Abstract
Background
Hereditary hemochromatosis (HH) is an autosomal recessive disorder that leads to iron overload and multiorgan failure.
Objectives
The aim of this systematic review was to provide up-to-date evidence of all the current data on the costs and cost effectiveness of screening and treatment for HH.
Methods
We searched PubMed, Cochrane Library, National Health Service Economic Evaluation Database (NHSEED), Cost-Effectiveness Analysis Registry (CEA Registry), Health Technology Assessment Database (HTAD), Centre for Reviews and Dissemination (CRD), and Econlit until April 2023 with no date restrictions. Articles that reported cost-utility, cost-description, cost-minimization, cost-effectiveness, or cost-benefit analyses for any kind of management (drugs, screening, etc.) were included in the study. Patients with HH, their siblings, or individuals suspected of having HH were included in the study. All screening and treatment strategies were included. Two authors assessed the quality of evidence related to screening (either phenotype or genotype screening) and treatment (phlebotomy and electrophoresis). Narrative synthesis was used to analyse the similarities and differences between the respective studies.
Results
Thirty-nine papers were included in this study. The majority of the studies reported both the cost of phenotype screening, including transferrin saturation (TS), serum ferritin, and liver biopsy, and the cost of genotype screening (HFE screening, C282Y mutation). Few studies reported the cost for phlebotomy and erythrocytapheresis treatment. Data revealed that either phenotype or genotype screening were cost effective compared with no screening. Treatment studies concluded that erythrocytapheresis might be a cost-effective therapy compared with phlebotomy.
Conclusions
Economic studies on either the screening, or treatment strategy for HH patients should be performed in more countries. We suggest that cost-effectiveness studies on the role of deferasirox in HH should be carried out as an alternative therapy to phlebotomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Screening of blood donors for hereditary hemochromatosis (HH) can decrease third-party payer health care costs in the long-term. |
Population screening programs for HH are cost effective compared with no screening. |
Pharmacoeconomic studies for the screening and treatment of HH patients should be performed in more countries. |
1 Introduction
Hereditary hemochromatosis (HH) is a genetic disease mainly affecting Caucasian populations, characterized by iron overload as a result of excessive iron intestinal absorption in the duodenum [1]. The disease was named by von Recklinghausen in 1889 due to the pigment that he thought was of blood origin [2]. The prevalence of HH is 1 in 300–500 individuals [3].
Mutation of the hemochromatosis gene (HFE: C282Y [main mutation], S65C, H63D) is the most common cause of HH [4] that contributes to iron overload in heart, liver, pancreas, and other organs, leading to multiorgan failure. Mutations of transferrin receptor 2, ferroportin protein, or hepcidin antimicrobial peptide (HAMP) are other causes of HH, whereas arrhythmias, diabetes mellitus, arthralgia, impotence, hypermelanotic pigmentation of the skin, cirrhosis of the liver, lethargy, cardiomyopathy, arthritis, and pancreatic disease are some of the complications of HH. Hepatocellular carcinoma is also another result of irreversible damage caused by HH [5, 6]. Men can develop more severe symptoms than women [7].
Since the discovery of HFE gene in 1996, DNA analysis was introduced as a diagnostic strategy for HH; however, different studies revealed that HH is often a neglected or missed diagnosis [8, 9]. The symptoms of the disease become apparent in women later than in men because of iron excretion associated with menstruation, and early detection can improve life expectancy and prevent complications [3].
Serum transferrin saturation (TS), serum ferritin, and unsaturated iron-binding capacity (UIBC) are some of the biochemical tests used for diagnosis and that are further confirmed with HFE genotyping. Studies have reported that TS is increased by 10 years of age [10].
Genetic screening has a relatively low cost [11]. Liver biopsy can be performed to assess the liver damage in severe cases. Screening for HH can contribute to the early detection of patients who are homozygous for the HFE gene, and hence can reduce their risk for severe irreversible diseases [12].
HH treatment is focused on iron excretion. Transferrin saturation screening can be used as an early indicator of the disease, and for the initiation of phlebotomy [13]. Ferritin levels are an indicator for the initiation and frequency of phlebotomies and are used to prevent complications. Therapeutic phlebotomy aims to reduce serum iron indices and iron overload [14]. Removing the excess iron before severe tissue damage significantly increases the survival rate.
