Abstract
Background
The aim of this study was to assess health care resource utilization (HRU) and costs associated with delayed pulmonary arterial hypertension (PAH) diagnosis in the United States.
Methods
Eligible adults with newly diagnosed PAH from Optum’s de-identified Clinformatics® Data Mart Database (2016–2021) were assigned to mutually exclusive cohorts based on time between first PAH-related symptom and first PAH diagnosis (i.e., ≤12 months’ delay, >12 to ≤24 months’ delay, >24 months’ delay). All-cause HRU and health care costs per patient per month (PPPM) were assessed during the first year following diagnosis and compared across cohorts using regression analysis adjusted for baseline covariates. Sensitivity analyses were conducted to assess outcomes during all available follow-up post-diagnosis.
Results
Among 538 patients (mean age: 65.6 years; 60.6% female), 60.8% had ≤12 months’ delay, 23.4% had a delay of >12 to ≤24 months, and 15.8% had >24 months’ delay. Compared with ≤12 months, delays of >12 to ≤24 months and >24 months were associated with increased hospitalizations (incidence rate ratio [95% confidence interval]: 1.40 [1.11–1.71] vs 1.71 [1.29–2.12]) and outpatient visits (1.17 [1.06–1.30] vs 1.26 [1.08–1.41]). Longer delays were also associated with more intensive care unit (ICU) stays and 30-day readmissions. Diagnosis delays translated into excess costs PPPM of US$3986 [1439–6436] for >12 to ≤24 months and US$5366 [2107–8524] for >24 months compared with ≤12 months’ delay; increased hospitalization costs (US$3248 [1108–5135] and US$4048 [1401–6342], respectively) being the driver. Sensitivity analyses yielded similar trends.
Conclusions
Delayed PAH diagnosis is associated with significant incremental economic burden post-diagnosis, driven by hospitalizations including ICU stays and 30-day readmissions, highlighting the need for increased awareness and a potential benefit of earlier screening.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This real-world study found that delayed diagnosis of pulmonary arterial hypertension (PAH) is common among patients in routine clinical practice in the US, resulting in a considerable economic burden from the healthcare payer perspective. |
Delayed time to diagnosis in PAH was associated with increased health care resource utilization and costs among patients primarily driven by hospitalizations, including intensive care unit stays and hospital readmissions, as well as more outpatient visits. |
Our findings underscore the need for timely PAH diagnosis through increased clinician awareness, earlier screening, and potential use of novel tools for PAH detection in order to minimize the downstream economic burden associated with delayed PAH diagnosis. |
1 Background
Pulmonary arterial hypertension (PAH) is a rare subgroup of pulmonary hypertension (PH) characterized by high mean pulmonary arterial pressure and increased vascular resistance [1, 2], with an estimated prevalence of 12.4 cases per million adult inhabitants in the United States (US) [1]. Despite advances in treatment, PAH remains a progressive and potentially fatal disease [3].
The 2022 guidelines of the European Society of Cardiology and the European Respiratory Society (ESC/ERS) recommend initial dual therapy with phosphodiesterase type 5 inhibitors (PDE5i) and endothelin receptor antagonists (ERA) for non-vasoreactive patients with idiopathic, heritable, or drug-associated PAH (I-/H-/D-PAH) or PAH associated with connective tissue disease (PAH-CTD), presenting at low or intermediate risk of death and without cardiopulmonary comorbidities [4]. Combination therapy with PDE5i, ERA, and prostacyclin pathway agents (PPA) have been shown to improve clinical outcomes among patients with PAH with higher-risk disease [4,5,6]. Thus, the guidelines recommend this triple combination therapy as initial treatment when patients without cardiopulmonary comorbidities present at intermediate-high or high risk, and when patients on initial dual therapy present at intermediate-low, intermediate-high, or high risk in follow-up evaluations [4]. For patients with cardiopulmonary comorbidities, initial monotherapy with a PDE5i or an ERA is recommended, although additional PAH medications may be considered on an individual basis for patients at intermediate or high risk after initial monotherapy [4].
