Abstract
Objectives
Infertility imposes considerable clinical and economic burden, and the high costs of fertility care are a major barrier to payers. This study assessed the cost differences of highly purified human menopausal gonadotropin (HP-hMG) versus recombinant follicle stimulating hormone (rFSH) for controlled ovarian stimulation (COS) protocols in predicted high-responders from the US payer perspective.
Methods
A discrete event simulation model was built to perform a cost-comparison analysis of HP-hMG versus rFSH in a cohort of predicted high-responders undergoing up to three embryo transfer cycles, informed by efficacy data from the MEGASET-HR trial. The model considered an event-based treatment pathway and transition probabilities were derived from MEGASET-HR. A variable time horizon was employed, and deterministic and probabilistic sensitivity analyses conducted.
Results
Subjects undergoing COS with HP-hMG and rFSH demonstrated comparable live birth rates following three in vitro fertilization (IVF) cycles, with 161 live births with HP-hMG and 152 live births with rFSH, per 310 high-responders. The total cost saving per live birth in subjects receiving HP-hMG versus rFSH was US$3024. These cost savings were largely driven by the need for fewer embryo transfers to achieve similar efficacy outcomes and a reduced rate of ovarian hyperstimulation syndrome. Following deterministic sensitivity analysis, HP-hMG remained cost saving in all baseline parameter variations. No parameters led to rFSH providing cost savings when compared with HP-hMG.
Conclusion
Comparable clinical outcomes can be achieved at a lower cost when using HP-hMG versus rFSH based COS protocols in a cohort of predicted high-responders. Such cost savings may reduce the economic burden infertility currently presents to US healthcare providers and those seeking fertility care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The high cost of fertility treatment is a major barrier to US payers; therefore, there is an unmet need for effective and more affordable fertility treatment options. |
This cost-comparison demonstrated that predicted high-responder subjects treated with HP-hMG required fewer embryo transfers to achieve similar live birth rates following up to three IVF cycles, when compared with rFSH. |
The results of the analysis suggest HP-hMG offers important cost savings when compared to rFSH, with these savings largely attributed to the need for fewer embryo transfers and a reduction in ovarian hyperstimulation events. |
1 Introduction
A striking reduction in US fertility rates has been observed in recent years [1], with reports showing these have decreased by 4% from 2019 to 2020 alone [2]. Several factors are likely to contribute toward this trend, including societal factors, which have resulted in a growing tendency to delay childbearing [3, 4], and biological factors, such as a possible 60% decline in sperm counts over the last 50 years [5]. With this in mind, it is understandable that the number of fertility clinics and the need for assisted reproductive technologies (ART), such as in vitro fertilization (IVF), continues to rise [6]. In fact, of all the infants born in the USA between 2017 and 2018, ART procedures contributed to 2% of these births [7]. This growing need for ART has highlighted the requirement to continually improve the efficiency of fertility treatment regimens to satisfy the demand for effective, safe, and affordable fertility care.
Despite the evident demand for ART, the high cost of fertility care is a major concern to payers and can often limit the accessibility of treatment [8]. As of 2022, mandated insurance laws including IVF coverage were reported in just 14 US states and public funding for ART is currently not available [9]. Those affected by infertility often value ART as the only means to have children; however, the lack of funding for fertility care in the USA commonly results in patients paying privately for treatment. This poses high out-of-pocket costs for those seeking fertility care [8], and with the average cost of one fresh IVF cycle approximating US$12,400 [10], many people do not have the financial means necessary to access appropriate treatment. Understandably, the cost of fertility treatment is a well-recognized barrier and this may explain why ART utilization in the USA is among the lowest seen in Western countries [11].
