Abstract
Introduction
The efficacy and safety of empagliflozin in the treatment of heart failure with preserved ejection fraction (HFpEF) were demonstrated in the EMPEROR-Preserved trial, which showed a 21% reduction in combined risks of cardiovascular death or HF hospitalization [hazard ratio (HR) 0.79; 95% confidence interval (CI) 0.69–0.90, p < 0.001] and a 27% reduction in the total number of HF hospitalizations (HR 0.73; 95% CI 0.61–0.88, p < 0.001) compared with placebo. On the basis of these results, the present study aimed to assess the cost-effectiveness of empagliflozin + standard of care (SoC) compared with SoC alone in the treatment of HFpEF.
Methods
A published Markov model was adapted to compare the health and economic outcomes in France, considering a collective perspective, in patients treated with empagliflozin in addition to SoC versus patients treated by SoC alone. The model simulated the intention-to-treat (ITT) population of the trial, transitioning between four mutually exclusive health states representing the quartiles of the Kansas City Cardiomyopathy Questionnaire-Clinical Summary Score (KCCQ-CSS). For each arm, the model estimated (over a lifetime time horizon) the economics and the health outcomes (HF hospitalizations avoided, and life years and quality-adjusted life years (QALYs) gained) to calculate the incremental cost-effectiveness ratios (ICERs). The resources used were derived by pairing the FREnch Survey on HF (FRESH) cohort data to French health insurance claims data, and the utilities were derived on the basis of the EQ-5D-5L questionnaire valued on the French tariff. Both economic and health outcomes were discounted at a 2.5% annual rate.
Results
The model predicted that treatment of HFpEF patients with empagliflozin would prevent, for 1000 patients treated, 74 HF hospitalizations and 15 deaths attributable to cardiovascular events, resulting on average in a gain of 1 month in overall survival (7.24 versus 7.16 years with placebo) and 0.11 QALYs (6.14 versus 6.03 with placebo). Empagliflozin costs were partially offset by the cost savings from avoided hospitalizations. The ICERs were €18,597 per life year gained and €13,980 per QALY gained. The sensitivity analyses conducted showed that empagliflozin has a 65% probability to be cost-effective under the €25,000/QALY threshold.
Conclusions
The base-case results showed that empagliflozin is a cost-effective strategy for management of HFpEF, in addition to the impact on public health by preventing HF-hospitalizations and deaths in France. Sensitivity analyses suggest that 65% of simulations are under the €25,000/QALY threshold.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Empagliflozin in the treatment of heart failure with preserved ejection fraction (HFpEF) would prevent 74 HF hospitalizations and 15 deaths attributable to cardiovascular events for 1000 patients treated with empagliflozin + standard of care (SoC) versus SoC alone. |
Empagliflozin costs were partially offset by cost savings from avoided hospitalizations (up to €166,000 for 1000 patients treated). |
Empagliflozin is a cost-effective strategy for management of HFpEF in addition to the impact on public health by preventing HF hospitalizations and deaths in France with a slight uncertainty described in a sensitivity analysis. |
1 Introduction
Heart failure (HF) is a clinical syndrome characterized by symptomatic left ventricle (LV) dysfunction involving a broad range of complex mechanisms that originate from structural or functional ventricular impairment. Although the manifestations of heart failure are diverse, two main phenotypes can be identified: heart failure with reduced ejection fraction (HFrEF, when the ejection fraction is less than or equal to 40%) and heart failure with preserved ejection fraction (HFpEF, when the ejection fraction is greater than or equal to 50%). Patients suffering from these two forms present different comorbidities and respond differently to treatments [1, 2]. While HFrEF treatments exist to attenuate the overactivation of endogenous neurohormonal systems, therapeutic options for patients with HFpEF are limited. Some benefits have been observed with mineralocorticoid-receptor antagonists, but the magnitude of the effect was modest [3,4,5] and currently no therapies have demonstrated a substantial reduction in mortality in patients with HFpEF. The standard of care (SoC) consists of beta-blockers (BB), associated with an angiotensin-converting enzyme inhibitor (ACEi), or angiotensin receptor blockers (ARB), mineralocorticoid receptor antagonists (MRA), or an angiotensin receptor neprilysin inhibitor (ARNi; for patients with heart failure with mildly reduced ejection fraction only). Recently, expert consensus statement established that there is currently no SoC beyond sodium-glucose cotransporter 2 (SGLT2) inhibitor for HFpEF [6]. Heart failure (HF) is a major and escalating public health problem affecting over 26 million people worldwide [7], among whom 50% have a preserved ejection fraction [8]. In addition to the significant impact on public health, HF is associated with substantial reductions in patient quality of life (QoL) and a financial burden for healthcare systems [1, 2].