A major challenge in PAH clinical practice remains the common phenomenon of delayed diagnosis [6]. The time between initial symptom presentation and PAH diagnosis can range from 2.5 to 3.9 years on average [7,8,9,10], and a study based on US registry data (i.e., REVEAL) reported that up to one in five patients experience a diagnosis delay of >2 years [11]. Several factors may contribute to these significant delays in PAH diagnosis, including non-specific symptoms (e.g., chest pain, fatigue) that can be subtle until the disease becomes more severe [2, 12]. Furthermore, there is a lack of any straightforward test that can be performed to diagnose PAH early in the course of the illness [6]. Right heart catheterization (RHC), the gold standard for PAH diagnosis recommended by current guidelines, is highly invasive, while non-invasive transthoracic echocardiography is less accurate [4]. As a result of diagnosis delays, patients may only be diagnosed and treated when the disease is more severe, potentially leading to poorer treatment responses, faster disease progression, and worse clinical outcomes [6, 13]. Indeed, delayed diagnosis has been associated with a higher mortality in patients with PAH, with a 2-year delay associated with increased mortality rates by 11% and a 5-year delay with 29% increased mortality [7].
While diagnosis delay in PAH has been previously described, there is limited information on the impact of delayed PAH diagnosis on the burden to the health care system, as measured by health care resource utilization (HRU) and health care costs. Accordingly, this study aimed to characterize the HRU and health care cost burden associated with delayed diagnosis of PAH in the US using retrospective administrative claims data.
2 Methods
2.1 Data Source
Data was obtained from Optum’s de-identified Clinformatics® Data Mart (CDM) Database between October 2015 and September 2021. CDM is derived from a database of administrative health claims for members of large commercial and Medicare Advantage health plans across a geographically diverse population, spanning all 50 states. The CDM database is statistically de-identified under the Expert Determination method in a manner consistent with the Health Insurance Portability and Accountability Act (HIPAA) of 1996 and managed according to Optum’s customer data use agreements.
2.2 Study Design and Sample Selection
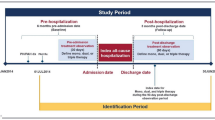
A retrospective cohort study design was used to address the study objective (Fig. 1). Patient selection criteria were based on diagnoses and procedures recorded in claims data and prescription fills. The date of the first medical claim with a recorded diagnosis for a PAH symptom after ≥12 months of continuous health insurance eligibility was defined as PAH symptom onset. The ≥12-month period of continuous eligibility was required as a ‘washout’ period to increase the likelihood of capturing the first symptom. PAH symptoms considered included ascites, chest pain, cyanosis, dizziness, edema, fatigue, syncope, tachycardia/palpitation, and unspecified dyspnea and were identified based on ICD-10-CM codes. The baseline period was defined as the 12 months before PAH symptom onset. The index date was defined as the date of the first PH-related diagnosis recorded on a medical claim (ICD-10-CM: I27.0, I27.20, I27.21, I27.89) following PAH symptom onset; patients whose first recorded PH-related diagnosis occurred prior to PAH symptom onset were excluded (i.e., approximately one third of patients; Fig. 2). The pre-diagnosis period was defined as the period between PAH symptom onset and the index date. The study period spanned from the index date until the earliest of 12 months, end of continuous health insurance eligibility, and the end of data availability; no minimum duration was required for the study period.
Study design. HRU health care resource utilization, PAH pulmonary arterial hypertension, RHC right heart catheterization. [1] Symptoms included ascites, chest pain, cyanosis, dizziness, edema, fatigue, syncope, tachycardia/palpitation, and unspecified dyspnea
Sample selection. CTEPH chronic thromboembolic pulmonary hypertension, ERA endothelin receptor antagonist, PAH pulmonary arterial hypertension, PDE5i phosphodiesterase type 5 inhibitor, PPA prostacyclin pathway agent, RHC right heart catheterization, sGCS soluble guanylate cyclase stimulator. [1] PAH-related treatments include PDE5is (sildenafil [excluding dosage corresponding to Viagra] or tadalafil [excluding dosage corresponding to Cialis]), ERAs (bosentan, ambrisentan or macitentan), sGCS (riociguat), PPAs (epoprostenol, iloprost, treprostinil, or selexipag). [2] PAH symptoms included ascites, chest pain, cyanosis, dizziness, edema, fatigue, syncope, tachycardia/palpitation, and unspecified dyspnea. [3] Procedures included pulmonary endarterectomy or balloon pulmonary angioplasty. [4] This criterion ensures that patients in all cohorts can have an index year during the same period (since patients in the >24 months’ diagnosis delay group can have an index year starting only in 2017 given the start of the data in 2015)
As shown in the sample selection flowchart (Fig. 2), eligible adult patients had (1) one or more PH-related diagnosis recorded on a medical claim in an inpatient setting or two or more PH-related diagnoses recorded on a medical claim in an outpatient (OP) setting on distinct dates, with the first diagnosis (i.e., index date) on or after January 1, 2017, (2) two or more PAH symptoms recorded on a medical claim ≥60 days apart, with the first PAH symptom prior to the index date, and (3) ≥12 months of continuous health insurance eligibility before PAH symptom onset. To increase the likelihood of identifying patients with PAH instead of other forms of PH, eligible patients were also required to have one or more prescription fill for a PAH-related treatment on or after the index date, one or more procedure claim for RHC at any time, and no documented claim for chronic thromboembolic pulmonary hypertension (CTEPH) or a CTEPH-related procedure at any time (list of diagnosis and procedure codes available in Supplementary Tables 1, 2 and 3, see electronic supplementary material [ESM]).
