Abstract
Background
Crohn’s-related rectovaginal fistulas (RVF) greatly impact quality of life and are notoriously difficult to treat. The aim of this study was to assess the burden of recurrent episodes of care for RVF and its economic impact.
Methods
A retrospective observational cohort study of administrative US claims databases was conducted. Eligible patients were female adults, with a diagnosis code for Crohn’s disease with or without a diagnosis/procedural code for RVF. For the RVF cohort, rates of recurrence of RVF episodes of care were estimated using Kaplan–Meier analyses. Healthcare resource utilization (HCRU) and direct healthcare costs were compared between the RVF cohort and RVF-free cohort.
Results
Mean ages in the RVF cohort (n = 963) and RVF-free cohort (n = 56,564) were 47.2 and 50.8 years, with a mean follow-up period of 58.7 and 49.8 months, respectively. For the RVF cohort, the probability of having a second RVF episode of care within 2 years of the first one was estimated to be 35.9% and of having a third episode within 2 years of the second was 47.8%. During the first 2 years, the RVF cohort had 67% more inpatient admissions than the RVF-free cohort with each RVF episode of care being associated with 16% more admissions. The estimated incremental cost associated with having RVF was US$17,561, with an incremental cost of US$11,607 for each additional RVF episode of care.
Conclusions
This real-world study highlights the significant impact of RVF in patients with Crohn’s disease with regard to repeat interventions and associated HCRU and direct healthcare costs, suggesting novel therapeutics are needed in this patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This is the first real-world study to assess the recurrence of Crohn’s-related rectovaginal fistula episodes of care, treatment patterns, and healthcare resource and economic burden using administrative claims databases. |
This study illustrates the impact of repeated interventions in the management of rectovaginal fistulas, in terms of increased healthcare resource utilization and costs, in patients with Crohn’s-related rectovaginal fistulas compared with those with Crohn’s disease without rectovaginal fistulas. |
1 Introduction
Crohn’s disease (CD) is a chronic inflammatory condition that can affect any part of the gastrointestinal tract [1]. Owing to the characteristic transmural inflammatory behaviour of CD, fistulas are a common phenotype affecting up to 50% of patients with CD within 20 years of initial diagnosis [2]. Rectovaginal fistulas (RVF) occur between the anal canal/rectum and vagina, and represent 9% of all cases of CD-related fistulas in females [2]. The incidence of RVF in females with CD has been reported to be 3–10% [3]. Patients with RVF may experience passage of flatus, stool, and other discharges from the rectum into the vagina, resulting in a feculent odour, recurrent vaginal mucosal inflammation, recurrent vaginal or urinary tract infections, and perineal pain [4, 5]. RVF can lower patients’ self-esteem, prevent successful intimate relationships, and lead to considerable social embarrassment. [5, 6]. RVF are associated with a significant negative impact on quality of life and have a high psychosocial burden which is reflected in the lower health-related quality of life scores reported for patients with non-healed and healed RVF compared with the general population [7].
The treatment goals for RVF are symptom improvement and fistula closure. However, conventional medical therapy is associated with limited efficacy in the treatment of RVF; therefore, many patients require multiple pharmacological treatments and surgical interventions, indicating a need for novel therapeutics to treat RVF [4, 8, 9]. Medical management of RVF usually involves immunomodulator and biological therapy [e.g. anti-tumor necrosis factor (TNF) therapies, natalizumab, ustekinumab, and vedolizumab] to reduce inflammation in the bowel mucosa and enable subsequent surgical intervention where required [10]. Antibiotics may also be useful to treat local sepsis around the fistula [5, 11]. Abscess drainage and seton placement may also be used to manage perianal infection when present [5].
When luminal inflammation and infection are well controlled, surgical interventions to close RVF may include the interposition of healthy tissue between the rectum and vagina from labial fat or gracilis muscle [10]. Alternatively, less-invasive advancement flaps, fistula plugs, or fistula ligation may be performed to close RVF [5, 6, 12]. Unfortunately, both the aforementioned pharmacological and surgical interventions are associated with variable success rates [6, 13]. Reported healing rates across heterogeneous studies of multiple surgical interventions range from 14% to 81% [14]. This wide range in success rate is likely attributed to the variation in chosen surgical procedures, variable definitions of success/remission, small study sizes, and variable follow-up durations.