In France, it is estimated that 2.3% of the population suffer from HF, and 10% among those are over 70 years old, leading to more than 160,000 hospitalizations and 70,000 deaths each year [9].
Empagliflozin is an orally bioavailable, highly effective, reversible, and selective inhibitor of SGLT2, initially approved for the treatment of type II diabetes. Although the mechanism of empagliflozin’s cardioprotective and nephroprotective effects are unknown on a molecular level, several distinct possibilities have been identified and are currently being investigated, such as triggering osmotic diuresis and natriuresis, improving myocardial and renal metabolism, preventing adverse cardiac remodeling through inhibition of inflammation, directly inhibiting the Na+/H+ exchanger in the myocardium, and preventing ischemia/reperfusion injury through a decrease in calmodulin kinase II activity [10].
The efficacy and safety of empagliflozin (Jardiance®) in HF have been studied in the EMPEROR trials, including two large-scale clinical trials in patients with HF with EF ≤ 40% (EMPEROR-Reduced, NCT03057977) [11] and HF with EF > 40% (EMPEROR-Preserved, NCT03057951) [12]. EMPEROR-Preserved is a phase III, randomized, double-blind trial including adults with New York Heart Association (NYHA) functional class II–IV chronic heart failure (cHF) and left ventricular ejection fraction (LVEF) of more than 40%. After a screening period, participants from 622 centers in 23 countries were randomly assigned in 1:1 ratio to receive either placebo or empagliflozin (10 mg per day), in addition to SoC. The primary outcome was time to the first event of adjudicated cardiovascular (CV) death or adjudicated hospitalization for heart failure (HHF). The key secondary outcomes were occurrence of adjudicated hospitalization for heart failure (including first and recurrent events), and the second one was the rate of decline in the estimated glomerular filtration rate (eGFR) [11, 12].
Over the 26-month follow-up period, 8.6% (259/2997) of the patients receiving empagliflozin were hospitalized owing to heart failure versus 11.8% (352/2991) in the placebo arm, and 7.3% (219/2997) died from cardiovascular causes versus 8.2% (244/2991) in the placebo arm; i.e., a reduction of 29% (HR 0.71; 95% CI 0.60–0.83) and 9% [HR 0.91; 95% confidence interval [CI] 0.91 (0.76–1.09)], respectively, establishing empagliflozin as an interesting option in the management of HF across the spectrum of LVEF [11, 12].
The aim of this study is to assess the public health impact and the cost-effectiveness of the introduction of empagliflozin in association with SoC in the treatment of HFpEF in French practice. The analysis compares the health and economic outcomes that would occur in an arm treated with empagliflozin in association with SoC and in an arm treated by SoC alone.