Diagnosis delay was defined as the time between PAH symptom onset and the date of the first PH-related diagnosis claim. Patients were classified into three mutually exclusive study cohorts based on their diagnosis delay, namely ≤12 months, >12 to ≤24 months (hereafter, 12–24 months), and >24 months.
2.3 Measures and Outcomes
Patient characteristics were measured during the baseline period, overall and stratified by study cohort, and included demographics, Quan-Charlson comorbidity index (i.e., adaptation of the Charlson comorbidity index using ICD-10-CM diagnosis codes) [14], specific comorbidities, common pharmacological treatments, HRU, and health care costs. The symptoms present at PAH symptom onset and during the pre-diagnosis period in the overall sample were also reported.
All-cause and PH-related HRU per patient per month (PPPM) were measured during the study period (i.e., up to 1 year post-diagnosis) and included hospitalization stays and hospitalization days, intensive care unit (ICU) stays and ICU days, readmission within 30 days of discharge, emergency department (ED) visits, OP visits, and other visits (e.g., home care). In addition, specialist visits (i.e., cardiologist, pulmonologist) and PH-related diagnostic tests in any setting were reported (Supplementary Table 3, see ESM). All-cause and PH-related health care costs PPPM were measured during the study period and included total costs, medical costs (i.e., hospitalization, ICU, ED, OP, other, specialist, PH-related diagnostic test costs), and pharmacy costs based on standardized costs in health insurance claims. All-cause HRU and medical costs were identified based on all medical claims; all-cause pharmacy costs were defined as pharmacy claims for any treatment. PH-related HRU and medical costs were defined based on medical claims with a PH-related diagnosis; PH-related pharmacy costs were defined as pharmacy claims for PAH-related treatments (i.e., PDE5i [sildenafil, tadalafil; excluding dosage consistent with Viagra or Cialis], ERA [ambrisentan, bosentan, macitentan], soluble guanylate cyclase stimulators [riociguat], and PPA [epoprostenol, iloprost, treprostinil or selexipag]). Costs were inflated to 2021 US dollars based on the Medical Care component of the US Bureau of Labor Statistics Consumer Price Index (https://www.bls.gov/cpi/).
2.4 Statistical Analyses
Patient characteristics were described overall and by study cohort using means, standard deviations (SDs), and medians for continuous variables, and frequencies and proportions for categorical variables. Standardized differences were used to evaluate differences in patient characteristics between study cohorts [15].
Time from PAH symptom onset to first PAH diagnosis was assessed using Kaplan-Meier analysis. The association between diagnosis delay and HRU and health care costs during the study period was assessed using regression models adjusting for baseline covariates (i.e., demographics, clinical characteristics, HRU and health care costs) to control for observable potential confounders at PAH symptom onset. Nonparametric bootstrap procedures with 500 replications were used to evaluate statistical significance and 95% confidence intervals (CIs) of incidence rate ratios (IRRs) for HRU and mean cost differences for health care costs. To investigate whether delayed diagnosis was associated with increased HRU and health care costs beyond the first year post-diagnosis, a sensitivity analysis was conducted where the study period spanned from the index date until the end of continuous insurance eligibility or data availability. All analyses were conducted using SAS Enterprise Guide 7.15.
3 Results
3.1 Study Sample
After applying the eligibility criteria, the final study sample comprised 538 patients, including 327 (60.8%) with a diagnosis delay of ≤12 months, 126 (23.4%) with a diagnosis delay of 12–24 months, and 85 (15.8%) with a diagnosis delay of >24 months (Fig. 2).
3.2 Baseline Characteristics
Patient characteristics overall and stratified by study cohorts are presented in Table 1. In the overall sample, patients had a mean age at PAH symptom onset of 65.6 years and 60.6% were female. The mean Quan-Charlson comorbidity index score was 2.7, with systemic hypertension (70.3%), diabetes mellitus (39.4%; either type 1 or 2), congestive heart failure (26.4%), obesity (26.0%), and chronic obstructive pulmonary disease (24.0%) being the most common comorbidities. Most patients were on antihypertensive agents (75.3%), while other common pharmacological treatments included antidepressants and oral steroids. In the overall sample, mean (median) total all-cause health care costs during the baseline period were US$2030 ($799) PPPM, largely driven by medical costs.