Fertility treatments involving the handling of oocytes (commonly referred to as eggs) and/or embryos through careful application of protocols involving medication, as well as surgical and laboratory techniques to optimize the chance of pregnancy, are recognized as ART [7]. Typically, controlled ovarian stimulation (COS) protocols are used to facilitate the recruitment and retrieval of multiple oocytes using several different hormone preparations, including recombinant follicle-stimulating hormone (rFSH), highly purified human menopausal gonadotropin (HP-hMG), or combinations thereof [12]. Following retrieval, oocytes are subsequently fertilized in the laboratory and the resulting embryos are transferred to the recipient sequentially based upon measurements of their quality via fresh and/or frozen cycles until a successful pregnancy is achieved [13].
Multiple clinical trials have established the efficacy and safety of HP-hMG and rFSH for COS [14,15,16,17]. MEGASET-HR (NCT02554279) [17] was a phase 4, randomized, assessor-blind trial that compared HP-hMG (Menopur®) and rFSH (Gonal-f®) in a gonadotropin-releasing antagonist cycle with single blastocyst transfer in a high-responder subject population [patients with antimüllerian hormone (AMH) level of ≥ 5 ng/mL] in the USA. The MEGASET-HR trial demonstrated that HP-hMG was noninferior to rFSH based upon the rate of ongoing pregnancy after fresh transfer per cycle start. A cost-minimization analysis from the US perspective and based on data from the MEGASET-HR trial demonstrated that treatment with HP-hMG (Menopur®) achieved a live birth after first transfer at a lower cost than rFSH [18]. However, the study used a static probability decision tree to assess the clinical and economic outcomes following first transfer alone, thus did not fully account for the real-world patient experience. Therefore, in this analysis, a simulation model was developed that considered patient baseline characteristics and the potential clinical differences between fresh and frozen embryo transfers, and allowed for multiple transfer cycles. The overall aim was to perform a cost-comparison analysis from the US private payer perspective to determine the overall cost difference between HP-hMG and rFSH following up to three embryo transfer cycles (one fresh and two frozen transfers) in predicted high-responder subjects.
2 Methods
2.1 Model Overview
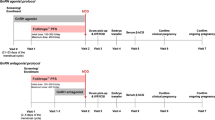
To represent the heterogeneity of the predicted high-responder population, a discrete event simulation framework that allowed event likelihoods to be estimated by the individual patient characteristics recorded at baseline was employed. A discrete event simulation model was built in Microsoft Excel 365® to conduct a cost-comparison analysis of a cohort of high-responder subjects (n = 310) undergoing HP-hMG versus rFSH based treatment protocols in up to three transfer cycles (one fresh and two frozen cycles) in a US treatment setting (Fig. 1). All analyses in this economic evaluation are in line with the consolidated health economic evaluation reporting standards (CHEERS 2022) statement [19]. A health economic analysis plan was not developed for this study as it is a retrospective analysis of the clinical trial data collected in MEGASET-HR.
All subjects entered the model upon initiation of gonadotropin treatment with HP-hMG or rFSH, followed by oocyte retrieval and embryo transfer if fertilization was achieved, and any resultant embryos were of good quality. A successful pregnancy was indicated by a positive beta-human chorionic gonadotropin (hCG) pregnancy test and was considered an ongoing pregnancy with the presence of a viable fetus 10 weeks following a positive result. At the event of a live birth or pregnancy loss, the treatment pathway was terminated. If pregnancy loss occurred, the subject could undergo a transfer of a cryopreserved embryo if available (up to two cycles), or they would exit the treatment pathway. Meanwhile, if a subject experienced an unsuccessful embryo transfer, they could undergo further transfer cycles of cryopreserved embryos if available or exit the treatment pathway. Following the stimulation cycle, any excess embryos of sufficient quality were assumed to be cryopreserved. An assumption was made that only euploid embryos were transferred; the model did not capture double embryo transfers or genetic testing costs. This assumption aligns with the MEGASET-HR trial protocol that ensured euploid embryos were primarily transferred. Primary outcomes included the overall cost of a live birth and cost of ongoing pregnancies. These outcomes were chosen to demonstrate the benefit of the treatments included in this analysis as they represent a key objective of payers funding ART and allow for a direct comparison between HP-hMG versus rFSH.