2 Methods
2.1 Pharmacoeconomic Model
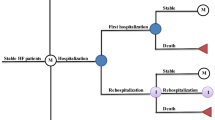
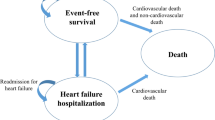
The model was built in Excel on the basis of a Markovian structure previously developed to assess the cost-effectiveness of empagliflozin in the treatment of HFrEF and adapted for HFpEF. This model has been methodologically accepted by the National Institute for Health and Care Excellence (NICE) as part of a medico-economic evaluation [13]. Four mutually exclusive health states were defined based on Kansas City Cardiomyopathy Questionnaire-Clinical Summary Score (KCCQ-CSS) quartiles. The KCCQ-CSS score is a patient-reported outcome instrument that allows assessment of the symptoms and physical limitations experienced by the patients. A diminution in the score, therefore, translates into a deterioration of the patient’s physical function and a worsening of the symptoms. The cohort simulated is the intention-to-treat (ITT) population of EMPEROR-Preserved (Table 1). At each cycle of 1 month, patients transitioned between the health states based on time-varying and treatment-specific transition probabilities. The monthly event rates (i.e., HF hospitalization rate, CV-related mortality, and all-cause (AC) mortality) were then applied (Fig. 1). In each treatment arm, the number of hospitalizations, deaths, QALYs lost, and costs associated with these events were generated throughout the patient’s life (lifetime time horizon). In addition, QALYs lost and costs attributable to the management of the adverse events were also accounted for. To compare the two strategies, incremental cost-effectiveness ratios (ICERs) were calculated, one informing the incremental cost per QALYs gained and a second informing the incremental cost per life year (LY) gained. Both health and economic outcomes were discounted considering a 2.5% annual rate as recommended in French guidelines [14]. Deterministic and probabilistic sensitivity analyses were conducted to assess the uncertainty around the model parameters.
Model structure. CV cardiovascular, HHF hospitalization for heart failure, KCCQ-CSS Kansas City Cardiomyopathy Questionnaire-Clinical Summary Score, PXY transition probability from X to Y, QX Quartile X, r intercurrent event probability, \({m}_{CV}\) cardiovascular mortality, \({m}_{ACM}\) all-cause mortality
2.2 Clinical Data
For each arm, the transition probabilities between the KCCQ-CSS quartiles were derived from an analysis of the EMPEROR-Preserved trial data for the following time points: months 1 to 3, months 4 to 8, and months 9 and higher (Table 2). To estimate the risk of HHF events and the CV and AC mortality over time, a distinct set of survival, for AC death and CV death, and risk equations for HHF were derived. These equations consider a coefficient depending on the KCCQ-CSS quartiles, as a time-varying predictor, and the treatment effect, in the empagliflozin arm. The inclusion of the coefficients was supported by statistical analyses on the EMPEROR trial data and key opinion leaders' (KOL) opinions. There were a total of 12 KOLs, including 1 pharmacist, 2 HF nurse specialists, 2 general practitioners (GP), 3 consultant cardiologists, 1 consultant nephrologist, 1 cardiology professor, 1 metabolic medicine professor, and 1 cardiologist professor and researcher. The KOLs were asked to confirm if they supported the derived risk equation for HHF based on KCCQ-CSS quartiles as a time-varying predictor. All KOLs agreed with the proposed risk equations. As a result, for the AC mortality, no treatment effect was considered as it was considered as not clinically relevant by the KOL interrogated. Then, the monthly rate of first and recurrent HHF (Fig. 2), mortality (CV, Fig. 3 and AC, Fig. 4), and treatment discontinuation were extrapolated beyond the EMPEROR-Preserved trial duration by applying parametric distributions to the data. For HHF, a Poisson regression with generalized estimating equations (GEEs) was considered. For both CV and AC mortality, a Weibull distribution was selected on the basis of the statistical fit and the plausibility of the results generated. The coefficients generated for the survival equation are presented in the Supplementary Material. For treatment discontinuation, a generalized gamma distribution was selected as it was the most conservative model and was the closest fitting to the trial data on visual inspection. In all the regression models, the treatment-specific and time-varying KCCQ-CSS health states were used as predictors of the disease progression. The treatment-specific rates of AEs were derived from the EMPEROR-Preserved trial and included if the difference in event rates between the two comparators was > 1%. The AEs included are presented in Table 1.