Much research has been conducted in relation to HH but only a few recent pharmacoeconomic studies have been carried out. The aim of this review was to summarize and provide up-to-date evidence of all the current data on the costs and cost-effectiveness of screening and treatment for HH. These data can help policy makers to evaluate the cost effectiveness of HH screening and treatment.
2 Methods
2.1 Literature Search and Presentation of the Full Search Strategies for All Databases
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify economic studies on hemochromatosis [15]. A protocol was not prepared and this review was not registered.
We searched the following databases: PubMed, Cochrane Library, National Health Service Economic Evaluation Database (NHSEED), Cost-Effectiveness Analysis Registry (CEA Registry), Health Technology Assessment Database (HTAD), Centre for Reviews and Dissemination (CRD), and Econlit between inception and April 2023, with no date restrictions. For each database, we used the following keywords (‘hemochromatosis’) and (‘economic evaluation’ OR ‘cost-effectiveness analysis’, OR ‘cost analysis’, OR ‘cost benefit’, OR ‘cost utility’, OR ‘direct cost’, OR ‘indirect cost’, OR ‘health economic’).
2.2 Study Design
Articles carried out in any country were included in the study if they contained cost-utility, cost-minimization, cost-description, cost-effectiveness, or cost-benefit analyses for any type of management (drugs, screening, etc.) or intervention. We excluded abstracts, conference papers, reviews, systematic reviews, posters, protocols, and letters to the editors. Two of the reviewers (MH, BZ) independently screened all articles and agreement was reached by consensus. Only articles published in English were included in this systematic review.
2.3 Eligibility
Original articles on hemochromatosis were considered eligible if they reported a full or partial economic estimation comparing intervention(s) and comparator(s) in outcomes and costs. As defined by Drummond et al. [16], a partial economic evaluation study reports the cost examination and/or consequences of one or more interventions, while a full economic evaluation study reports a comparison of either costs or consequences of two or more interventions [16]. All articles that described hemochromatosis as the main outcome with no interventions for treatment or screening were excluded. Studies that did not report health economic data were excluded.
2.3.1 Population
Our population included patients with HH, their siblings, or individuals suspected of having HH. Studies using hypothetical populations in decision models were also included.
2.3.2 Intervention
The interventions were kept broad, and all screening and treatment strategies were included.
2.3.3 Comparators
Sequential screening (phenotype and genotype screening) and therapy (phlebotomy, erythrocytapheresis) were used as interventions/comparators. A no-screening strategy was also used as a comparator.
2.4 Data Extraction
The data extracted from each study included author names, year of publication, country, target group, sample size, time frame, study type, duration, discount rate, comparators, intervention, and outcomes, etc. Two authors collected the data. Incremental cost-effectiveness ratios (ICERs) were extracted from all studies reporting the cost effectiveness of drugs.
2.5 Synthesis (Methods)
Narrative synthesis was used to assess the similarities and differences between the respective studies. Due to the heterogeneity of the studies, we classified them into either screening or treatment studies. The screening strategies studies were synthesized into two different tables—CEA or non-CEA studies. The information recovered from the studies was synthesized in different columns in the respective tables to make it easier for readers to view the similarities and compare the data. Discrepancies were double-checked and discussed between MH and BZ.
2.6 Effect Measures
Health economic metrics such as ICER and quality-adjusted life-years (QALYs) were reported for the CEA studies. In addition, we reported all cost values in the original currency, as well as in current (Euros [€]) currency (year 2023).
2.7 Outcomes
The mean cost and cost effectiveness of phlebotomy and erythrocytapheresis are reported as the main outcomes in the economic studies on the treatment strategies included in this review. Moreover, the phenotype versus genotype screening costs are also reported as the main outcomes in the economic studies on the screening strategies included in this article.
2.8 Risk-of-Bias Assessment and Quality Assessment
Two reviewers independently selected the studies and assessed the respective interventions and outcomes of the studies—either the reported outcomes, or the missing outcomes. Agreement was reached by consensus. The sample size may have introduced bias in different studies, estimating the cost to the population level.
