Abstract
Background
Non-small cell lung cancer (NSCLC) is the predominant histological subtype of lung cancer and is the leading cause of cancer-related deaths globally. Quality of life is an important consideration for patients and current treatments can adversely affect health-related quality of life (HRQoL).
Objective
The objectives of this systematic literature review (SLR) were to identify and provide a comprehensive catalogue of published health state utility values (HSUVs) in patients with early-stage NSCLC and to understand the factors impacting on HSUVs in this indication.
Methods
Electronic searches of Embase, MEDLINE and Evidence-Based Medicine Reviews were conducted via the Ovid platform in March 2021 and June 2022 and were supplemented by grey literature searches of conference proceedings, reference lists, health technology assessment bodies, and other relevant sources. Eligibility criteria were based on patients with early-stage (stage I–III) resectable NSCLC receiving treatment in the adjuvant or neoadjuvant setting. No restriction was placed on interventions or comparators, geography, or publication date. English language publications or non-English language publications with an English abstract were of primary interest. A validated checklist was applied to conduct quality assessment of the full publications.
Results
Twenty-nine publications (27 full publications and two conference abstracts) met all eligibility criteria and reported 217 HSUVs and seven disutilities associated with patients with early NSCLC. The data showed that increasing disease stage is associated with decreasing HRQoL. It was also indicated that utility values vary by treatment approach; however, the choice of treatment may be influenced by the patients’ disease stage at presentation. Few studies aligned with the requirements of health technology assessment (HTA) bodies, indicating a need for future studies to conform to these preferences, making them suitable for use in economic evaluations.
Conclusions
This SLR found that disease stage and treatment approach were two of several factors that can impact patient-reported HRQoL. Additional studies are warranted to confirm these findings and to investigate emerging therapies for early NSCLC. In collecting a catalogue of HSUV data, this SLR has begun to identify the challenges associated with identifying reliable utility value estimates suitable for use in economic evaluations of early NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Quality of life in patients with early non-small cell lung cancer can be negatively impacted by many factors, including stage of disease and the treatment administered. |
Robust utility data aligned with global health technology assessment (HTA) body requirements is scarce and thus further appropriately designed studies are required. |
1 Introduction
Globally, lung cancer is the second most diagnosed cancer (11.4% of all cases) and is the leading cause of cancer-related deaths (18% of all cancer deaths) [1]. The predominant histological subtype is non-small cell lung cancer (NSCLC), which represents 80–85% of all lung cancer cases [2]. Patients presenting with early-stage disease (stage I/II/resectable III) are typically treated surgically [3, 4]. However, 30–55% of patients will experience recurrence within 5 years of complete surgical resection [5]. In addition, the 5-year survival rate for these patients is approximately 64%, indicating that more than a third of patients die despite curative therapy [6, 7].
Because of continued risk of relapse after surgical resection, adjuvant chemotherapy has become the established standard of care following surgery in patients with stage II–III NSCLC, according to treatment guidelines [4, 8]. However, adjuvant chemotherapy confers a limited survival advantage, and chemotherapy along with surgery and radiotherapy are associated with adverse events which negatively impact patients’ quality of life (QoL) post-treatment [9, 10]. The impact of progressive disease stage and currently available treatments on patient QoL has highlighted an unmet need for alternative treatments in this patient population. This is driving the introduction of new therapies, including targeted therapies and immunotherapies, which are currently under investigation in prospective clinical trials [11].
Any new therapies must be proven to be cost effective, and an essential component of cost-effectiveness analyses is patient-reported QoL. The impact of a health technology on health-related QoL (HRQoL) can be estimated using health state utility values (HSUVs). HSUVs represent the preference of the general public for different patients' health states and are an essential parameter of model-based economic evaluations of health technologies. HSUVs can be derived through several instruments, but methodologies acceptable to health technology assessment (HTA) bodies must be used to produce an accurate estimate of cost effectiveness [12,13,14,15,16]. The current systematic literature review (SLR) has three principal aims: first, to identify published HSUVs associated with patients with early-stage NSCLC; second, to present a comprehensive catalogue of available HSUV data for use in future health economic modelling in this indication; and third, to understand the factors impacting on HSUVs in early-stage NSCLC.