2.3 Health-Related Quality of Life
Health-related quality of life (HrQoL) inputs associated with KCCQ-CSS health states, HHF, and adverse events were obtained from the EMPEROR-Preserved trial. The data were collected using the EQ-5D-5L administered at baseline, at weeks 12, 32, 52, and 100, and/or at treatment discontinuation; and 30 days post completion. A linear mixed model was fit to account for repeated measures on some patients. The model incorporated time-varying indicators for HHF in 0–1 month, 1–2 months, 2–4 months, and 4–12 months prior or not to being hospitalized, as well as time-varying levels of KCCQ-CSS quartiles. The estimates were valued based on the French tariff [15]. The impact of HHF on quality of life was captured as a one-off QALY loss and calculated based on HRQoL decrements measured at four different time points (0–1 month, 1–2 months, 2–4 months, and 4–12 months), after patients experienced HHF. Similarly, a utility decrement was considered for each adverse event and applied during one cycle of the model (Table 3).
2.4 Resource Use and Economic Inputs
The pharmacoeconomic model accounts for the cost of drug acquisition, disease management (medical consultations and examinations), event management (HHF and CV deaths), and adverse events management. The costs were derived considering a collective perspective [14], thus expenses covered by the different insurance schemes and the patients’ participation were accounted for. All costs are expressed in 2021 Euros (€), adjusted for inflation using the French Consumer price index for health services and products (base 2015, all households), when necessary [16].
2.5 Drug Costs
Drug costs for empagliflozin 10 mg and SoC therapies were extracted from the French Health Insurance drug and pricing information database. For each of them, the latest price published by the official instance was considered for the indicated dosages, as reported in the Summary of Product Characteristics (SmPC) [17]. In addition, dispensing fees were accounted for, i.e., €1.02 per pack, €0.51 per prescription, and an additional €1.58 specific to prescriptions dispensed to individuals aged 70 years and over (the mean age at model entry was 72 years) [18]. For the SoC, an overall treatment cost was estimated by calculating the average treatment cost weighted by distribution of the patients among the different pharmacologic classes presented in Table 3. It was however assumed that the different drugs among the pharmacological classes were distributed uniformly among the patients. Monthly treatment costs were applied until treatment discontinuation.
2.6 Disease Management
The cost of HFpEF management includes medical consultations, medical examinations, and technical procedures carried out as part of the follow-up of the patient and their disease. The specific medical investigations, procedures, and treatments, and their frequencies, were identified via the national guidelines published by the French Health agency and the FREnch Survey on HF (FRESH)-SNDS study, an observational study using secondary data extracted from the FRESH clinical cohort linked to the French administrative healthcare database (SNDS, Système National des Données de Santé) conducted from 2014 to 2018 (with data available from 2012) [19]. The average consultation fee (including the patient’s out-of-pocket) for each specialist (general practitioner, cardiologist) was calculated by the ratio of the total fees received and the total activity. Biological examinations were valued by using the French quotation system using the National Biology Coding Table [20]. The national tariff as reported in the French classification for clinical procedures published in 2022 CCAM (Classification Commune des Actes Médicaux) was considered for the other medical procedures.
2.7 Events Management
The expenses incurred for the management of HHF and CV death were valued on the basis of the National Cost Survey (ENC, Etude nationale des coûts) providing the production cost per diagnosis-related group (DRG) [21]. When the production costs could not be used (because the quality of the estimation was insufficient or the sample was too small), the DRG tariff (amount perceived by the hospital) was applied instead [22]. For HHF, the DRGs associated with the 10th revision of the International Classification of Diseases (ICD-10) code I50 (“heart failure”) were considered and the average cost weighted by the activity was calculated. Similarly, for CV deaths, the weighted average cost associated with the DRGs 05M22E (“myocardial infarction leading to death”) and 05M22E (“cardiovascular event leading to death”) was calculated. The costs associated with non-CV deaths were not accounted for. The costs associated with the management of treatment-related adverse events differ according to the setting. For the adverse events treated in the outpatient setting, the cost of a visit to a GP was considered. When hospitalization is required, management costs were derived either from the literature or by considering the production costs, as per the approach described for the CV events. The sources and assumptions are described in Table 4.