There are limited real-world data on the burden of RVF in patients with CD and on RVF management and outcomes. What remains apparent in the literature is the high frequency of repeat surgical intervention and stoma procedures in patients with RVF despite the use of biologics and multiple other interventions [15, 16]. The aim of this study was to assess the recurring episodes of care for RVF, treatment patterns, and associated healthcare resource utilization (HCRU) and direct healthcare costs using real-word data from administrative claims databases for patients with CD and RVF in the USA. This information will improve the understanding of disease burden of RVF in patients with CD.
2 Methods
2.1 Study Design
A retrospective, observational, US database analysis was conducted using data from Truven Health MarketScan® Commercial Claims and Encounters database and Truven MarketScan Medicare Supplemental and Coordination of Benefits database, from 1 January 2001 to 31 December 2019 (Fig. 1). All data were de-identified and comply with the confidentiality requirements of the Health Insurance Portability and Accountability Act [17]. This report complies with the Equator network guidelines on Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). The STROBE checklist for cohort studies is provided (Supplementary Table 1 in the Online Resource).
2.2 Study Population
Patients eligible for inclusion in this study were females aged ≥ 18 years with ≥ 1 medical claim with a diagnosis code for CD [International Classification of Diseases (ICD)-9 and ICD-10; Supplementary Table 2]. Eligible patients had a first diagnosis for CD and continuous health plan enrollment with both medical and pharmacy coverage of ≥ 180 days before, and ≥ 720 days after, the index date (defined below).
The study population consisted of two cohorts: patients with CD and RVF (‘RVF cohort’) and patients with CD without RVF (‘RVF-free cohort’). Patients in the RVF cohort experienced ≥ 1 ‘RVF episode of care’ (see Sect. 2.3), a period requiring healthcare visits identified via medical service claims with diagnosis or procedure codes for RVF (Supplementary Table 3). Patients in the RVF-free cohort had no RVF-specific diagnosis/procedure codes at any time.
The index date for patients in the RVF cohort was the date of the start of the first RVF episode of care on or after the first observed CD diagnosis in claims, and the index date for patients in the RVF-free cohort was a randomly selected date after the first observed CD diagnosis in claims. The baseline period was a 180 day period before index date for both cohorts. The length of the follow-up period (which varied by patient) was the time from the index date until the end of continuous health plan enrollment or end of data availability, whichever occurred first.
2.3 Identification of RVF Episodes of Care
On the basis of observations from clinical practice and a review of data from the databases, a claims-based algorithm was developed to identify RVF episodes of care. The first RVF episode of care started on the date of the first RVF-related code after CD diagnosis. For the purpose of this study, RVF-related codes ≤ 90 days apart were considered part of the same RVF episode of care. A subsequent RVF-related code > 90 days after the previous RVF-related code marked the start of a new RVF episode of care. It should be noted that this algorithm does not aim to identify episodes of RVF disease, but episodes of care received for RVF. Accordingly, one prolonged non-healed RVF episode could involve more than one distinct episode of care. Some RVF-related procedure codes may also be used for other conditions, such as perianal fistulas; hence, the definition of an RVF episode of care included ≥ 1 RVF-specific diagnosis/procedure code. Episodes of care < 14 days were adjusted to 14 days if there were no procedure codes for RVF, on the basis that a minimum of 2 weeks to stabilize RVF in patients receiving pharmacological treatments for RVF has been typically observed by the authors in clinical practice. Episodes of care < 14 days that had a procedure code were not adjusted because RVF symptoms may disappear immediately after surgery.
2.4 Patient Variables and Outcomes
For patients in both cohorts, data were extracted for demographic and clinical characteristics, and treatments received during the 180 day baseline period including conventional therapies (aminosalicylates, antibiotics, corticosteroids, immunomodulators/immunosuppressives), biological therapies, and CD-related surgery. All-cause HCRU (comprised of inpatient stays, days with outpatient services, and emergency department visits) and total direct healthcare costs (comprised of medical costs for inpatient stays, outpatient and emergency department visits, and pharmacy costs) during the baseline period were also assessed separately for patients in both cohorts.
For patients in the RVF cohort, the characteristics of the RVF episodes of care experienced and the time to recurrence of an RVF episode of care, defined as the time from the end of an RVF episode of care to the start of a subsequent RVF episode of care requiring access to medical services, was assessed (i.e. time from first to second and time from second to third RVF episode of care).