2.2 Patient Population
The modeled population reflects the inclusion criteria of the MEGASET-HR trial [17]. This was defined as subjects aged 21–35 years with a body mass index (BMI) between 18 and 30 kg/m2, regular menstrual cycles of 21–45 days, and infertility ≥ 12 months. Additionally, the MEGASET-HR inclusion criteria stated subjects must have day 2 or 3 serum FSH levels of 1−12 IU/L and serum AMH ≥ 5 ng/mL at the time of screening.
Baseline patient age was 30.25 ± 3.04 years, mean infertility duration was 37.01 ± 1.54 months, mean baseline prolactin was 13.67 ± 0.38 μg/L, mean baseline FSH was 6.11 ± 0.1 U/L, and mean baseline AMH was 7.67 ± 0.17 ng/mL. Further adjustments were not made to the patient population as predicted high-responders are the population of interest for this study.
2.3 Comparators and Treatment Sequence
This analysis compared popular gonadotropin preparations used in the USA for COS during IVF; HP-hMG (Menopur®) or rFSH (Gonal-F®). Efficacy data were sourced from the MEGASET-HR trial [17] where interventions were used according to label indications and modeled clinical outcomes mirrored those of the trial. Risk equations were derived from datasets to determine the impact of baseline characteristics on outcomes such as the number of oocytes retrieved, number of blastocytes, number of good quality blastocysts, number of cryopreserved embryos, and the probability of over stimulation. Adjusting outcomes for patient characteristics reduces statistical power, as such, large numbers of events are required to develop risk equations. Given the small numbers in the stratification by transfer method (fresh or frozen transfer 1 or 2), it was not feasible to develop adjusted risk equations from the datasets. Instead, the unadjusted observed probabilities for the probability of a transfer, probability of a positive beta-HCG test, probability of ongoing pregnancy, and the probability of a live birth were used, and these were stratified by transfer method.
A linear model was used to estimate the continuous variables, whereas logistic regression was used to determine dichotomous variables. Step-wise analysis was used to determine the variables included. A univariate analysis was conducted in the first instance and all variables that were statistically significant (p < 0.05) were included in the multivariate analysis with all variables remaining significant retained in the final model.
To reflect that the treatment pathway for assisted reproduction is event based, the model employed an event-based treatment pathway and baseline patient characteristics, including age, BMI, prolactin, FSH, AMH, and previous history of fertility treatment, defined the likelihood of an event. Transition probabilities were derived from the probability of an event occurring in the MEGASET-HR trial dataset [17].
The model employed a variable time horizon which considered the number of transfer cycles and treatment success (i.e., achieving a live birth). Simulations were limited to a maximum of three transfer cycles, one fresh and two frozen, to align with the perspective of the US private payer; however, some patients had less either because early transfers were successful or because they did not have sufficient embryos to allow for three transfers.
2.4 Cost and Resource Use
This economic evaluation was conducted from the US private payer perspective to demonstrate the potential cost benefits to patients in the USA. A previously published study that contained the results of a cost survey conducted at MEGASET-HR trial sites was used to obtain most cost inputs for procedures [18], all of which were inflated to 2022 US$ according to reported inflation rates in the USA.[20] Pharmacy selling price was used for drug costs. Costs were not discounted as the model did not track costs for patients beyond 1 year. The cost inputs for the model are reported in Table 1. Resource use included embryo cryopreservation, downregulation duration (days), number of ultrasounds (stimulation and pregnancy), percentage with AMH testing, number of antenatal appointments, percentage experiencing vaginal delivery, percentage experiencing caesarean section. To better demonstrate the impact of HP-hMG on the direct costs of IVF protocols, all economic outcomes are reported with and without the inclusion of costs of delivery.