3 Results
3.1 Base Case Analysis
The discounted results of the simulations show that the introduction of empagliflozin in 1000 patients in French practice would prevent 74 hospitalizations attributable to heart failure and 15 deaths due to a cardiovascular event. On average, this would translate into 0.08 life years and 0.11 QALYs per patient. The higher number of QALYs gained emphasizes the positive impact of the treatment with empagliflozin on the patient’s quality of life. In addition, the distribution of patients in the different KCCQ-CSS quartiles shows that, in the arm treated with empagliflozin, a higher proportion of patients stayed in the higher quartiles’ health states (Fig. 5) and hence presented fewer symptoms and physical limitations.
Over a lifetime horizon, adding empagliflozin to SoC would represent an additional treatment cost of €1641 per patient, as empagliflozin is an add-on and not a replacement to the existing strategy. On average, patients are estimated to be treated with empagliflozin for 3.81 years and, in both arms, continue SoC indefinitely. In addition, as patients are living longer, higher disease management costs were also observed (+€42 per patient). However, this investment is partially offset by the savings attributable to the hospitalization and CV deaths prevented (−€169 per patient) and, to a lesser extent, to the management of the AEs (−€29 per patient). Indeed, if empagliflozin presents deleterious side effects (e.g., infections of the genitourinary system, hypotension), its impact on the glycemic regulation and nephro- and hepatoprotective effect would allow preventing serious adverse events such as renal and hepatic failure. Overall, the incremental cost of introducing empagliflozin is estimated at €1485, leading to an ICER of €13,980 per QALY or €18,597€ per LY gained. The discounted results are presented in Table 5.
3.2 Sensitivity Analysis
The results of deterministic sensitivity analyses show that the most impactful parameter was the treatment effect associated with empagliflozin on HF hospitalizations. When no treatment effect was considered on HHF, the resulting ICER was €18,184/QALY, i.e., a 30% increase from the base case. Other impactful parameters included the disutility for HHF, the treatment effect associated with CV mortality, whether the model considers that patients discontinue empagliflozin, the utility values considered in the model for the health state, and the cost associated with the management of HHF (Fig. 6). The model outcomes generated €14,050/QALY as mean ICER in the probabilistic analysis. This result was close to the deterministic ones (Table 5). Most of the simulations (74%) were in the north-east quadrant of the cost-effectiveness plane, indicating that the association of empagliflozin with SoC is a more costly but also more effective strategy. The probability for empagliflozin to be the most cost-efficient strategy is about 65% for a willingness to pay of €25,000/QALY (Fig. 7).
4 Discussion
Chronic heart failure is an important public health issue in France, as it was estimated in 2020 that more than 669,000 individuals were affected, representing a financial burden of €1.55 billion, including €171 million attributable to hospitalizations [2, 25]. In France in recent years, observational studies have led to the conclusion that the prevalence of the phenotype with preserved ejection fraction particularly is increasing, exacerbating the challenges associated with HFpEF. First, its diagnosis can be difficult to establish as HFpEF is not a homogeneous disease but a syndrome associated with multiple symptoms and various comorbidities. The physiopathology is complex, and it can originate from different etiologies. As a result, unlike what is observed in HFrEF, none of the available treatments has for decades managed to show a significant reduction in the morbidity or mortality of HFpEF [2].
The efficacy and safety of empagliflozin were assessed in the EMPEROR-Preserved trial [12], with it being the first therapeutic option demonstrating a significant reduction in HF hospitalization and CV deaths. The present study estimates that the introduction of empagliflozin would allow the prevention of 74 additional hospitalizations and 15 deaths associated with a CV event for 1000 patients treated. This would result in an average gain of 0.08 years in patient survival and 0.11 QALYs, including 0.018 attributable to the reduction in hospitalizations, emphasizing the benefit that empagliflozin brings on patient quality of life. Although treating HFpEF patients would request an additional investment (€1641 incremental cost) owing to the treatment acquisition and the longer life expectancy of the patients, this would be partially offset by the savings engendered mostly by the hospitalizations and CV deaths prevented. Overall, empagliflozin showed to be a cost-effective strategy with ICERs at €18,597/LY gained and €13,980/QALY gained.
