-allocation-d-education-de-l-enfant-handicape-aeeh-par-caf Accessed 15 May 2022.
Direction de la Recherche des Etudes de l'Evaluation et des Statistiques (DREES). Beneficiaries of departmental social assistance for the elderly or disabled (APA, PCH, ASH, Household help, etc.) [Les bénéficiaires de l'aide sociale départementale aux personnes âgées ou handicapées (APA, PCH, ASH, Aides ménagères, …)]. 2022. Available from: https://data.drees.solidarites-sante.gouv.fr/explore/dataset/les-beneficiaires-de-l-aide-sociale-departementale-aux-personnes-agees-ou-handic/information/. Accessed 24 Jun 2022.
Direction de la Recherche des Etudes de l'Evaluation et des Statistiques (DREES). Disability compensation benefit (PCH) and compensation allowance for third person (ACTP) [La prestation de compensation du handicap (PCH) et l’allocation compensatrice pour tierce personne (ACTP)]. 2020. Available from: https://drees.solidarites-sante.gouv.fr/prestation-de-compensation-du-handicap-pch-et-allocation-compensatrice-pour-tierce-personne-actp. Accessed 24 Jun 2022.
Ministère des Solidarités et de la Santé. INSTRUCTION No. DGS/SP/2018/163 of July 27, 2018 relating to prophylaxis invasive meningococcal infections [INSTRUCTION N° DGS/SP/2018/163 du 27 juillet 2018 relative à la prophylaxie des infections invasives à méningocoque]. 2018. Available from: https://sante.gouv.fr/IMG/pdf/instruction_dgs_sp_2018_163.pdf. Accessed 7 Sep 2023.
Dejour Salamanca D, Tararbit K, Prévosto F, et al. Community outbreak of serogroup B invasive meningococcal disease in Beaujolais (Rhône, France) 2016: organization of the immunization campaign and results [Epidémie d’infections invasives à méningocoque B dans le Beaujolais (Rhône), 2016: organisation de la vaccination et résultats]. Bull Epidémiol Hebdo. 2018;30–31:620–7.
Agence Régionale de Santé (ARS) Bourgogne-Franche-Comté. Exceptional vaccination campaign on campus of Dijon: First assessment [Campagne de vaccination exceptionnelle sur le campus de Dijon : premier bilan]. 2017. Available from: http://www.prefectures-regions.gouv.fr/bourgogne-franche-comte/content/download/30803/210379/file/20170214_bilan_campagne_vaccination.pdf. Accessed 15 May 2022.
Agence régionale de Santé (ARS) Bourgogne-Franche-Comté. Meningococcal W vaccination [Vaccination contre le méningocoque W]. 2019. Available from: https://www.bourgogne-franche-comte.ars.sante.fr/vaccination-contre-le-meningocoque-w. Accessed 15 May 2022.
Nord E, Johansen R. Concerns for severity in priority setting in health care: a review of trade-off data in preference studies and implications for societal willingness to pay for a QALY. Health Policy. 2014;116(2–3):281–8. https://doi.org/10.1016/j.healthpol.2014.02.009.
Read RC, Baxter D, Chadwick DR, et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. The Lancet. 2014;384(9960):2123–31. https://doi.org/10.1016/S0140-6736(14)60842-4
Shen J, Bouee S, Aris E, et al. Long-term mortality and state financial support in invasive meningococcal disease-real-world data analysis using the French National Claims Database (SNIIRAM). Infect Dis Ther. 2022;11(1):249–62. https://doi.org/10.1007/s40121-021-00546-z.
Institute for Clinical and Economic Review (ICER). ICER 2019 Perspectives on Cost-Effectiveness Threshold Ranges. 2019. Available from: https://icer.org/wp-content/uploads/2023/08/ICER_2019_Perspectives-on-Cost-Effectiveness-Threshold-Ranges.pdf. Accessed 8 Feb 2024.
Hanquet G, Christensen H, Trotter C, et al. A quadrivalent vaccine against serogroup B meningococcal disease: a cost-effectiveness study. In: (KCE) BHCKC, editor., et al., Health technology assessment (HTA). Brussels: Belgian Health Care Knowledge Centre (KCE); 2014.
Attema AE, Brouwer WBF, Claxton K. Discounting in economic evaluations. Pharmacoeconomics. 2018;36(7):745–58. https://doi.org/10.1007/s40273-018-0672-z.
Medicines Australia. Submission to the PBAC on the Base Case Discount Rate. 2022. Available from: https://www.biointelect.com/submission-to-the-pbac-on-the-base-case-discount-rate/. Accessed 16 Apr 2024
Annemans L, Beutels P, Bloom DE, et al. Economic Evaluation of Vaccines: Belgian Reflections on the Need for a Broader Perspective. Value Health. 2021;24(1):105–11. https://doi.org/10.1016/j.jval.2020.09.005.