Post-index treatment patterns for the RVF-cohort, including RVF-related procedures conducted after the start of the first RVF episode of care were described. All-cause HCRU and total direct healthcare costs up to 2 years after the index date were assessed for patients in both cohorts. Direct healthcare costs were adjusted for inflation using the US Medical Care Consumer Price Index from the Bureau of Labor statistics, US Department of Labor, and reported in 2022 US$.
2.5 Statistics
Patient demographics, clinical characteristics, characteristics of RVF episodes of care, treatment patterns, HCRU, and direct healthcare costs were summarized using means, standard deviations (SDs), and medians for continuous variables, and frequency counts and percentages for categorical variables. Comparisons of baseline characteristics between the RVF-free and RVF cohorts were conducted using Wilcoxon rank-sum tests for continuous variables and Chi-squared tests for categorical variables.
For the RVF cohort, Kaplan–Meier (KM) analyses of time to recurrence of an RVF episode of care, taking into account the right censoring of patients with RVF but without a subsequent RVF episode of care (i.e. censored at the end of their follow-up period), were used to estimate the rates of having a second or third RVF episode of care at key time points (e.g. 3, 6, 9, 12, 24, 60, 84, and 96 months). KM rates of having a third RVF episode of care were assessed among patients with ≥ 2 RVF episodes of care. KM curves, the number of patients still at risk at key time points, and median time to a subsequent RVF episode of care, if achieved, were reported.
To estimate the burden of recurrence of RVF episodes of care, the impact on each of the HCRU categories and direct healthcare cost components was assessed during a 2 year fixed period after the index date for patients in the RVF cohort and in those in the RVF-free cohort. A multivariable Poisson regression model was used to assess the burden of RVF on HCRU, and incidence rate ratios (IRRs) were reported. The burden of RVF on direct healthcare costs was assessed using a two-part model, a logistical model with binomial distribution, and a generalized linear model with a log link and gamma distribution, and incremental costs were reported. For both models, p-values and 95% confidence intervals were estimated using a non-parametric bootstrap resampling technique [18].
Both the HCRU and the direct healthcare cost models included two independent variables: (1) a ‘binary’ variable indicating the cohort (with the RVF-free cohort as the reference cohort); and (2) a ‘count’ variable indicating the number of RVF episodes of care during the 2 years post-index (0 for patients in the RVF-free cohort). For HCRU analyses, two IRRs were thus reported: (1) an IRR assessing the impact of having a first RVF episode of care compared with being RVF free; and (2) a second IRR assessing the impact of having one additional RVF episode of care among patients in the RVF cohort. Similarly, for the healthcare cost analyses, two mean cost difference measures were reported: (1) the mean cost difference related to having a first RVF episode of care as compared with being RVF free, and (2) the mean cost difference related to having one additional RVF episode of care among patients in the RVF cohort.
Both models were adjusted for an a priori list of potential confounding factors including age, region, health insurance plan type, and calendar year measured at the index date. The models were also adjusted for patient characteristics that were clinically relevant, measured during the 180 day baseline period (other autoimmune diseases, Quan Charlson Comorbidity Index, end-stage renal disease, liver transplant, perianal abscess, fistulizing disease other than RVF, CD-related surgery, CD-related infections, use of conventional therapies, use of biological therapies, cancer in the pelvic region, number of inpatient admissions, number of emergency department visits, and number of days with outpatient services).
3 Results
3.1 Study Population
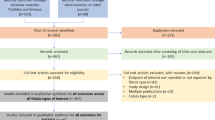
Between 2001 and 2019, 672,294 patients with ≥ 1 medical claim with a diagnosis for CD were included in the Truven Health MarketScan databases (Fig. 2). A total of 963 patients met the eligibility criteria to be included in the RVF cohort and 56,564 patients were eligible to be included in the RVF-free cohort.
Patient disposition. aCD diagnosis codes listed in Supplementary Table 1. bRVF-specific codes include diagnosis/procedure codes listed in Supplementary Table 2. cThe first RVF episode of care started on the date of the first RVF-related code after CD diagnosis. CD Crohn’s disease, RVF rectovaginal fistula
3.2 Patient Characteristics and Treatments at Baseline
The full list of patient baseline characteristics that were assessed (including those that were accounted for in the adjustments of the HCRU and cost models) are reported in Table 1 and Supplementary Tables 4 and 5.