The sensitivity analyses indicated that the robustness of the results was fairly satisfactory. The results of the deterministic sensitivity analyses showed that few parameters or assumptions dramatically impact the results. Only one analysis significantly impacted the results (30% increase in the ICER), when no additional treatment effect was assumed on the HHF in the arm treated with empagliflozin. However, this extreme scenario is highly implausible as the effect of empagliflozin on the reduction of hospitalization has been clinically proven. The important impact of the treatment effect on the model’s results in comparison with other outcomes is explained by the magnitude of the treatment effect on the HHF: the coefficient of −0.254 in the HHF risk equation, versus −0.082 on CV death, and 0 on AC deaths. This translates into 74 hospitalizations and 15 deaths due to CV events prevented when treated with empagliflozin for 1000 patients treated with empagliflozin. Therefore, the results were not only mainly driven by reductions in mortality but also by improvements in HRQoL and reductions in hospitalizations as in McMurray et al. [26].
The other parameters tested showed a limited impact on the model results and led to ICER variation of up to 13%. In addition, the outcomes generated in the probabilistic analyses were close to the deterministic ones. On the CE acceptability curve, the beginning of a plateau occurs after willingness to pay ≥ €20,000. This is due to a significant proportion of the simulations showing a CV benefit in favor of the placebo arm despite the clinical irrelevance confirmed by the experts.
Nevertheless, certain modeling choices and assumptions and their representativeness of French practice should be discussed. Mainly, the model considers the SoC as observed in the EMPEROR-Preserved trial, implying that the estimation of the cost of treatment with SoC is based on the distribution of the drugs provided to the trial participants. Although this choice remains the best option to ensure the homogeneity of clinical inputs considered and the estimation of the cost, EMPEROR-Preserved is an international multicentric trial, thus the practice could vary on a national level. The treatment included in the SoC in the model was therefore confronted with the local guidelines, and the distribution of the patients was presented to French clinical experts. If the composition of the SoC was consistent with the French recommendation, however, the clinical experts deemed that the distribution of the treatments could be different in France, but no local data were available to quantify it. Despite the uncertainty surrounding the SoC in France, it is not expected to substantially bias the results of the analysis as the same assumptions are made in both arms.
In addition, in the EMPEROR trial, the primary outcome was time to first event of CV death or HHF. However, in the model, patients transition between the different health states representing the KCCQ-CSS quartiles, and then a monthly event rate is applied to the population using the distribution of the patients within the different quartiles as a predictor. The transition probabilities considered in the model were then obtained from a post hoc analysis of the ITT population of the trial reassigned within those quartiles based on the KCCQ-CSS collected in the trial. However, a multistate Markov model based on a patient’s disease severity is appropriate compared with a two-state model that would directly consider the results of EMPEROR-Preserved for several reasons. First, if the model was to consider only two states (alive and dead) and constant event rates, it would equally assume that the treatment efficacy is constant over time. On contrary, the structure presented allows for explicit modeling of the relationship between disease progression and clinical outcomes (e.g., different rates of HHF and CV death can be specified). It also allows tracking resources used and HrQoL in the different health states, thus capturing a diminution of the quality of life as the patient’s condition worsens. Finally, this structure better captures the heterogeneity between patients with HFpEF and key disease characteristics.
Unlike other published models in HF [26,27,28], the health states were based on the KCCQ score, an established and prognostically important measure of health status in patients with HF that allows capturing the patients’ perception of their condition, unlike instruments informed by clinicians such as the NYHA score. Studies comparing both instruments showed that the KCCQ score provided a functional assessment similar to the NYHA, although people reporting good or excellent health status tend to be assigned to a wide range of NYHA classes by clinicians [29, 30]. A proposed explanation is the heterogeneity in the clinical assessment as based on nonsystematic interviews, and it is argued that the KCCQ score would allow for building more homogeneous cohorts. In HFrEF, the KCCQ proved to be even more accurate at predicting mortality, independently of the patients’ NYHA class, and was more sensitive to clinically meaningful changes [31].