2.5 Approach to Sensitivity Analysis
To assess the influence of individual parameters on the modeled outcomes, deterministic sensitivity analyses (DSA) were conducted using upper and lower values of the base cost values (± 20% variation of the costs) and 95% CI for clinical parameters (Online resource, Table S1). To measure the combined uncertainty of different model inputs, a probabilistic sensitivity analysis (PSA) was conducted with a total of 1000 iterations. Details of the type of distribution and distribution parameters used for this analysis are presented in Table S2 (Online resource).
3 Results
3.1 Clinical Outcomes
These economic analyses predicted that high-responder subjects treated with HP-hMG-based COS protocols required fewer embryo transfers to achieve comparable live birth rates to those receiving rFSH (Table 2). Modeled event rates per 310 high-responder subjects using HP-hMG were 279 embryo transfers, 169 ongoing pregnancies, and 161 live births following three IVF cycles. For those treated with rFSH, respective event rates were 310, 157, and 152 per 310 high-responder subjects (Table 2).
This model also estimated the rate of pregnancy loss and ovarian hyperstimulation syndrome (OHSS) as secondary outcomes. For subjects treated with HP-hMG-based COS protocols, there were fewer cases of OHSS when compared with those who received rFSH over three IVF cycles, with 26 and 65 events per 310 subjects, respectively (Table 2). Meanwhile, fewer pregnancy losses were also seen in the HP-hMG treatment arm, with 26 events per 310 subjects versus 60 events in the rFSH arm (Table 2). Subjects who received HP-hMG had more successful fresh cycle embryo transfers compared with rFSH, implying a reduced time to pregnancy and time to live birth.
3.2 Economic Outcomes
When excluding cost of delivery, the model estimated an average cost per patient starting a COS cycle of US$19,761 for HP-hMG and US$20,139 for rFSH. When including the cost of delivery, the average cost per patient starting a COS cycle for HP-hMG versus rFSH was US$27,154 and US$27,119, respectively. For subjects treated with HP-hMG-based COS protocols, the model estimated a total cost saving per live birth of US$3024 (including cost of delivery) compared with those receiving rFSH (Table 3). When excluding the cost of delivery, the model estimated an average cost per live birth of US$38,048 for HP-hMG and US$41,073 for rFSH, resulting in a cost saving of US$3024 per live birth associated with HP-hMG (Table 3). In addition, the model estimated an average cost per ongoing pregnancy of US$36,247 for HP-hMG and US$39,765 for rFSH when excluding cost of delivery, leading to a cost saving of US$3517 per ongoing pregnancy associated with HP-hMG (Table 3). The cost savings demonstrated with HP-hMG were largely a result of the reductions in OHSS events and the need for fewer embryo transfers to achieve similar live birth rates in subjects treated with HP-hMG versus rFSH-based COS protocols.
3.3 Sensitivity Analyses
Results of the DSA demonstrated that these findings were robust to changes in all baseline parameters, where HP-hMG continued to provide cost savings when compared with rFSH (Fig. 2). Daily cost of HP-hMG and rFSH were the most influential parameters, whereby HP-hMG remained cost saving versus rFSH despite variations in these parameters. No parameters led to rFSH providing cost savings. Results of the PSA demonstrated some uncertainty in the outcomes of the cost-comparison analysis, as evidenced by the spread of data shown in the cost-comparison plane (Fig. 3). However, 78.2% of the PSA simulations resulted in HP-hMG providing cost savings (when cost of delivery was excluded). The economic outcomes of the PSA demonstrate that in scenarios where the total cost per live birth for rFSH increased, the proportion of probabilistic results where HP-hMG was cost saving versus rFSH was higher (Fig. 4). The PSA results of the modeled clinical outcomes and economic outcomes are presented in Tables 4 and 5, respectively.