The mean (SD) age of patients in the RVF cohort and RVF-free cohort on the index date was 47.2 (13.9) and 50.8 (15.5) years, respectively, and the proportion residing in each US region was similar across both cohorts (Table 1 and Supplementary Table 4). The most frequent health insurance plan types in the RVF and RVF-free cohorts were preferred provider organizations (54% for both cohorts) and health maintenance organizations (15% and 14%, respectively; Supplementary Table 4). The mean (SD) duration of the follow-up period was 58.7 (32.6) and 49.8 (26.5) months for the RVF and RVF-free cohorts, respectively (Table 1).
Compared with patients in the RVF-free cohort, a higher proportion of patients in the RVF cohort had indicators of CD severity during the baseline period (perianal abscess, fistulising disease other than RVF, and CD-related infections). The use of conventional therapies, biological therapies, and CD-related surgery during the baseline period was also higher in the RVF cohort than in the RVF-free cohort (Table 1 and Supplementary Table 5). Likewise, the RVF cohort had significantly higher HCRU and total direct healthcare costs than the RVF-free cohort during the baseline period (Table 1).
3.3 Characteristics of RVF Episodes of Care and Treatment Patterns (RVF Cohort)
During the entire follow-up period, 533/963 patients (55.3%) had one RVF episode of care. The mean duration of the first RVF episode of care was 46.4 days. Among patients who experienced a second RVF episode of care (430/963 patients; 44.7%), the mean time from the end of first RVF episode of care to the start of the second episode of care was 15.5 months and the mean duration of the second episode of care was 47.7 days. For patients who experienced a third RVF episode of care (217/963 patients; 22.5%), the mean time from the end of second RVF episode of care to the start of the third episode of care was 12.9 months and the mean duration of the third episode of care was 55.3 days (Table 2).
Almost all patients in the RVF cohort (929/963; 96.5%) were treated with conventional therapies at any time after the index date, and approximately half were treated with biological therapies (494/963; 51.3%). At any time after the index date, approximately 60% of patients in the RVF-cohort underwent CD-related surgery (575/963) or RVF-related surgery (587/963) (Supplementary Table 6).
3.4 Time to Subsequent RVF Episode of Care (RVF Cohort)
Using KM analyses of time to next RVF episode of care, the probability of having a second RVF episode of care was estimated to be 28.5% and 35.9% within 1 year and 2 years after the end of the first RVF episode of care, respectively (Fig. 3). The probability of having a third RVF episode of care was 37.4% and 47.8% at 1 year and 2 years after the end of the second RVF episode of care, respectively.
Probability of having a subsequent RVF episode of care in patients with CD, using Kaplan–Meier analysis. aThe median time to a second RVF episode of care was 79.8 months and from a second to a third RVF episode of care was 27.1 months. The KM rates should be interpreted with caution after the 24 month time point, because these values were based on a small proportion of patients still at risk. CI confidence interval, KM Kaplan–Meier, RVF rectovaginal fistula
3.5 HCRU and Direct Healthcare Costs (RVF and RVF-Free Cohorts)
Analyses to evaluate the burden of RVF on HCRU showed that patients in the RVF cohort (regardless of the number of RVF episodes of care) had 67% more inpatient admissions than those in the RVF-free cohort during the 2 years after index date: adjusted IRR 1.67 (95% confidence interval, 1.29–2.17, p < 0.01). Each additional RVF episode of care was associated with 16% (adjusted IRR = 1.16; p < 0.05) more inpatient admissions during the 2 year period. Thus, patients having a first RVF episode of care had 94% (adjusted IRR 1.67 × 1.16 = 1.94) more inpatient admissions during the 2 year post-index period than those who were RVF free. The numbers of emergency department visits (adjusted IRR 0.89; p = 0.37) and days with outpatient services (adjusted IRR 1.02; p = 0.68) were not statistically different between patients in the RVF cohort and RVF-free cohort. Likewise, the incremental numbers of emergency department visits (adjusted IRR 1.04; p = 0.60) and days with outpatient services (adjusted IRR 1.08; p = 0.06) associated with an additional RVF episodes of care were not statistically significant (Table 3).
In the first 2 years after the index date, the adjusted incremental increase in total direct healthcare costs associated with having RVF (regardless of the number of RVF episodes of care) versus the RVF-free cohort was $17,561 (p < 0.01), mainly driven by increased inpatient costs ($9152; p < 0.01), and the adjusted incremental cost associated with each additional RVF episode of care was $11,607 (p < 0.01), mainly driven by incremental outpatient costs ($9265; p < 0.01). Thus, the adjusted incremental total costs associated with having a first RVF episode of care versus not having RVF was $29,168 ($17,561 + $11,607; Table 4).