The British Medical Journal (BMJ) checklist [17] was used to assess the quality of the economic studies included in this current study. The BMJ checklist is made up of 35 items, each of which require a ‘yes’, ‘no’, or ‘not applicable’ answer, which were each given a score of ‘1’ when the task was carried out and ‘0’ when the task was not executed. The total scores were converted and reported in percentages.
We assessed the certainty of evidence as high, moderate, or low quality. High-quality studies were considered as those with a total percentage of 75% from the BMJ checklist, moderate-quality studies as those with 50–75%, and low-quality studies as those with a total percentage of <50% from the BMJ checklist [18]. The relevant information is reported in Tables 1, 2, and 3 in the Results section.
2.9 Reporting Bias Assessment
Multiple databases with no date restrictions were used to recover the data. MH and BZ double-checked the papers to avoid potential duplication. Tables were used to report and compare the BMJ checklist, and the outcomes for each eligible study were included in this review.
3 Results
3.1 Overview of Selected Studies
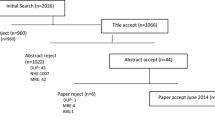
As shown in Fig. 1, we identified 590 articles, of which 252 were duplicates and were hence removed. Fifty-five articles were excluded based on title and abstract screening. Other studies that did not fulfil the eligibility criteria, i.e. review articles, systematic reviews, abstracts, poster presentations, and studies not reporting full or partial economic evaluation of HH, were also excluded (n = 244). Of 590 identified records, 39 were selected for inclusion in our systematic review.
A summary of the study characteristics of all the articles included in this current study is reported in Tables 1, 2, and 3. The studies were variable and the study design included population, intervention, comparator, intervention duration, outcomes, perspectives, and ICER; the QALYs were heterogeneous.
Thirty-three percent of the studies evaluated the modeled screening programs over a lifetime [19,20,21,22,23,24,25,26,27,28,29,30,31]. Six studies were conducted in Canada [7, 19,20,21, 23, 35], 13 in the US [24, 25, 30, 34, 37, 39, 40, 45, 48, 49, 52, 53, 55], 3 in Germany [22, 31, 47], 2 in Norway [32, 33], 3 in The Netherlands [29, 50, 51], 4 in the UK [28, 36, 38, 41], 6 in Australia [26, 27, 42,43,44, 46], 1 in Switzerland [54], and 1 in Italy [56]. The timeline of publications was from 1992 to 2020, and the currency evaluated was €, Canadian dollars (CAN$), US dollars (US$), Australian dollars (AUS$), and Great Britain pounds (£). Other than the original cost values in the original currency (outcome, ICER), we also added two additional columns reporting all the current cost values in the original currency, as well as the cost values in the current currency (€). Taking into consideration the heterogeneity of all the economic published data, we expressed the cost data in the same year using the standard inflator for the country on which the analysis is focused. The average daily exchange rates for the period from 1 January 2023 to 30 June 2023 were taken into consideration when calculating the cost values in the current (€) currency.
The majority of the studies reported both phenotype screening, including TS, serum ferritin, and liver biopsy, and genotype screening (HFE screening, C282Y mutation) [7, 20,21,22,23, 25,26,27, 29, 30, 32, 35, 36, 38, 42]. A few studies reported on phlebotomy and erythrocytapheresis treatment [50,51,52,53,54,55,56].
Of the 39 papers accepted, most studies used a cost-effectiveness analysis (n = 20) [7, 19, 21,22,23,24,25,26,27, 29, 31, 32, 34, 36,37,38, 41, 48, 49]; 23.1% of studies used a decision tree (n = 9) [17, 18, 21, 24, 25, 30, 35, 37, 40]. There were eight non-experimental screened studies that included a cost description [28, 30, 42, 43, 45, 47, 52, 53]. Four studies employed a cost-utility analysis [19, 20, 33, 44], and a Markov model was applied in four studies [22, 24, 27, 33].
Overall, 12.8% of studies reported an annual discount rate of 3%, while other studies reported a discount rate of 5% [31] or 0–7% [27]. No discounting of costs was reported in 80.5% of screening studies [7, 21, 28,29,30, 32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56].