4 Discussion
The results presented here demonstrate that treatment protocols with HP-hMG reduced the cost per pregnancy and cost per live birth in a population of predicted high-responders over three transfers, compared with treatment with rFSH from the US perspective. The cost savings associated with HP-hMG use were influenced by the need for fewer embryo transfers for subjects treated with HP-hMG-based COS protocols and a reduction in OHSS events.
Insurance coverage for infertility treatment is absent or inadequate for many in the USA; therefore, the cost of treatment is a significant barrier to those seeking fertility care [11]. Identifying cost saving treatment protocols to improve the affordability of ART may increase the accessibility of fertility treatment [21, 22]. This economic evaluation demonstrates that treatment protocols using HP-hMG alone can result in an overall cost saving of US$3024 per live birth compared with the use of rFSH, equating to a 7.4% reduction in the total cost of treatment (excluding delivery costs) per live birth at a cohort level of predicted high-responders. These cost savings may lessen the economic burden incurred by those financing fertility treatment, while also informing healthcare providers of more affordable protocols to improve the availability of ART and better satisfy the growing demand for fertility services in the USA.
These findings are consistent with previous economic evaluations, which have demonstrated cost benefits associated with HP-hMG treatment compared with rFSH for COS [23,24,25]. From the US perspective, Robins et al. [18] performed a cost-minimization analysis based on the MEGASET-HR trial and showed that the cost to achieve a live birth following the first transfer was reduced by US$3310 with HP-hMG treatment versus rFSH [18]. Using a discrete event simulation model, the cost-comparison presented here considers the impact of patient baseline characteristics on clinical outcomes, thus represents the heterogeneity within the high-responder population, while also modeling the clinical differences between fresh and frozen transfer cycles. This approach differs from prior analyses but has arrived at a similar conclusion, thus provides additional supporting evidence that HP-hMG offers considerable cost offsets.
In addition to the direct costs associated with treatment, the indirect costs associated with fertility care are also a concern to many patients. The time spent receiving fertility treatment can greatly impact patient productivity, leading to work absences and wage loss, for example. In fact, an analysis conducted in the Netherlands demonstrated that patients undergoing IVF/intracytoplasmic sperm injection report an average productivity loss of €596 per cycle [26]. This adds to the economic burden of those seeking fertility care [27], which may influence whether a patient continues treatment. This analysis demonstrated there were numerically more successful fresh cycle embryo transfers for subjects undergoing HP-hMG treatment compared with rFSH, reducing the need for subsequent transfer cycles, thus resulting in an implied reduction in time to pregnancy and time to live birth. This suggests that for predicted high-responders, protocols that use HP-hMG treatment may reduce the time burden that fertility care imposes on patients and minimize further financial strain caused by work absences, for example.
There are limitations to this economic evaluation. The analyses use prospective trial data whereby patients were randomized to gonadotropin treatment arms. However, in real-world clinical settings, gonadotropin treatment administration is more variable. The treatment pathways observed in the clinical trial were simplified for this simulation model. For instance, the model did not consider double embryo transfers nor the costs of genetic testing and assumed that only euploid embryos were transferred. This assumption was justified as the MEGASET-HR trial protocol ensured mostly euploid embryos were transferred. Additionally, the model does not allow for elective “freeze-all” protocols that are increasingly being utilized in the USA as a means to avoid OHSS, a risk for high-responders [28]. Further, the model does not consider the concomitant use of HP-hMG and rFSH, which is common in the USA. Lastly, given this model is informed by trial data in the predicted high-responder population and outcomes are specific to the US private payer, these findings may not be generalizable to other populations, payer groups, or geographies.
This economic evaluation highlights that a comparable number of live births can be achieved at a reduced overall cost when using HP-hMG compared with rFSH-based COS protocols in a cohort of predicted high-responders. These findings may be informative for decision-making by healthcare providers. Further studies are required to assess whether the cost offsets associated with HP-hMG protocols may alleviate some of the direct cost burden that fertility treatment often imposes on payers.