When stratifying by study cohorts, patients with ≤12 months’ delay were on average younger than those with 12–24 months’ and >24 months’ delay, but had numerically higher total all-cause health care costs during the baseline period (US$2165 vs US$1708 and US$1990 PPPM, respectively; Table 1).
3.3 Pre-Diagnosis Characteristics
Mean (median) diagnosis delay was 4.0 (2.9) months in the ≤12 months’ delay cohort, 17.4 (17.7) months in the 12–24 months’ delay cohort, and 33.5 (31.7) months in the >24 months’ delay cohort (Table 1), with overall diagnosis delay of 11.8 (8.3) months (Fig. 3).
Patients had an average of 1.2 distinct PAH symptoms recorded at PAH symptom onset (patients may have had more than one recorded symptom on the date of symptom onset) and 2.7 distinct PAH symptoms recorded between PAH symptom onset and PAH diagnosis (i.e., the pre-diagnosis period). Unspecified dyspnea was the most common symptom recorded both at symptom onset and during the pre-diagnosis period (51.3% and 88.1%, respectively), followed by chest pain, fatigue, and edema (>15% and >35%, respectively; Fig. 4).
3.4 Association Between Diagnosis Delay and Health Care Resource Utilization and Costs
The mean (median) duration of the study period was 11.5 (12.0) months in the ≤12 months’ delay cohort, 10.9 (12.0) months in the 12–24 months’ delay cohort, and 9.7 (12.0) months in the >24 months’ delay cohort. During the study period, patients in the ≤12 months’ delay cohort had lower all-cause HRU PPPM than those in the 12–24 and >24 months’ delay cohorts (hospitalization: 0.12 vs 0.16 and 0.19 stays; ICU: 0.07 vs 0.10 and 0.14 stays; OP: 4.01 vs 4.75 and 4.62 visits, respectively). Most of the hospitalizations were driven by PH-related HRU (Table 2).
In adjusted regression analyses, patients in the 12–24 months’ and >24 months’ delay cohorts incurred higher rates of all-cause HRU compared with those in the ≤12 months delay cohort, including more hospitalizations, ICU stays, and OP visits; similar trends were observed for PH-related HRU (all p < 0.05). Compared with patients in the ≤12 months’ delay cohort, those in the >24 months’ delay cohort also had higher rates of all-cause ED visits and readmission within 30 days of discharge. Longer delays were also associated with higher rates of PH-related specialist visits and PH-related diagnostic tests (Table 2).
During the study period, all-cause health care costs PPPM averaged US$12,907 for patients in the ≤12 months’ delay cohort compared with US$15,829 and US$16,312 in the 12–24 and >24 months’ delay cohorts, respectively. In adjusted regression analyses, total all-cause health care costs PPPM were US$3986 (95% CI 1439–6436) higher in the 12–24 months’ delay cohort and US$5366 (2107–8524) higher in the >24 months’ delay cohort compared with the ≤12 months’ delay cohort, driven largely by hospitalization costs. Similar results were found for PH-related health care costs. Costs for PH-related specialist visits and PH-related diagnostic tests (in any setting) were also significantly higher in longer delay cohorts (Table 3).
3.5 Sensitivity Analyses
When prolonging the study period to the end of health insurance eligibility or data availability, the duration of the study period was 30.3 (32.3) months in the ≤12 months of diagnosis delay cohort, 23.9 (22.7) months in the 12–24 months’ delay cohort, and 14.9 (12.5) months in the >24 months’ delay cohort. Despite differences in the overall duration of the study period, similar trends were found in the sensitivity analyses.
In adjusted regression analyses, patients in the 12–24 and >24 months’ delay cohorts incurred higher rates of all-cause HRU compared with those in the ≤12 months’ delay cohort, including more hospitalizations, OP visits, and PH-related diagnostic tests (all p < 0.05). Similar trends were found for PH-related HRU. This increased HRU translated into total health care costs PPPM that were US$2043 (95% CI −356 to 4285) higher in the 12–24 months’ delay cohort and US$3082 (−20 to 6139) higher in the >24 months’ delay cohort compared with the ≤12 months’ delay cohort.
4 Discussion
In this retrospective, claims-based study among patients with PAH, delayed diagnosis was associated with significantly increased HRU and health care costs during the year following the first PH-related diagnosis, particularly for hospitalizations. These results were confirmed in a sensitivity analysis showing that the impact of delayed diagnosis on HRU and health care cost burden lasted beyond the first year post-diagnosis.