3.2 Screening
Thirty-two studies that reported the screening strategy were identified (Tables 1, 2). The majority of the studies reported a sequential screening, a combination of both the genotypic and phenotypic screening programs. We identified 10 CEA studies on screening, which are all reported in Table 1, whereas Table 2 reports all the economic studies, other than CEA studies on the screening strategies included in this review. The phenotype screening included TS, UIBC, and serum ferritin, with a confirmation of liver biopsy. HFE mutation identification was used to confirm the HH diagnosis.
The QALYs or quality-adjusted life-days (QALDs) were reported in a few studies [19, 20, 27, 33]. Adams et al. showed that in a hypothetical cohort of 10,000 blood donors and siblings, the screening of blood donors showed a QALY of 0.84, versus a QALY of 1.18 for the screening of asymptomatic homozygous siblings [19]. In line with these findings, Adams and Valberg showed a QALD of 2.75 per genotypic screening per person screened in a hypothetical cohort of 10,000 blood donors and their siblings [20]. In both studies, a discount rate of 3% was reported and the third-party payer perspective was reported. The incremental cost saving per person was US$0.97 in 1999 for phenotyping TS versus US$151 for genotyping, in comparison with the no-screening strategy [20]. However, when converted to the current currency (€), the genotyping cost was €168.88 of incremental cost saving/person screened versus the no-screening strategy. The incremental cost savings were higher for homozygous siblings screened versus blood donors, and when the values were converted to Euros (current currency), the incremental cost savings were €14.91 per person screened versus €3.78 for the screening of blood donors [19].
A cost-utility analysis carried out in a cohort of 30-year-old men in Norway reported a QALY of 7.65 for phenotype screening, with a screening cost of US$250 per QALY gained [33]. Using a DNA test as the primary screening test resulted in a higher ICER (€210,434.18) in comparison with sequential TS/HFE screening (€162,073.53/life-year gained [LYG]) [22]. The incremental cost of US$1.50 per specimen could be added to other chemistry tests if the serum iron test is used as a preliminary screening test [37]. In a cost analysis, it was reported that a new HH diagnosis results in an additional health care cost of US$3118/patient yearly [40]. Other cost-description studies showed that the estimated cost for newly identified HH patients was US$390 [32], or £117 for each HH patient identified, which, when converted to Euros in the current currency, would be €621.26/newly identified HH subject and €287.23/HH patient identified, respectively [38]. The TS strategy was cost effective, with an ICER of AUS$10,195/QALY gained [27]. In line with these findings, UIBC was determined to be a cost-effective screening, with an incremental cost of US$3.19/each donor [49]. Other cost-description papers compared phenotype versus genotype screening [7, 21, 23, 36, 38]. Genotyping the spouse of a homozygote is the best cost-efficient strategy in pedigree studies [21]. The cost of phenotype screening for UIBC per HH detected was reported in different studies [7, 23]. In a quasi-experimental cost-analysis study of 105 siblings with a median age of 55 years from 35 proband cases of HH, it was revealed that the screening of siblings with ferritin and TS may be adequate in many families, with a total cost of US$1800–$2100/screening of a family with four members [35]. However, other studies showed that the uptake of screening with the genotypic strategy was not inferior to that in the phenotypic strategy [36]. The cost for different screening programs was reported in a few studies conducted in four different countries (USA, Germany, UK, Australia) [27, 28, 30, 42, 45, 47]. A cost-utility analysis reported that the symptomatic stages of HH and the presence of multiple self-reported symptoms were associated with decreasing utility [44]. In addition, we noticed a lack of utility weight sources in the cost-utility studies on either phenotype, or genotype screening, despite reporting the utility weights for diabetes, heart failure, and cirrhosis [19, 20, 33]. In their cost-of-illness study, De Graaff et al. reported for the first time the HH cost estimate for the Australian population, showing that reducing the clinical penetrance of HH can result in a significant reduction in cost [44].
3.3 Treatment
This review identified eight economic evaluation studies on HH treatment (Table 3). The most recent study, a randomized, crossover clinical trial was carried out in 2016. Two studies concluded that whole blood donation (WBD) was a more cost-effective treatment than double erythrocytapheresis (DEC). Gribble et al. concluded that in an economic study performed in HH blood donors from a social provider perspective during the period January 2008–December 2008, the total cost for WBD was US$6000 versus US$23,595 for DEC [55].