References
Eisenberg ML, Thoma ME, Li S, McLain AC. Trends in time-to-pregnancy in the USA: 2002 to 2017. Hum Reprod. 2021;36(8):2331–8.
Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 20202021. https://stacks.cdc.gov/view/cdc/109213. Accessed 12 May 2022.
Khandwala YS, Zhang CA, Lu Y, Eisenberg ML. The age of fathers in the USA is rising: an analysis of 168 867 480 births from 1972 to 2015. Hum Reprod. 2017;32(10):2110–6.
Matthews TJ, Hamilton BE. Delayed childbearing: more women are having their first child later in life. NCHS Data Brief. 2009;21:1–8.
Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 2017;23(6):646–59.
Sunderam S, Kissin DM, Crawford SB, Folger SG, Boulet SL, Warner L, et al. Assisted reproductive technology surveillance—United States, 2015. MMWR Surveill Summ. 2018;67(3):1–28.
Sunderam S, Kissin DM, Zhang Y, Jewett A, Boulet SL, Warner L, et al. Assisted reproductive technology surveillance—United States, 2018. MMWR Surveill Summ. 2022;71(4):1–19.
Kissin DM, Boulet SL, Jamieson DJ. Fertility treatments in the United States: improving access and outcomes. Obstet Gynecol. 2016;128(2):387–90.
RESOLVE: The National Infertility Association. Insurance coverage by state 2022. https://resolve.org/learn/financial-resources-for-family-building/insurance-coverage/insurance-coverage-by-state/.
American Society for Reproductive Medicine. Is in vitro fertilization expensive? 2022. https://www.reproductivefacts.org/faqs/frequently-asked-questions-about-infertility/q06-is-in-vitro-fertilization-expensive/.
Hammoud AO, Gibson M, Stanford J, White G, Carrell DT, Peterson M. In vitro fertilization availability and utilization in the United States: a study of demographic, social, and economic factors. Fertil Steril. 2009;91(5):1630–5.
Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, Ovarian Stimulation T, et al. ESHRE guideline: ovarian stimulation for IVF/ICSI(†). Hum Reprod Open. 2020;2020(2):hoaa009.
Huang JYJ, Rosenwaks Z. Assisted reproductive techniques. In: Rosenwaks Z, Wassarman PM, editors. Human fertility: methods and protocols. New York: Springer; 2014. p. 171–231.
Efficacy and safety of highly purified menotropin versus recombinant follicle-stimulating hormone in in vitro fertilization/intracytoplasmic sperm injection cycles: a randomized, comparative trial. Fertil Steril. 2002;78(3):520-8.
Andersen AN, Devroey P, Arce JC. Clinical outcome following stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF: a randomized assessor-blind controlled trial. Hum Reprod. 2006;21(12):3217–27.
Devroey P, Pellicer A, Nyboe Andersen A, Arce JC. A randomized assessor-blind trial comparing highly purified hMG and recombinant FSH in a GnRH antagonist cycle with compulsory single-blastocyst transfer. Fertil Steril. 2012;97(3):561–71.
Witz CA, Daftary GS, Doody KJ, Park JK, Seifu Y, Yankov VI, et al. Randomized, assessor-blinded trial comparing highly purified human menotropin and recombinant follicle-stimulating hormone in high responders undergoing intracytoplasmic sperm injection. Fertil Steril. 2020;114(2):321–30.
Robins JC, Khair AF, Widra EA, Alper MM, Nelson WW, Foster ED, et al. Economic evaluation of highly purified human menotropin or recombinant follicle-stimulating hormone for controlled ovarian stimulation in high-responder patients: analysis of the Menopur in Gonadotropin-releasing Hormone Antagonist Single Embryo Transfer-High Responder (MEGASET-HR) trial. F S Rep. 2020;1(3):257–63.
Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II Good Practices Task Force. Value Health. 2022;25(1):10–31.