Prior studies have reported average diagnosis delays ranging from 2.5 to 3.9 years, which is longer than what we observed [7,8,9,10, 16]. Our study was not designed to assess the average diagnosis delay and thus did not require a minimal follow-up duration, unlike other studies which did have this requirement [16]. Therefore, in our study, it is likely that some patients were lost to follow-up prior to being diagnosed, resulting in a sample more skewed towards shorter delay than in other studies. Regarding symptoms prior to PAH diagnosis, we found that unspecified dyspnea was experienced by the vast majority of patients (>80%) at any time prior to diagnosis and was the most common symptom present at PAH symptom onset. This finding is consistent with those reported by other retrospective studies of PAH populations [8, 16].
There is currently scarce information on the association between diagnosis delay and HRU and costs among patients with PAH. As such, this study provides important context to the findings of previous studies on the economic burden of PAH overall. Prior evidence indicates that diagnosed PAH is associated with a substantial economic burden in the US [17,18,19,20,21]. In a retrospective administrative claims-based study of patients with diagnosed PAH in the US from 2015 to 2020, mean total all-cause health care costs after PAH treatment initiation (mean follow-up of 1.5 years) were US$14,201 PPPM [21], which is consistent with the mean total all-cause health care costs obtained in our overall sample (US$14,129 PPPM). Previous studies further suggest that hospitalizations are the main driver of the substantial HRU and cost burden among patients with PAH [6, 18, 20, 22,23,24,25,26,27,28]. Indeed, several studies have shown that average total health care costs (ranging between US$2023 and US$9353 PPPM across studies) were driven by hospitalization costs (between 40% and 60% of total costs across studies), while pharmacy costs accounted for a lower proportion of costs (between 15% and 40% of total costs across studies) [18, 24,25,26,27]. Moreover, patients in prior studies have been shown to incur substantial HRU and costs prior to receiving a diagnosis of PAH, primarily due to high rates of hospitalization [24, 29]. Consistent with this prior evidence, the present study observed substantial total all-cause health care costs following PAH diagnosis, which were largely driven by the high costs of hospitalization. Our study adds to this previous body of literature by highlighting the economic consequences of delayed diagnosis, namely an incremental rise in HRU and costs with longer diagnosis delay.
In the year following diagnosis, our findings suggest that diagnosis delays of 12–24 months and >24 months are associated with increased costs of US$47,832 and US$64,392 per patient, respectively, relative to a ≤12 months delay. Given a PAH incidence of 2.0 per million adult individuals [30] and the US population [31], it is estimated that more than 500 patients could be diagnosed with PAH in a given year. Based on these prior estimates, our findings suggest that diagnosing individuals within 12 months of their PAH symptom onset could lead to a reduction in health care costs of more than US$20 million in the year following their diagnosis.
A primary goal in PAH clinical practice is to reduce the burden of hospitalization among patients, as it is among the key indicators of disease progression. Our results highlight the importance of early PAH diagnosis as delays are associated with downstream increases in HRU costs following diagnosis, particularly due to high rates of hospitalization. Evidence suggests that the burden of PAH could be mitigated through a wide range of recommended PAH treatments with demonstrated efficacy, including combination therapy targeting the nitric oxide, endothelin, and prostacyclin pathways [4]. In addition to their positive impact on survival, these PAH treatments have been shown to reduce the frequency of hospitalizations among patients in a number of clinical trials [5, 32,33,34]. Further, PH-related pharmacy costs in the present study made up approximately one fifth of the total health care costs, suggesting that investment in early treatment could eventually be offset by a downstream reduction in hospitalization costs. Thus, earlier screening and diagnosis could ensure that patients gain prompt access to effective PAH treatments, potentially mitigating the high HRU and cost burden associated with more advanced, severe PAH [6, 13, 35].
In terms of priorities, greater attention should be focused on identifying patients who present with more subtle and non-specific symptoms, as they are more likely to be overlooked for PAH screening in routine clinical practice. Given the difficulty in identifying such patients, algorithms for the early detection of PAH are currently being developed to help to streamline the process and address gaps in care. For instance, a machine-learning model recently developed using retrospective health insurance claims data was able to distinguish between patients with PAH and controls, correctly identifying 73% of patients with PAH 6 months prior to a confirmed PAH diagnosis [36]. Moreover, PAH diagnosis could be expedited through earlier screening with commonly available tests, including an artificial intelligence-driven algorithm for electrocardiogram data [37, 38] and an automated decision support tool for echocardiograms (Us2.ai) [39, 40]. Additionally, clinicians may benefit from learning tools (EchoRight app) that could enable them to better evaluate signs of PH based on echocardiography data [41]. By helping to identify patients that should be further evaluated for PH [37, 38], these novel algorithms and learning tools have the potential to further reduce the economic burden of PAH on the health care system, particularly due to delayed diagnosis.