4 Discussion
To our knowledge, this is the first real-world study to assess the recurrence of RVF episodes of care, treatment patterns, and economic burden for patients with CD-related RVF using administrative claims databases. This retrospective, longitudinal, administrative, US claims database study demonstrates that patients with CD, who go on to develop RVF, experience increased disease burden, as measured by medication use and CD-related surgery, compared with patients with CD who do not develop RVF.
Compared with patients in the RVF-free cohort, a higher proportion of patients in the RVF cohort had indicators of increased CD severity at baseline, suggesting that patients who have more severe CD are more likely to go on to develop RVF than those who have less severe CD. This is in line with the observation that a greater proportion of patients in the RVF cohort had received conventional and biological therapies and underwent CD-related surgery than those in the RVF-free cohort during the baseline period. For both cohorts, conventional non-biologics were the most frequently used pharmacological treatments, followed by biological therapies, and then CD-related surgeries, during this period.
It was estimated that more than one-third of patients who experience an RVF episode of care experience a second RVF episode of care within 2 years, and almost half of these would experience a third RVF episode of care within the same time period. After the start of the first RVF episode of care, almost all patients in the RVF cohort had received conventional therapies, more than half received biological therapies, and nearly two-thirds underwent CD-related surgery. These findings demonstrate the high treatment burden for patients with CD-related RVF and the need for novel therapeutics to treat RVF. It is worth noting that a number of therapies may be used for CD treatment rather than specifically for the treatment of RVF; for example, infliximab is an anti-TNF approved for the treatment of RVF in CD, but this therapy is also used for the treatment of patients with CD without RVF [19]. Although the use of infliximab has shown some efficacy in the treatment of RVF, the closure rate of RVF treated with infliximab has been reported to be significantly lower than that of other Crohn’s fistulas [20]. Furthermore, long-term closure with anti-TNF therapies such as infliximab requires maintenance treatment, which may be associated with safety concerns in some patient groups owing to an increased risk of infection [21]. In general, RVF treatment requires surgical intervention and selected techniques are dependent on location of RVF and the extent of disease activity [22]. The success rate of surgical treatments of RVF vary widely and multiple procedures are often required to achieve long-term fistula closure [22].
Nearly two-thirds of patients with CD-related RVF underwent RVF-related surgery any time after the index date. This study also found that, after adjusting for potential confounding factors at baseline (including but not limited to CD severity indicators), patients with CD-related RVF had a greater use of HCRU and incurred significantly higher total direct healthcare costs than patients with CD without RVF. This was mainly driven by inpatient admissions. For example, patients having a first RVF episode of care had almost double the inpatient admissions of the RVF-free cohort and an incremental total cost of $29,168 during the first 2 years. While the medical practices and specific costs associated with RVF treatment may vary between countries and regions, the impact of RVF treatment on HCRU is a global concern. Although there are no published reports on the specific costs of RVF treatment in other countries, the impact of Crohn’s disease fistulas on healthcare costs and utilization has been reported to be higher than for patients with Crohn’s disease without fistulas [23, 24].We therefore anticipate that the healthcare costs in countries outside of the USA would also be higher for patients with CD and RVF compared with those with CD without RVF, although further research is required to demonstrate this.
This study was subject to common limitations that are inherent in retrospective observational studies using claims databases and was limited to commercially insured patients in the USA. Claims databases only include diagnostic/procedure codes recorded for reimbursement purposes and reasons for diagnostic/procedure codes are not available. While the definition of an RVF episode of care in this study included at least one diagnosis/procedure code specific to RVF, it could not be determined whether some surgical procedures not specific to RVF were used to treat RVF or, for example, procedures to treat Crohn’s perianal fistulas (CPF), as having RVF is not mutually exclusive from having CPF. Additionally, laboratory tests and characteristics of RVF severity (e.g. location, size) were not available. The claims databases used in this study allowed HCRU and direct healthcare costs to be comprehensively assessed in patients with CD; nevertheless, no causal relationship between HCRU, costs, and RVF episodes of care can be inferred. It should be noted that the claim-based algorithm developed for the purpose of identifying RVF episodes of care in this study was not validated and is not intended to be used in clinical practice. A further potential limitation of the study design is the risk of ‘immortal time bias’ as patients in the RVF cohort were considered to not have RVF during the time between CD diagnosis and their first RVF episode of care and thus their HCRU and costs during that period were not accounted for. However, it is worth noting that the time from CD diagnosis to index date was similar for both cohorts. It is also possible that patients in the RVF-free cohort could go on to develop RVF after the end of the follow-up period; however, patients were required to have a minimum follow-up period of 2 years to be included in the study, which ensured that patients were observed for a sufficient period to be included in the RVF-free cohort. Finally, this study focuses on the costs and HCRU associated with RVF in patients with CD and does not reflect the wider burden of RVF in these patients, such as the impact on patient quality of life [14, 25].