2 Methods
2.1 Study Design
An SLR was conducted to identify published HSUV data associated with patients with early NSCLC receiving treatment in the adjuvant or neoadjuvant setting. Disease-specific/generic HRQoL data were also sought but were not reported further in this article beyond a list of citation details. The searches for the current review were run in March 2021 and June 2022, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17]. The protocol for the current SLR was drafted to conform to PRISMA-P guidelines and was approved between Mtech Access and Roche. The protocol is not registered in a publicly available database but can be made available upon request to the authors.
2.2 Data Sources and Search Strategy
The following databases were searched on 18 March 2021 via the Ovid platform: Embase; MEDLINE (including Epub ahead of print, in-process and other non-indexed citations, and daily update) and Evidence-Based Medicine (EBM) Reviews (incorporating the Cochrane Database of Systematic Reviews, American College of Physicians [ACP] Journal Club, Database of Abstracts of Reviews of Effects [DARE], Cochrane Clinical Answers, Cochrane Central Register of Controlled Trials [CENTRAL], Cochrane Methodology Register, HTA database, and the National Health Service Economic Evaluation Database [NHS EED]). These searches were updated on 22 June 2022. The full search strategies are available in Online Resource 1 (see electronic supplementary material [ESM]). Additional searches of conference proceedings, reference lists of included publications, HTA bodies, and additional sources and websites were conducted (Online Resource 2, see ESM) using free-text terms which included, but were not limited to, ‘non-small cell lung cancer’, ‘NSCLC’, ‘NSCLC AND early’, ‘NSCLC AND quality of life’ and ‘utility’.
2.3 Eligibility Criteria
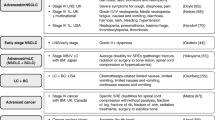
Eligibility criteria for the SLR were defined by the PICO (population, interventions, comparators, and outcomes) framework and study design (Table 1). There were no restrictions in terms of publication date or study country. Reference lists of review publications were checked to ensure any relevant primary studies were considered for inclusion. Full publications reporting HSUVs were selected for further analysis. Conference abstracts reporting HSUVs and full publications reporting on non-preference–based HRQoL only were not interrogated further and mapping to preference-based measures was not conducted as it was anticipated that sufficient published utility data would be available.
2.4 Study Selection and Data Extraction
Screening was completed by two independent analysts at title/abstract stage and at the full publication stage. Any disputes were referred to a third analyst and resolved by consensus.
Data extraction was conducted by a single analyst and 100% of data elements were checked by a second analyst. Disputes were referred to a third analyst and resolved by consensus. The extracted parameters included study characteristics (i.e. study design, patient population, sample size), HSUVs, methods/results of regression analyses, and a summary of the study-reported conclusions and limitations.
2.5 Quality and Relevance Assessment
During data extraction, the relevance of utilities and the quality of the studies generating them were assessed and recorded, and the quality of any mapping algorithms examined. This process is as recommended in the National Institute for Health and Care Excellence (NICE) technical support documents 8–10 [18,19,20] and enables justification of the use/non-use of different utility values or mapping algorithms in an economic model. In particular, the following issues were addressed: whether response rates, loss to follow-up, or amount of missing data are likely to threaten the validity of the utility estimate; whether the selection criteria yield a population similar to that being modelled; whether the utility value incorporated a decrement for QoL loss resulting from adverse events (AEs); whether adequate details of the sample sizes of patients were reported during the study recruitment process and whether the sample sizes of patients who were analysed were small (total study population ≤ 100 patients).
2.6 Health Technology Assessment (HTA) Relevant Assessment Methods
The appropriateness of the utilities reported was assessed, based on how they conformed to the requirements of HTA body reference cases, specifically NICE [12, 13], the Scottish Medicines Consortium (SMC) [14], The Canadian Agency for Drugs and Technologies in Health (CADTH) [15], and the Pharmaceutical Benefits Advisory Committee (PBAC) [16] (Table 2). The following four questions were answered for each included study:
-
1.
Was a generic preference-based instrument used to describe health states?
-
2.