4.1 Limitations
The present study was subject to certain limitations. Patients may have been misclassified in a given cohort due to inaccuracies in health insurance claims data. For instance, it is possible that some patients might have been incorrectly diagnosed with PAH and treated with a PAH-related medication when in fact they might have had other types of PH. Several diagnosis codes are used in clinical practice for PAH, such that the population of interest may not be identified with a single ICD-10-CM diagnosis code. Therefore, a broader set of PH-related diagnosis codes were used to identify the relevant patient population. To further increase the likelihood that the study sample would capture patients with PAH, at least one claim for PAH-related treatments and RHC were also required for inclusion in the study, while patients with a claim for CTEPH or a CTEPH-related procedure were excluded. However, this may have led to the exclusion of true PAH patients who did not undergo RHC such that the sample may not be representative of the overall PAH population. Additionally, since diagnosis delay could not be measured directly, it was identified based on information available in health insurance claims data such as diagnosis codes. For instance, although PAH symptom onset was defined as the date of the first PAH symptom claim recorded in the claims data, it is possible that the PAH symptom started before the patient sought care for it. Moreover, approximately one third of patients did not have a PAH symptom recorded on a medical claim before their first PAH diagnosis. Since it was unclear whether these patients had experienced any PAH symptoms prior to diagnosis, they were not included in the study. Thus, the results of this study should be interpreted as the delay in diagnosis after the patient sought medical attention for a PAH symptom rather than the delay following symptom diagnosis. This study was also unable to capture patients with PH-related symptoms who died without ever receiving a PH-related diagnosis or treatment, leading to potential survival bias.
While the analysis adjusted for potential confounders to account for differences in patient groups, there may have been some residual confounding or unmeasured confounding due to unobservable factors; thus, causal inferences may not be drawn from this study. In particular, disease severity information (e.g., WHO functional class) was not available in the claims database, and hence could not be adjusted for. This represents an important limitation of the present study. In a study by Dufour et al. [35], greater PAH severity, as indicated by higher WHO functional class, was associated with an increased likelihood of hospitalization and significantly higher PH-related costs. Thus, the increase in HRU costs with diagnosis delay might reflect greater PAH severity among patients with longer delays, as their disease has remained uncontrolled for an extended period. On the other hand, all-cause total health care costs PPPM during the baseline period were highest among the ≤12 months’ delay cohort, which could reflect greater disease severity at symptom onset leading to an earlier diagnosis. To the extent that patients in the shorter delay cohort had more severe disease, this may have led to an underestimation of the impact of diagnosis delay on health care costs at follow-up. However, this hypothesis cannot be confirmed given lack of disease severity information in claims data.
The costs presented reflected standardized costs recorded in claims data, which may not equate to actual costs or paid amounts. In addition, PH-related HRU and costs were identified based on diagnosis recorded in claims, but it is not a confirmation that the medical services were sought specifically due to PAH. Finally, given that our study population was predominantly from the South and enrolled in commercial or Medicare Advantage plans, the results may not be generalizable to patients in the rest of the US, including those with other types of health insurance plans or without health insurance.
5 Conclusions
Delayed PAH diagnosis was associated with an increased economic burden post-diagnosis that was primarily driven by hospitalizations, whereas PH-related pharmacy costs were a relatively small portion of this burden. Moreover, longer delay in PAH diagnosis was associated with an incremental risk of ICU stays and 30-day readmissions, which are also relevant from a payer, health system, and policy perspective. These findings highlight the need for increased awareness and earlier screening in routine clinical practice, as this could provide an opportunity for earlier treatment which might lead to better clinical outcomes among patients with PAH and reduced economic burden for payers, employers, and society at large.
References
Leber L, Beaudet A, Muller A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: identification of the most accurate estimates from a systematic literature review. Pulm Circ. 2021;11(1):2045894020977300.
Frost AE, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US Contemporary Registries. Chest. 2011;139(1):128–37.
Thenappan T, et al. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360: j5492.
Humbert M et al., 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2022.
Burger CD, et al. Early intervention in the management of pulmonary arterial hypertension: clinical and economic outcomes. Clinicoecon Outcomes Res. 2017;9:731–9.
Burgoyne DS. Reducing economic burden and improving quality of life in pulmonary arterial hypertension. Am J Manag Care. 2021;27(3 Suppl):S53–8.