In line with these findings, Stefashyna et al. showed that the cost of a single DEC was higher (US$238) in respect to WBD (US$186) [54]. In a randomized controlled trial carried out in HH patients from three hospitals, the mean treatment costs for phlebotomy was lower (€235) than the cost for erythrocytapheresis (€511); the results showed that erythrocytapheresis is the preferred treatment method [51]. In line with this study, Mariani et al. showed that in a non-experimental descriptive case-series study that included three patients with severe HH, the total mean costs for erythrocytapheresis was higher (€602) in respect of phlebotomy (€35) [56]. Rombout et al., reported that erythrocytapheresis might be a cost-saving therapy [50]. The mean cost of phlebotomy varied from US$90 in hospitals to US$52 in blood centers, which, when converted to the current currency (€), would be €152.4 and €88.05, respectively [52]. Discounting costs are not reported in all studies. Two of the selected studies were randomized clinical trials, both performed in The Netherlands, with a duration of 3 years, and from a societal perspective [50, 51]. The cost-analysis studies were carried out in Italy and Switzerland, both from a service provider perspective [54, 56]. A further two cost-description papers were identified, one of which reported a financial gain of US$36,000 for the therapeutic phlebotomy program during the 13-month study period conducted in a rural hospital in the US [53, 55]. One of the studies was included in both Table 1 and Table 3 because it reported data from both a screening and treatment strategy point of view [24]. In that study, a Markov model was used in a group of males aged ≥25 years, with no pre-existing conditions that would predispose to iron loading. The data showed that early detection and treatment was slightly more costly than treatment at the onset of symptoms, with an average cost of US$605 per life-year gained [24]. However, the cost-effectiveness results obtained with this hypothetical cohort of 25-year-old males were based on certain assumptions, some of which have moderate-to-high degrees of uncertainty.
In conclusion, treatment economic studies performed in four different countries showed that erythrocytapheresis was more costly than phlebotomy [50,51,52, 56]; however, the most recent economic study on the treatment strategy of HH included in this review was published in 2016 [51].
4 Discussion
This systematic review summarizes all health economics data, either full or partial economic evaluations on the screening (phenotypic and genetic) and treatment of HH. No recent systematic reviews have been conducted in this field; to our knowledge, the latest systematic review in this field was published in 2015 [18]. Our review reports additional economic evaluation studies published until April 2023, either on phenotype or genotype screening, or treatment of HH, that were classified into two groups, i.e. screening or treatment economic studies. We were unable to perform a meta-analysis due to the heterogeneity of the studies.
Most of the studies reported the screening strategies. Studies have mostly shown that either phenotype or genotype screening were cost effective compared with no screening. In addition, treatment studies concluded that erythrocytapheresis might be a cost-effective therapy compared with phlebotomy. Phenotype screening with a confirmation of genetic screening is an optimal strategy for HH diagnosis. Rombout et al. revealed that erythrocytapheresis is a highly effective treatment to reduce iron overload and might potentially also be a cost-saving therapy compared with phlebotomy [50]. In addition, phenotyping with transferrin saturation and genotyping are cost-saving strategies compared with the no-screening strategy [19, 20]. The studies were heterogeneous, including either individuals suspected of having HH or patients with HH, or their siblings; however they all concluded that population screening programs for HH are cost-effective compared to no screening. El-Serag showed that HFE gene testing was less costly compared with serum iron screening [25]. In an Australian decision model study with a hypothetical cohort, it was shown that asymptomatic hemochromatosis subjects had higher costs than symptomatic patients, reflecting the low clinical penetrance estimate used. The authors showed that health sector and the time related to the productivity were the main cost drivers, and that the clinical penetrance estimate had a significant role on the assessment of cost effectiveness [23].
In a German cost-description study in which the presence of C282Y mutation was tested using different methods, such as PCR and restriction digest, reverse allele-specific oligonucleotide hybridization, solid-phase oligonucleotide ligation assay (SPOLA), and microarray (DNA-chip) [47], the respective costs were reported. Elsaid et al. reported that the annual health care costs were higher in HH patients with hypertension, arthritis, type 2 diabetes, and chronic kidney disease, but without HH [40].
Only a few cost-utility studies were observed and future studies should include reliable utility weights. The majority of the studies modeled screening programs over a lifetime.