Were HSUVs assessed for patients?
-
3.
Were appropriate societal preferences used to value health states?
-
4.
Was the time trade-off/standard gamble (TTO/SG) method used to value health states?
3 Results
3.1 Search Yield
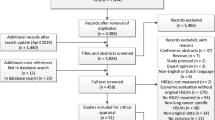
The electronic database search identified 1987 citations (Fig. 1). After removal of duplicates, 1723 titles and abstracts were screened, from which 95 citations were deemed potentially relevant. Following full-paper review, 15 records reported relevant HSUVs and were eligible for inclusion. Grey literature searches yielded 12 additional relevant publications and therefore 27 records (25 full publications and two conference abstracts) were included. The updated search yielded two additional relevant full publications. The two conference abstracts were not considered further due to limited reporting (citation details provided in Online Resource 3, see ESM) and therefore a total of 27 full publications reporting relevant HSUVs were considered in the current SLR. A further 155 records reporting generic and/or disease-specific HRQoL data were tagged, but are not reported further in this article (citation details provided in Online Resource 4, see ESM).
3.2 Description of Identified Studies
A range of QoL instruments were reported across the 27 eligible full publication studies measuring patient utilities and disutilities (Online Resource 5, see ESM). Of the 21 studies administering a questionnaire, the majority utilised a single instrument (n = 17), with four studies employing a combination of instruments. A single study retrieved proxy-derived patient HSUVs utilising standard gamble and the visual analogue scale (general population as proxies for patients). Three studies used published mapping algorithms to map utility values from disease-specific measures to generic preference-based measures (EORTC-QLQ-C30 to EQ-5D [3L and/or 5L]). Two studies derived SF-6D utility values using SF-12 and SF-36. Follow-up times ranged from 2 weeks to 6 years. Studies generally reported utility data from a single country (n = 25), with a minority presenting multi-national datasets (n = 2). Generally, studies reported populations covering multiple or individual stages of NSCLC and different treatment regimens.
3.3 Health State Utility Values by Disease Stage
A total of 17 studies reported utility values by disease stage [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. The EQ-5D was the most commonly used instrument (EQ-5D-3L, n = 9; EQ-5D-5L; n = 2, version not specified, n = 2), followed by SF-6D, n = 2; visual analogue scale (VAS), n = 1; 15 Dimensions (15D), n = 1; Assessment of Quality of Life, n = 1; Functional Assessment of Cancer Therapy—Lung Utility Index (Fact-U), n = 1; and Multi-Attribute Utility Theory (MAUT) based index, n = 1). Two studies did not report the instrument used, but rather the method—standard gamble (SG). Of the 17 studies, only four had a sample size of > 100 in all disease-stage groups (range: 105–1292) [29, 34, 36, 39], while 12 studies had a sample size of < 100 in most disease-stage groups (range: 16–99) [23, 24, 26,27,28, 30,31,32,33, 35, 37, 38]. One study did not report the number of patients within each disease stage [25].
As expected, utility values appear to decrease with advancing disease, as was the case from the perspective of both patients and proxy respondents (general public) (Fig. 2). Individual studies reporting on multiple disease stages generally showed a reduction in utilities from Stage I to Stage III disease; however, substantial variation in estimates was observed across the studies (Stage I: 0.48–0.89; Stage II: 0.38–0.83; Stage III: 0.27–0.83). HSUVs for stage IA and stage IB disease were comparable; however, only two estimates were available for each of these stages (0.696–0.718 and 0.711–0.727, respectively).
Utilities by disease stage. The three estimates for ‘Stage IA–IIIB’ are derived from the study by Andreas et al (2018) [23]. All patients underwent complete surgical resection. Of these patients, some remained disease free (0.72), but others relapsed and progressed to locoregional recurrence (0.62) or distant metastasis/terminal disease (0.67), forming the three distinct data points. 15D 15 Dimensions, AQoL Assessment of Quality of Life, EQ-5D European Quality of Life-5 dimensions, EQ-5D-3L European Quality of Life -5 dimensions 3 levels, EQ-5D-5L European Quality of Life -5 dimensions 5 levels, FACT-U Functional Assessment of Cancer Therapy—lung utility index, MAUT Multi-Attribute Utility Theory, NR not reported, SF-6D Short Form-6 dimensions, SG standard gamble, VAS visual analogue scale
A single study reported on patients who had undergone resection and were disease free versus those with recurrence [13]. Patients with stage IB to IIIA disease who were disease-free (n = 238) had a higher utility (0.72) versus those who progressed to locoregional recurrence (0.62; n = 19) or distant metastasis/terminal disease (0.67; n = 32), indicating that disease progression negatively impacts HSUVs.