Khou V, et al. Diagnostic delay in pulmonary arterial hypertension: Insights from the Australian and New Zealand pulmonary hypertension registry. Respirology. 2020;25(8):863–71.
Strange G, et al. Time from symptoms to definitive diagnosis of idiopathic pulmonary arterial hypertension: The delay study. Pulmonary circulation. 2013;3(1):89–94.
Humbert M, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173(9):1023–30.
Badesch DB, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376–87.
Brown LM, et al. Delay in recognition of pulmonary arterial hypertension: factors identified from the REVEAL Registry. Chest. 2011;140(1):19–26.
Zanatta E, et al. Pulmonary arterial hypertension in connective tissue disorders: pathophysiology and treatment. Exp Biol Med (Maywood). 2019;244(2):120–31.
Studer SM, et al. Considerations for optimal management of patients with pulmonary arterial hypertension: a multi-stakeholder roundtable discussion. Am J Manag Care. 2017;23(6 Suppl):S95–104.
Quan H, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–82.
Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228–34.
Didden EM, et al. Time to diagnosis of pulmonary hypertension and diagnostic burden: a retrospective analysis of nationwide US healthcare data. Pulm Circ. 2023;13(1): e12188.
Studer S, et al. Treatment patterns, healthcare resource utilization, and healthcare costs among patients with pulmonary arterial hypertension in a real-world US database. Pulm Circ. 2019;9(1):2045894018816294.
Ogbomo A, et al. The direct and indirect health care costs associated with pulmonary arterial hypertension among commercially insured patients in the United States. J Manag Care Spec Pharm. 2022;28(6):608–16.
Sikirica M, et al. The economic burden of pulmonary arterial hypertension (PAH) in the US on payers and patients. BMC Health Serv Res. 2014;14:676.
Phatak H, et al. A systematic literature review of the economic burden and cost drivers in pulmonary arterial hypertension. Value in Health. 2021;24:S200–1.
Pizzicato LN, et al. Real-world treatment patterns, healthcare resource utilization, and cost among adults with pulmonary arterial hypertension in the United States. Pulm Circ. 2022;12(2): e12090.
Burke JP, et al. Characterizing pulmonary hypertension-related hospitalization costs among Medicare Advantage or commercially insured patients with pulmonary arterial hypertension: a retrospective database study. Am J Manag Care. 2015;21(3 Suppl):s47-58.
Burger CD, et al. Characterization of first-time hospitalizations in patients with newly diagnosed pulmonary arterial hypertension in the REVEAL registry. Chest. 2014;146(5):1263–73.
Copher R, et al. Treatment patterns and healthcare system burden of managed care patients with suspected pulmonary arterial hypertension in the United States. J Med Econ. 2012;15(5):947–55.
Said Q, et al. The cost to managed care of managing pulmonary hypertension. J Med Econ. 2012;15(3):500–8.
Kirson NY, et al. Excess costs associated with patients with pulmonary arterial hypertension in a US privately insured population. Appl Health Econ Health Policy. 2011;9(5):293–303.
Angalakuditi M, et al. Treatment patterns and resource utilization and costs among patients with pulmonary arterial hypertension in the United States. J Med Econ. 2010;13(3):393–402.
Gu S, Hu H, Dong H. Systematic review of the economic burden of pulmonary arterial hypertension. Pharmacoeconomics. 2016;34:533–50.
Bergemann R, et al. High levels of healthcare utilization prior to diagnosis in idiopathic pulmonary arterial hypertension support the feasibility of an early diagnosis algorithm: the SPHInX project. Pulm Circ. 2018;8(4):2045894018798613.
McGoon MD, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62(25 Suppl):D51–9.
U.S. Census Bureau. American Community Survey 1-Year Estimates. 2021 03-15-2023]; Available from: https://data.census.gov/table?q=Population+by+age&tid=ACSST1Y2021.S0101.
Galie N, et al. Initial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension. N Engl J Med. 2015;373(9):834–44.
Pulido T, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369(9):809–18.
Chin KM, et al. Three- Versus Two-Drug Therapy for Patients With Newly Diagnosed Pulmonary Arterial Hypertension. J Am Coll Cardiol. 2021;78(14):1393–403.
Dufour R, et al. Healthcare resource utilization and costs for patients with pulmonary arterial hypertension: real-world documentation of functional class. J Med Econ. 2017;20(11):1178–86.
Bettencourt, K.C., et al. A Claims-Based, Machine-Learning Algorithm to Identify Patients with Pulmonary Arterial Hypertension. in American Thoracic Society 2022. 2022. San Fransisco, CA, USA.