Deferasirox is an iron chelator administered orally once daily in patients with transfusion-dependent anemias and other iron overload syndromes. We identified a review on the pharmacoeconomic benefits of deferasirox, but unfortunately it did not meet the eligibility criteria of this study. Furthermore, we did not identify any original articles on the economic aspects of deferasirox as a potential alternative therapy to phlebotomy in HH patients [57]. Various studies have been carried out on the role of deferasirox in different iron overload syndromes, but no cost-effectiveness, cost-analysis, or cost-utility studies have been conducted in HH patients.
There are limitations of this current review that warrant consideration. First, the quality of the data was variable, and an evaluation of the quality of the studies and the credible measurement of costs should be reported in the future. Second, the search was limited to articles published in English only, and including articles in other languages would have extended our results.
5 Conclusions
This systematic review provides up-to-date evidence on the economic data regarding either screening or treatment for HH. We noted that the current studies were only performed in a few countries. The lack of high-quality economic studies is an obstacle for population screening programs, which are considered as an approach to reduce clinical penetrance.
No original article on the economic aspects of deferasirox as a potential alternative therapy to phlebotomy in HH patient was found. We believe that despite assessing the cost of erythrocytapheresis and phlebotomy, it would be of great interest to carry out cost-effectiveness studies on the role of deferasirox in HH other than in different iron overload syndromes. There are still evidence gaps that need to be addressed.
References
Powell LW, Seckington RC, Deugnier Y. Haemochromatosis. Lancet. 2016;388(10045):706–16.
von Recklinghausen FD. Uber Hamochromatose. Tagebl Versamml Natur Arzte. 1889;62:324.
Porter JL, Rawla P. Hemochromatosis. Treasure Island (FL): StatPearls Publishing; 2023 Jan. https://www.ncbi.nlm.nih.gov/books/NBK430862/
Hanson EH, Imperatore G, Burke W. HFE gene and hereditary hemochromatosis: a HuGE review. Human Genome Epidemiology. Am J Epidemiol. 2001;154(3):193–206.
Niederau C, Fischer R, Pürschel A, Stremmel W, Häussinger D, Strohmeyer G. Long-term survival in patients with hereditary hemochromatosis. Gastroenterology. 1996;110(4):1107–19.
Niederau C, Strohmeyer G, Stremmel W. Epidemiology, clinical spectrum and prognosis of hemochromatosis. Adv Exp Med Biol. 1994;356:293–302.
Adams PC, Kertesz AE, McLaren CE, Barr R, Bamford A, Chakrabarti S. Population screening for hemochromatosis: a comparison of unbound iron-binding capacity, transferrin saturation, and C282Y genotyping in 5,211 voluntary blood donors. Hepatology. 2000;31(5):1160–4.
Fairbanks VF, Baldus WP. Hemochromatosis: the neglected diagnosis. Mayo Clin Proc. 1986;61(4):296–8.
Crosby WH. Hemochromatosis: the missed diagnosis. Arch Intern Med. 1986;146(6):1209–10.
Bassett ML, Halliday JW, Ferris RA, Powell LW. Diagnosis of hemochromatosis in young subjects: predictive accuracy of biochemical screening tests. Gastroenterology. 1984;87(3):628–33.
Whitlock EP, Garlitz BA, Harris EL, Beil TL, Smith PR. Screening for hereditary hemochromatosis: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2006;145(3):209–23.
Edwards CQ, Kushner JP. Screening for hemochromatosis. N Engl J Med. 1993;328(22):1616–20.
Raddatz D, Legler T, Lynen R, Addicks N, Ramadori G. HFE genotype and parameters of iron metabolism in German first-time blood donors—evidence for an increased transferrin saturation in C282Y heterozygotes. Z Gastroenterol. 2003;41(11):1069–76.
Phatak PD, Barton JC. Phlebotomy-mobilized iron as a surrogate for liver iron content in hemochromatosis patients. Hematology. 2003;8(6):429–32.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-94.
Drummond MF, et al. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ. 1996;313(7052):275–83.
De Graaff B, et al. A systematic review and narrative synthesis of health economic studies conducted for hereditary haemochromatosis. Appl Health Econ Health Policy. 2015;13(5):469–83.