In this study, the median age at index was higher than expected based on observations from clinical practice and reported studies where the age range is 20–50 years [16, 26]; therefore, initial stages of RVF management may not have been captured for a portion of the patients in this study, which could influence the data (such as the proportion of patients who underwent RVF-related surgery, which may have been higher than the proportion extracted from the databases). This could potentially lead to an underestimation of treatments received, HCRU, and direct healthcare costs in the RVF cohort. Given that the HCRU and direct healthcare costs increased with each additional RVF episode of care, it is likely that the HRCU and costs reported in this study are a conservative estimate.
In summary, management of RVF is complex and extremely challenging, and requires a multidisciplinary approach to at all stages of disease, from diagnosis and assessment through treatment planning and ongoing patient management. This study demonstrated that patients with CD who go on to develop RVF in a real-world setting have more severe disease at baseline in terms of clinical characteristics associated with CD (such as CD-related infections and other types of fistulising disease), and an increased burden on HCRU and direct healthcare costs compared with RVF-free patients with CD. After developing RVF, the requirement for repeated episodes of pharmacological and surgical care may reflect the limited options available for successful treatment of RVF and indicate an unmet need for innovative therapies targeting CD-related RVF. Further studies are required to gain a greater understanding of the requirements for effective treatment of CD-related RVF and to reduce the burden on HCRU.
References
Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380(9853):1590–605.
Schwartz DA, et al. Prevalence of fistulizing Crohn’s disease in the United States: estimate from a systematic literature review attempt and population-based database analysis. Inflamm Bowel Dis. 2019;25(11):1773–9.
Matsuzawa F, et al. Successful treatment of rectovaginal fistula and rectal stenosis due to perianal Crohn’s disease by dual-port laparoscopic abdominoperineal resection: a report of two cases. Surg Case Rep. 2016;2(1):83–83.
Nikolic M, et al. Allogeneic expanded adipose-derived stem cells in the treatment of rectovaginal fistulas in Crohn’s disease. Colorectal Dis. 2021;23(1):153–8.
Valente MA, Hull TL. Contemporary surgical management of rectovaginal fistula in Crohn’s disease. World J Gastrointest Pathophysiol. 2014;5(4):487–95.
Kniery KR, Steele SR. Operative considerations for rectovaginal fistulas. World J Gastrointest Surg. 2015;7(8):133–7.
Söderqvist EV, Cashin PH, Graf W. Surgical treatment of rectovaginal fistula-predictors of outcome and effects on quality of life. Int J Colorectal Dis. 2022;37(7):1699–707.
Kaimakliotis P, et al. A systematic review assessing medical treatment for rectovaginal and enterovesical fistulae in Crohn’s disease. J Clin Gastroenterol. 2016;50(9):714–21.
de la Poza G, et al. Genital fistulas in female Crohn's disease patients.: clinical characteristics and response to therapy. J Crohns Colitis. 2012;6(3):276–80.
Lichtenstein GR, et al. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113(4):481–517.
Mayo Clinic. Rectovaginal fistula: overview. https://www.mayoclinic.org/diseasesconditions/rectovaginal-fistula/symptoms-causes/syc-20377108. Accessed 12 Jan 2022.
Tuma F, Al-Wahab Z. Rectovaginal fistula. [Updated 2021 Oct 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022.
Göttgens KW, et al. Systematic review and meta-analysis of surgical interventions for high cryptoglandular perianal fistula. Int J Colorectal Dis. 2015;30(5):583–93.
Iglay K, et al. A systematic review of the patient burden of Crohn’s disease-related rectovaginal and anovaginal fistulas. BMC Gastroenterol. 2022;22(1):36.
Hauch A, et al. Rectovaginal fistula repair 1 year later: lessons learned. Ann Plast Surg. 2021;87(2):187–93.