Ultsch B, Damm O, Beutels P, et al. Methods for health economic evaluation of vaccines and immunization decision frameworks: a consensus framework from a European Vaccine Economics Community. Pharmacoeconomics. 2016;34(3):227–44. https://doi.org/10.1007/s40273-015-0335-2.
Haut Conseil de Santé Publique (HCSP). Vaccination with meningococcal serogroup C conjugate vaccine [Vaccination par le vaccin conjugué contre le méningocoque de sérogroupe C]. 2009. Available from: https://www.hcsp.fr/Explore.cgi/Telecharger?NomFichier=hcspr20090424_meningC.pdf. Accessed 24 Jun 2022.
Joint Committee on Vaccination and Immunisation (JCVI). Minutes of the meeting on 11 and 12 February 2014. 2014. Available from: https://app.box.com/s/iddfb4ppwkmtjusir2tc/file/229171703722. Accessed 15 May 2022.
Rodrigues F, Marlow R, Simoes MJ, et al. Protocol Summary : case-control study to evaluate the effectiveness of 4CMenB vaccine in the prevention of invasive meningococcal disease in Portugal—(EMGM 2019- 13358). 2019. 15th European Meningococcal and haemophilus Disease Society (EMGM) Congress. Lisbon, Portugal.
INFOVAC-FRANCE. Invasive meningococcal infections [Infections invasives à Méningocoque ], Bulletin 2. 2023. Available from: https://www.infovac.fr/actualites/bulletin-n-2-fevrier-2023. Accessed 8 Feb 2023.
Scholz S, Schwarz M, Beck E, et al. Public health impact and cost-effectiveness analysis of routine infant 4CMenB vaccination in Germany to prevent serogroup B invasive meningococcal disease. Infect Dis Therapy. 2022;11(1):367–87. https://doi.org/10.1007/s40121-021-00573-w.
Martinon-Torres F, Nolan T, Toneatto D, et al. Persistence of the immune response after 4CMenB vaccination, and the response to an additional booster dose in infants, children, adolescents, and young adults. Hum Vaccin Immunother. 2019;15(12):2940–51. https://doi.org/10.1080/21645515.2019.1627159.
Huang L, Balmer P, Farkouh R. Can current health economic modeling frameworks capture the unpredictability of invasive meningococcal disease? 2019. Meningitis Research Conference (MRC). London, UK. Available from: https://www.meningitis.org/getmedia/621715fb-bfde-4a1a-8a26-985e7c9cd954/All-Poster-Abstracts-2019_final?disposition=attachment. Accessed 16 Apr 2024.
Acknowledgments
The authors would like to thank Guillaume Dumenil (Institut Pasteur, Paris, France) and Patricia Merhant Sorel (Association Petit Ange, France), Valérie Grange (GSK), Patricia Pujol (GSK), Najida Begum (Independent consultant for GSK) and Kinga Meszaros (GSK) for their contribution and advice during study conduct and data analyses or interpretation. The authors would like to thank Business & Decision Life Sciences Medical Communication Service Center for editorial assistance and manuscript coordination, on behalf of GSK. Katrin Spiegel (independent, on behalf of GSK) provided medical writing support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This was a non-interventional study without the participation of human subjects. No ethics approval was required. The study only used anonymized patient data and was compliant with procedures outlined by the National Commission on Informatics and Liberty (Commission Nationale de l'Informatique et des Libertés – CNIL).
Consent to Participate
This was a non-interventional study which did not enroll patients. No informed consent was required.
Competing Interests
GdP received personal consulting fees from GSK for his involvement in advisory boards on health economic studies related to MenB (related to the present publication) and other vaccines. GdP also received honoraria from GSK for a press conference on MenB vaccination. MBB and LG are employees of Amaris Consulting. Amaris Consulting received financial compensation from GSK for performing the study related to this publication. VLP, EB, CP and GN are employed by and hold shares in GSK. The authors declare no other financial and non-financial relationships and activities.
Consent for Publication
This was a non-interventional study which did not enroll patients. No informed consent was required.
Funding
GlaxoSmithKline Biologicals SA funded this study (GSK study identifier: VEO 000057) and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also took charge of all costs associated with the development and publication of this manuscript.
Contributorship
All authors participated in the design, implementation, analysis, and interpretation of the study and the development of this manuscript. All authors had full access to the data and gave final approval before submission. All authors agree to be accountable for all aspects of the work.
Availability of Data and Material
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to https://www.gsk-studyregister.com/en/. To access data for other types of GSK-sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website.
Code Availability
The invasive meningococcal disease dynamic transmission model was programmed in MS Excel 2016 and updated in Excel Office 365. The model was developed by GSK and is proprietary in nature. Further information is available upon request from the corresponding author.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
de Pouvourville, G., Breau-Brunel, M., Loncle-Provot, V. et al. Public Health Impact and Cost-Effectiveness Analysis of 4-Component Meningococcal Serotype B Vaccination for Infants in France. PharmacoEconomics Open 8, 539–557 (2024). https://doi.org/10.1007/s41669-024-00488-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-024-00488-5