Wagner T et al. An Automated Screening Algorithm Using Electrocardiograms for Pulmonary Hypertension, in D3. D003 COME TOGETHER - CLINICAL ADVANCES IN PULMONARY HYPERTENSION: LESSONS FROM BEST ABSTRACTS. p. A1179-A1179.
Wagner TE et al. Early Detection of Pulmonary Arterial Hypertension Using a Pulmonary Hypertension Electrocardiogram Algorithm, in D96. EMBARCADERO: MACHINES AND DEEP PHENOTYPING OF THE PULMONARY CIRCULATION. p. A5300-A5300.
Us2.ai. Us2.ai receives FDA clearance for the first fully automated solution measuring both 2D and Doppler cardiac ultrasound images to produce a complete patient report. 2021 [cited 2023 June 9]; Available from: https://us2.ai/wp-content/uploads/2022/01/FDA-clearance-release-2.0.pdf.
Tromp J, et al. A formal validation of a deep learning-based automated workflow for the interpretation of the echocardiogram. Nat Commun. 2022;13(1):6776.
Maia M. EchoRight Pro app aims to speed pulmonary hypertension diagnosis: Game-style use of real-world echocardiography cases to teach signs of PH. 2023 [cited 2023 May 2]; https://pulmonaryhypertensionnews.com/news/echoright-app-aims-speed-pulmonary-hypertension-diagnosis/.
Acknowledgements
This study was funded by Janssen Scientific Affairs, LLC. The study sponsor was involved in several aspects of the research, including the study design, interpretation of data, writing of the manuscript, and decision to submit the manuscript for publication. Medical writing assistance was provided by professional medical writer, Mona Lisa Chanda, PhD, an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, which funded the development and conduct of this study and manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Janssen Scientific Affairs, LLC. The study sponsor was involved in several aspects of the research, including the study design, interpretation of data, writing of the manuscript, and decision to submit the manuscript for publication.
Medical writing
Medical writing assistance was provided by professional medical writer, Mona Lisa Chanda, PhD, an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, which funded the development and conduct of this study and manuscript.
Conflict of interest
HDG and SP were employees of Janssen Scientific Affairs, LLC at the time of the study conduct (now Johnson and Johnson Innovative Medicines) and may own stock/stock options. JL is an employee of Janssen-Cilag GmbH and may own stock/stock options. MGL, AS, AMM, MC, and PL are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, which funded the development and conduct of this study and manuscript. HMD has received research grants from Bayer Pharmaceuticals, consultancy fees from Janssen, Pharmaceutical Companies of Johnson & Johnson, and has served on advisory boards for Janssen Pharmaceutical Companies of Johnson & Johnson and United Therapeutics. RPF is scientific medical advisor to Altavant, ShouTi, Liquidia Technologies, Merck, Tenax Pharmaceuticals, and Janssen Pharmaceutical Companies of Johnson & Johnson and has received consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Gossamer Bio, Merck, Tenax, Insmed, and is on DSMB for Aerovate. His institution has received funding from Bayer and Gossamer Bio.
Author contributions
This study was conceptualized and designed by HDG, SP, MGL, AS, AMM, MC, and PL. Data analysis and interpretation were performed by HMD, HDG, SP, JL, MGL, AS, AMM, MC, PL, and RPF. All authors have made substantial contributions to the drafting of the manuscript and have revised it critically for important intellectual content. All authors have provided final approval of this version to be published and agree to be accountable for all aspects of the work.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to third-party restrictions. The data that support the findings of this study are available from Optum®. Restrictions apply to the availability of these data, which were used under license for this study. However, data could be available from the corresponding author on reasonable request, provided that permission has been granted by Optum®.
Code availability
All analyses were conducted using SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC, USA). The SAS programs are proprietary materials of Analysis Group, Inc.; therefore, restrictions apply to the access of these codes, which cannot be made available publicly.
Ethics approval and informed consent
This observational study was conducted using de-identified, commercially available data from a secondary health care database that complies with the requirements of the HIPAA of 1996. Therefore, ethics approval and consent to participate are not applicable for the current study per Title 45 of Code of Federal Regulation, Part 46.101(b)(4) (https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/#46.101). All methods were carried out in accordance with relevant guidelines and regulations, including the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Previous presentations
N/A.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
DuBrock, H.M., Germack, H.D., Gauthier-Loiselle, M. et al. Economic Burden of Delayed Diagnosis in Patients with Pulmonary Arterial Hypertension (PAH). PharmacoEconomics Open 8, 133–146 (2024). https://doi.org/10.1007/s41669-023-00453-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-023-00453-8