Tracanelli L, et al. Rectovaginal fistula in Crohn’s disease treatment: a low long-term success rate and a high definitive stoma risk after a conservative surgical approach. Tech Coloproctol. 2021;25(10):1143–9.
Health Insurance Portability and Accountabiity Act. https://www.cdc.gov/phlp/publications/topic/hipaa.html. Accessed 17 Jan 2022.
Andrews DWK, Buchinsky M. A three-step method for choosing the number of bootstrap repetitions. Econometrica. 2000;68(1):23–51.
US Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/103772s5389s5391s5394lbl.pdf. Accessed June 2021.
Parsi MA, et al. Type of fistula determines response to infliximab in patients with fistulous Crohn’s disease. Am J Gastroenterol. 2004;99(3):445–9.
D’Haens G, et al. Five-year safety data From ENCORE, a European Observational Safety Registry for adults with Crohn’s disease treated with infliximab [Remicade®] or conventional therapy. J Crohns Colitis. 2016;11(6):680–9.
Zhu YF, et al. Current treatment of rectovaginal fistula in Crohn’s disease. World J Gastroenterol. 2011;17(8):963–7.
Chaparro M, et al. Health care costs of complex perianal fistula in Crohn’s disease. Dig Dis Sci. 2013;58(12):3400–6.
Hirsch RD, et al. Direct health care costs of managing perianal Crohn’s disease in a population based cohort. Scand J Gastroenterol. 2022;57(4):432–8.
El-Gazzaz G, et al. Analysis of function and predictors of failure in women undergoing repair of Crohn’s related rectovaginal fistula. J Gastrointest Surg. 2010;14(5):824–9.
Milito G, et al. Surgical treatment of rectovaginal fistula in Crohn’s disease: a tertiary center experience. Surg Technol Int. 2017;30:113–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Takeda Pharmaceuticals. Medical writing support was provided by Sally McTaggart, PhD, of Oxford Pharmagenesis, Oxford, UK and was funded by Takeda Pharmaceuticals.
Conflict of interest
Chitra Karki is an employee and shareholder of Takeda Pharmaceuticals USA, Inc. Dominick Latremouille-Viau is an employee of Analysis Group, Inc., a company which received funding from Takeda Pharmaceuticals USA, Inc. for this study. Inmaculada Gilaberte is an employee of Takeda Madrid, Cell Therapy Technology Center. Gary Hantsbarger is an employee and shareholder of Takeda Pharmaceuticals USA, Inc. Hela Romdhani is an employee of Analysis Group, Inc., a company which received funding from Takeda Pharmaceuticals USA, Inc. for this study. Amy L Lightner was an employee of Cleveland Clinic at the time of this study, is a consultant for Takeda, Ossium Health, Mesoblast Inc, and Boomerang Health, and is Chief Medical Officer at Direct Biologics.
Ethics approval
Not applicable: this was a non-interventional retrospective study. All data collected from the US administrative claims databases were de-identified and comply with the confidentiality requirements of the Health Insurance Portability and Accountability Act.
Consent to participate
Not applicable: all data were collected retrospectively from administrative claims databases and de-identified.
Consent for publication
Not applicable: all data were collected retrospectively from administrative claims databases and de-identified.
Availability of data and material
The data that support the findings of this study are derived from Truven Health MarketScan®. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of Truven Health MarketScan®.
Code availability (software application or custom code)
Not applicable.
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Dominick Latremouille-Viau and Hela Romdhani. All authors contributed to the previous versions of the manuscript and approved the final manuscript.
Disclosure
Some findings in this article were previously presented at the ECCO 2021 virtual congress, 8–10 July 2021.
Ethical considerations
This was a non-interventional retrospective study. All data collected from the US administrative claims databases were de-identified and comply with the confidentiality requirements of the Health Insurance Portability and Accountability Act.
Additional information
Since the time of this study, Dr Lightner’s affiliation has changed to Direct Biologics LLC, Texas, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Karki, C., Latremouille-Viau, D., Gilaberte, I. et al. Disease Burden, Treatment Patterns, and Economic Impact of Rectovaginal Fistulas in Patients with Crohn’s Disease: Findings from a Retrospective, Observational, Longitudinal Study Based on US Claims Databases. PharmacoEconomics Open 7, 811–822 (2023). https://doi.org/10.1007/s41669-023-00424-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-023-00424-z