U.S. Bureau of Labor Statistics. Databases, tables & calculators by subject 2022. https://www.bls.gov/data/. Accessed 19 June 2023.
Chambers GM, Hoang VP, Sullivan EA, Chapman MG, Ishihara O, Zegers-Hochschild F, et al. The impact of consumer affordability on access to assisted reproductive technologies and embryo transfer practices: an international analysis. Fertil Steril. 2014;101(1):191-8.e4.
Collins J. An international survey of the health economics of IVF and ICSI. Hum Reprod Update. 2002;8(3):265–77.
Lloyd A, Kennedy R, Hutchinson J, Sawyer W. Economic evaluation of highly purified menotropin compared with recombinant follicle-stimulating hormone in assisted reproduction. Fertil Steril. 2003;80(5):1108–13.
Wechowski J, Connolly M, Schneider D, McEwan P, Kennedy R. Cost-saving treatment strategies in in vitro fertilization: a combined economic evaluation of two large randomized clinical trials comparing highly purified human menopausal gonadotropin and recombinant follicle-stimulating hormone alpha. Fertil Steril. 2009;91(4):1067–76.
Barriere P, Porcu-Buisson G, Hamamah S. Cost-effectiveness analysis of the gonadotropin treatments HP-hMG and rFSH for assisted reproductive technology in France: a Markov model analysis. Appl Health Econ Health Policy. 2018;16(1):65–77.
Bouwmans CAM, Lintsen BAME, Al M, Verhaak CM, Eijkemans RJC, Habbema JDF, et al. Absence from work and emotional stress in women undergoing IVF or ICSI: an analysis of IVF-related absence from work in women and the contribution of general and emotional factors. Acta Obstet Gynecol Scand. 2008;87(11):1169–75.
Wu AK, Elliott P, Katz PP, Smith JF. Time costs of fertility care: the hidden hardship of building a family. Fertil Steril. 2013;99(7):2025–30.
Li Z, Wang AY, Bowman M, Hammarberg K, Farquhar C, Johnson L, et al. Cumulative live birth rates following a ‘freeze-all’ strategy: a population-based study. Hum Reprod Open. 2019;2019:2.
Valencia Z, Bozzi D, Sen A, Martin K. Health Care Cost Institute: the price of childbirth in the U.S. tops $13,000 in 2020. 2022. https://healthcostinstitute.org/hcci-research/the-price-of-childbirth-in-the-u-s-tops-13-000-in-2020. Accessed 19 June 2023.
Acknowledgements
The authors thank Chloe Salter of Health Economics and Outcomes Research Ltd. for providing medical writing support and Jani Silvanto of Health Economics and Outcomes Research Ltd. for additional analytical support, which was funded by Ferring Pharmaceuticals in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Ferring Pharmaceuticals who provided support for the model development and medical writing for this study.
Conflicts of interest
AK, MM, CB, GD, and PH are employees of Ferring Pharmaceuticals. TB is an employee of Health Economics and Outcomes Research Ltd. who received fees from Ferring Pharmaceuticals in relation to this study.
Availability of data and material
The datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.
Author contributions
TB, MM, AK, CB, GD, and PH have been involved in study design and analysis plan from inception to completion. TB was responsible for data analysis. All authors contributed to interpretation of the results, preparation, and review of the manuscript, and approval of the final manuscript for publication.
Code availability (software application or custom code)
This model was developed in Microsoft Excel 365® using Visual Basic for Application (VBA) code, which is not publicly available.
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Khair, A., Brown, T., Markert, M. et al. Highly Purified Human Menopausal Gonadotropin (HP-hMG) Versus Recombinant Follicle-Stimulating Hormone (rFSH) for Controlled Ovarian Stimulation in US Predicted High-Responder Patients: A Cost-Comparison Analysis. PharmacoEconomics Open 7, 851–860 (2023). https://doi.org/10.1007/s41669-023-00429-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-023-00429-8