Adams PC, Kertesz AE, Valberg LS. Screening for hemochromatosis in children of homozygotes: prevalence and cost-effectiveness. Hepatology. 1995;22(6):1720–7.
Adams PC, Valberg LS. Screening blood donors for hereditary hemochromatosis: decision analysis model comparing genotyping to phenotyping. Am J Gastroenterol. 1999;94(6):1593–600. https://doi.org/10.1111/j.1572-0241.1999.1120_f.x.
Adams PC. Implications of genotyping of spouses to limit investigation of children in genetic hemochromatosis. Clin Genet. 1998;53(3):176–8.
Rogowski WH. The cost-effectiveness of screening for hereditary hemochromatosis in Germany: a remodeling study. Med Decis Making. 2009;29(2):224–38.
Gagné G, Reinharz D, Laflamme N, Adams PC, Rousseau F. Hereditary hemochromatosis screening: effect of mutation penetrance and prevalence on cost-effectiveness of testing algorithms. Clin Genet. 2007;71(1):46–58.
Buffone GJ, Beck JR. Cost-effectiveness analysis for evaluation of screening programs: hereditary hemochromatosis. Clin Chem. 1994;40(8):1631–6.
El-Serag HB, et al. Screening for hereditary hemochromatosis in siblings and children of affected patients. A cost-effectiveness analysis. Ann Internal Med. 2000;132(4):261–9.
Bassett ML, Leggett BA, Halliday JW, Webb SI, Powell LW. Analysis of the cost of population screening for haemochromatosis using biochemical and genetic markers. J Hepatol. 1997;27:517–24.
de Graaff B, et al. Cost-effectiveness of different population screening strategies for hereditary haemochromatosis in Australia. Appl Health Econ Health Policy. 2017;15(4):521–34.
Timms AE, et al. Genetic testing for haemochromatosis in patients with chondrocalcinosis. Ann Rheum Dis. 2002;61(8):745–7.
Jacobs EMG, et al. Impact of the introduction of a guideline on the targeted detection of hereditary haemochromatosis. Neth J Med. 2005;63(6):205–14.
Beutler E, Gelbart T. Large-scale screening for HFE mutations: methodology and cost. Genet Test. 2000;4(2):131–42.
Schoffski O, Schmidtke J, Stuhrmann M. Cost-effectiveness of population-based genetic hemochromatosis screening. Community Genet. 2000;3:2–11.
Asberg A, Hveem K, Thorstensen K, Ellekjter E, Kannelønning K, Fjøsne U, et al. Screening for hemochromatosis: high prevalence and low morbidity in an unselected population of 65,238 persons. Scand J Gastroenterol. 2001;36(10):1108–15.
Asberg A, Tretli S, Hveem K, Bjerve KS. Benefit of population-based screening for phenotypic hemochromatosis in young men. Scand J Gastroenterol. 2002;37(10):1212–9.
Baer DM, Simons JL, Staples RL, Rumore GJ, Morton CJ. Hemochromatosis screening in asymptomatic ambulatory men 30 years of age and older. Am J Med. 1995;98(5):464–8.
Adams PC, Kertesz AE. Human leukocyte antigen typing of siblings in hereditary hemochromatosis: a cost approach. Hepatology. 1992;15(2):263–8.
Patch C, Roderick P, Rosenberg W. Factors affecting the uptake of screening: a randomised controlled non-inferiority trial comparing a genotypic and a phenotypic strategy for screening for haemochromatosis. J Hepatol. 2005;43(1):149–55.
Balan V, Baldus W, Fairbanks V, Michels V, Burritt M, Klee G. Screening for hemochromatosis: a cost-effectiveness study based on 12,258 patients. Gastroenterology. 1994;107(2):453–9.
Bhavnani M, et al. Screening for genetic haemochromatosis in blood samples with raised alanine aminotransferase. Gut. 2000;46(5):707–10.
Montanez K, et al. Genetic testing costs and compliance with clinical best practices. J Genetic Counselling. 2020;29(6):1186–91.
Elsaid MI, et al. Health care utilization and economic burdens of hemochromatosis in the United States: a population-based claims study. J Manag Care Spec Pharm. 2019;25(12):1377–86.