5 Conclusions
Over a lifetime horizon, this analysis shows that empagliflozin would have a favorable impact on public health in France, in terms of HF hospitalizations and CV deaths avoided. In addition, the base-case results indicate that empagliflozin is a cost-effective strategy with both ICERs lower than €20,000/LY and €/QALY. Sensitivity analyses suggest that 65% of simulations are under the €25,000/QALY threshold.
References
Hajouli S, Ludhwani D. Heart failure and ejection fraction. Treasure Island (FL): StatPearls Publishing StatPearls; 2022 [updated 30 Apr 2022]. https://www.ncbi.nlm.nih.gov/books/NBK553115/. Accessed 13 July 2023.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726.
Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–92.
Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, et al. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016;37(5):455–62.
Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609–20.
Kittleson MM, Panjrath GS, Amancherla K, Davis LL, Deswal A, Dixon DL, et al. 2023 ACC expert consensus decision pathway on management of heart failure with preserved ejection fraction: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. 2023;81(18):1835–78.
Federation WH. ROADMAP FOR HEART FAILURE 2023. [accessed : 13 Jul 2023] https://world-heart-federation.org/cvd-roadmaps/whf-global-roadmaps/heart-failure/#:~:text=Due%20to%20the%20aging%20population,additional%20millions%20of%20undiagnosed%20cases.
Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10(4):401–10.
Insuffisance cardiaque: Santé publique France; 2019 [updated 17 Jun 2019]. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-cardiovasculaires-et-accident-vasculaire-cerebral/insuffisance-cardiaque. Accessed 13 July 2023.
Sizar O, Podder V, Talati R. Empagliflozin: StatPearls; 2018. https://www.ncbi.nlm.nih.gov/books/NBK532925/#_NBK532925_pubdet_. Accessed 13 July 2023.
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–24.
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61.
NICE. Empagliflozin for treating chronic heart failure with reduced ejection fraction [ID3826]. 2021.
Choix méthodologiques pour l'évaluation économique à la HAS: HAS; 2020. https://www.has-sante.fr/upload/docs/application/pdf/2020-07/guide_methodologique_evaluation_economique_has_2020_vf.pdf. Accessed 13 July 2023.
Andrade LF, Ludwig K, Goni JMR, Oppe M, de Pouvourville G. A French value set for the EQ-5D-5L. Pharmacoeconomics. 2020;38(4):413–25.
Indice des prix à la consommation - Base 2015 - Ensemble des ménages - France - Services de santé: Insee; 2022. https://www.insee.fr/fr/statistiques/3530261?sommaire=3530678. Accessed 13 July 2023.
BdM_IT : Recherche par code: L'assurance maladie; 2022. http://www.codage.ext.cnamts.fr/codif/bdm_it/index.php?p_site=AMELI. Accessed 13 July 2023.
Avis et communications. AVIS DIVERS. MINISTÈRE DES SOLIDARITÉS ET DE LA SANTÉ Journal officiel de la république française; 2020. https://www.ameli.fr/sites/default/files/Documents/694090/document/avenant-20-convention-nationale-pharmacien.pdf. Accessed 13 July 2023.
Health Data Hub system. FRESH-SNDS CHFpEF study. https://www.health-data-hub.fr/projets/fresh-snds-enrichissement-du-registre-fresh-un-appariement-avec-le-systeme-national-des. Accessed 13 July 2023.
Table Nationale de codage de Biologie: L'assurance maladie; 2022. http://www.codage.ext.cnamts.fr/codif/nabm/telecharge/index_tele.php?p_site=AMELI. Accessed 13 July 2023.
ENC MCO: ScanSanté; 2018. https://www.scansante.fr/applications/enc-mco. Accessed 13 July 2023.
Tarifs MCO et HAD: ATIH; 2021. https://www.atih.sante.fr/tarifs-mco-et-had. Accessed 13 July 2023.