Cooper K, et al. A decision analysis model for diagnostic strategies using DNA testing for hereditary haemochromatosis in at risk populations. QJM Monthly J Assoc Physicians. 2008;101(8):631–41.
Hickman PE, Hourigan LF, Powell LW, Cordingley F, Dimeski G, Ormiston B, et al. Automated measurement of unsaturated iron binding capacity is an effective screening strategy for C282Y homozygous haemochromatosis. Gut. 2000;46(3):405–9.
de Graaff B, et al. Quality of life utility values for hereditary haemochromatosis in Australia. Health Qual Life Outcomes. 2016;14(31):29.
de Graaff B, Neil A, Sanderson K, Yee KC, Palmer A. Costs associated with hereditary haemochromatosis in Australia: a cost-of-illness study. Aust Health Rev. 2016;41(3):254–67.
Stave GM, et al. Evaluation of a workplace hemochromatosis screening program. Am J Prev Med. 1999;16(4):303–6.
Dye DE, Brameld KJ, Maxwell S, Goldblatt J, O’Leary P. The impact of single gene and chromosomal disorders on hospital admissions in an adult population. J Community Genet. 2011;2(2):81–90.
Stuhrmann M, et al. Genotype-based screening for hereditary haemochromatosis. I: Technical performance, costs and clinical relevance of a German pilot study. Eur J Human Genet EJHG. 2005;13(1):69–78.
Barton JC, et al. Hemochromatosis detection in a health screening program at an Alabama forest products mill. J Occup Environ Med. 2002;44(8):745–51.
Smith BN, et al. Prevalence of hereditary hemochromatosis in a Massachusetts corporation: is celtic origin a risk factor? Hepatology. 1997;25(6):1439–46.
Rombout-Sestrienkova E, Nieman FH, Essers BA, van Noord PA, Janssen MC, van Deursen CT, et al. Erythrocytapheresis versus phlebotomy in the initial treatment of HFE hemochromatosis patients: results from a randomized trial. Transfusion. 2012;52(3):470–7.
Rombout-Sestrienkova E, Winkens B, Essers BA, Nieman FH, Noord PA, Janssen MC, et al. Erythrocytapheresis versus phlebotomy in the maintenance treatment of HFE hemochromatosis patients: results from a randomized crossover trial. Transfusion. 2016;56(1):261–70.
McDonnell SM, et al. A survey of phlebotomy among persons with hemochromatosis. Transfusion. 1999;39(6):651–6.
Flynn RC, Bryant BJ. Therapeutic phlebotomy procedures and their impact on a rural hospital’s red blood cell inventory and fiscal stature. Transfusion. 2011;51(12 Pt 2):2761–6.
Stefashyna O, et al. Pattern of care of blood donors with early-uncomplicated hereditary haemochromatosis in a Swiss blood donation centre. Vox Sang. 2014;106(2):111–7.
Gribble DM, et al. Cost-effectiveness of FDA variance for blood collection from individuals with hereditary hemochromatosis at a 398-bed hospital-based donor center. Immunohematology. 2009;25(4):170–3.
Mariani R, et al. Erythrocytapheresis plus erythropoietin: an alternative therapy for selected patients with hemochromatosis and severe organ damage. Haematologica. 2005;90(5):717–8.
Imran F, Pradyumna P. Pharmacoeconomic benefits of deferasirox in the management of iron overload syndromes. Expert Rev Pharmacoecon Outcomes Res. 2009;9(4):297–304.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Malvina Hoxha, Visar Malaj, and Bruno Zappacosta certify that they have no affiliations with, or involvement in, any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Availability of data and material
The authors confirm that the data supporting the findings of this study are available within the article.
Author contributions
Conceptualization and methodology: All authors. Database search, study selection, and data extraction: MH and BZ. Data synthesis: MH, BZ, and VM; First draft preparation: All authors. Draft review and editing: All authors. All authors read and approved the final manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication (from patients/participants)
Not applicable.
Funding
No funding was received to assist in the preparation of this article.
Code availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hoxha, M., Malaj, V. & Zappacosta, B. Health Economic Evaluations of Hemochromatosis Screening and Treatment: A Systematic Review. PharmacoEconomics Open 8, 147–170 (2024). https://doi.org/10.1007/s41669-023-00463-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-023-00463-6