Sullivan PW, Ghushchyan V. Preference-Based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410–20.
Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making. 2011;31(6):800–4.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263–421.
McMurray JJV, Trueman D, Hancock E, Cowie MR, Briggs A, Taylor M, et al. Cost-effectiveness of sacubitril/valsartan in the treatment of heart failure with reduced ejection fraction. Heart (British Cardiac Society). 2018;104(12):1006–13.
Rohde LE, Bertoldi EG, Goldraich L, Polanczyk CA. Cost-effectiveness of heart failure therapies. Nat Rev Cardiol. 2013;10(6):338–54.
Parizo JT, Goldhaber-Fiebert JD, Salomon JA, Khush KK, Spertus JA, Heidenreich PA, et al. Cost-effectiveness of dapagliflozin for treatment of patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2021;6(8):926–35.
Hawwa N, Vest AR, Kumar R, Lahoud R, Young JB, Wu Y, et al. comparison between the Kansas City cardiomyopathy questionnaire and New York Heart Association in assessing functional capacity and clinical outcomes. J Cardiac Fail. 2017;23(4):280–5.
Tran AT, Chan PS, Jones PG, Spertus JA. Comparison of patient self-reported health status with clinician-assigned New York Heart Association Classification. JAMA Netw Open. 2020;3(8): e2014319.
Greene SJ, Butler J, Spertus JA, Hellkamp AS, Vaduganathan M, DeVore AD, et al. Comparison of New York Heart Association class and patient-reported outcomes for heart failure with reduced ejection fraction. JAMA Cardiol. 2021;6(5):522–31.
Acknowledgements
The authors thank Mohammed Alshaikheid for medical writing support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Boehringer Ingelheim.
Declaration of competing interest
Laurent Fauchier: consultant and speaker activities for AstraZeneca, Bayer, BMS/Pfizer, Boehringer Ingelheim, Medtronic, Novartis, Novo, XO, and Zoll. Nicolas Lamblin: reports fees for advisory board and speaking for Janssen (Actelion), Akcea, Alnylam, AMICUS therapeutics, AstraZeneca, Bayer, BMS, Boehringer-Ingelheim, Bouchara-Recordati, LILLY, MSD, Novartis, PFIZER, and Sanofi-Aventis. Pierre Lévy: reports serving on a consultancy or paid advisory board for AstraZeneca, Bayer, BMS/Pfizer, Boehringer Ingelheim, Novartis, Novo Nordisk, Sanofi, and Servier. Jean TARDU and Lucile BELLIER were employed by Creativ-Ceutical at the time of manuscript submission. Creativ-Ceutical has since joined Putnam PHMR. Julien CHOLLET, Harinala GROYER and Deborah ITTAH are employed by Boehringer Ingelheim France. Stephan LINDEN is now a former employee of BI Intl GmbH.
Ethics approval
Not applicable.
Availability of data and material
No other data or material is available than the ones published in this manuscript.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability (software application or custom code)
No code or information from the cost-effectiveness model is available.
Authors’ contributions
Laurent Fauchier and Nicolas Lamblin were consulted as clinical experts to validate assumptions in the model and for its adaptation. Pierre Lévy was consulted as medico-economic expert to validate assumptions in the model and for its adaptation as well. These 3 experts also took part in the proofreading of the manuscript. Jean TARDU managed the project and adapted the cost-effectiveness model to the French adapted perspective. Lucile BELLIER and Jean TARDU both wrote the manuscript. Julien CHOLLET, Harinala GROYER, Stephan LINDEN and Deborah ITTAH proofread the manuscript. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fauchier, L., Lamblin, N., Tardu, J. et al. Public Health Impact and Cost-Effectiveness of Empagliflozin (JARDIANCE®) in the Treatment of Patients with Heart Failure with Preserved Ejection Fraction in France, Based on the EMPEROR-Preserved Clinical Trial. PharmacoEconomics Open 8, 19–30 (2024). https://doi.org/10.1007/s41669-023-00432-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-023-00432-z