3.4 Intervention-Specific Utilities
According to the European Society of Medical Oncology (ESMO) guidelines, treatment approach is dependent on disease stage, even within the early NSCLC population, with surgery being the earliest intervention (recommended for patients with stage I–II disease) and chemotherapy being utilised for later stages (stage IIB–III) [3, 4]. Unsurprisingly, early-stage patients treated with surgery had utilities in the range of 0.803–0.855, which is higher compared with patients treated with chemotherapy (0.60–0.75) and similar to patients treated with radiotherapy (0.83). Due to treatment approach being determined by disease stage, it was not appropriate to compare utilities across treatment approaches. Instead, utilities related to a particular treatment were compared over a range of follow-up times (Fig. 3).
HSUVs by intervention. † For studies that did not report follow-up times in weeks, these were converted up to 1 year of follow-up. ‡ A single study by Jang et al (2009) [41] provided the 6-year data for chemotherapy treatment. No baseline value is provided for this treatment as the three groups represented are post-treatment but different stages of toxicity: TWiST state (0.75); toxicity state (0.68) and relapse state (0.60). CGA comprehensive geriatric assessment, HSUVs health state utility values, NR not reported, SBRT stereotactic body radiation therapy, VATS video-assisted thoracoscopic surgery
Seven studies reported intervention-specific utilities for patients with early NSCLC, across a number of different interventions including video-assisted thoracoscopic surgery (VATS) (n = 3) [24, 30, 40], thoracotomy (n = 2) [24, 40], vinorelbine plus cisplatin chemotherapy (n = 1) [41], stereotactic body radiation therapy (SBRT) plus comprehensive geriatric assessment (CGA) (n = 1) [42], SBRT alone (n = 1) [42], mixed surgeries (n = 1) [31], and chemo-radiation (n = 1) [37]. The EQ-5D (EQ-5D-3L, n = 2; EQ-5D-5L, n = 2; version not specified, n = 1) was the most commonly used tool to measure intervention-specific HSUVs. The Assessment of Quality of Life (AQoL) and 15 Dimensions (15D) were also utilised (both n = 1). Follow-up times ranged between 2 weeks and 6 years. The majority of studies had < 100 patients in each group (range: 20–83). Only two of the seven studies included > 100 patients per group (range: 180–482) [40, 41].
Across the range of follow-up periods reported, the utility values remained broadly constant, although some trends could be seen for individual treatments. For patients treated surgically (VATS or thoracotomy), utility values declined post-operatively compared with baseline (0.81–0.89 for VATS and 0.86 for thoracotomy at baseline; 0.74–0.78 for VATS and 0.73 for thoracotomy at post-operative follow-up of 2 weeks) [24, 30, 31, 40]. Despite this initial post-surgical decrease, a prolonged follow-up period demonstrated a return to values comparable with baseline by 52 weeks post-surgery (0.86 and 0.84 for VATS and thoracotomy, respectively) [24]. The study of Manser et al. (2006) [31] reported utilities for a mixed surgical population; the utility values for this population declined from 0.67 at baseline to 0.55 as 12 weeks’ follow-up and 0.59 at 26 weeks’ follow-up.
A single study reported on radiotherapy [42]. Utility values for both SBRT alone and SBRT plus CGA fluctuated slightly during the follow-up periods of 5, 12, 26, 39, and 52 weeks. From baseline to 52 weeks post-treatment, the utility changed from 0.71 to 0.67 in the SBRT-alone group, and from 0.77 to 0.75 in the SBRT plus CGA group, indicating that utilities post-treatment were largely unchanged versus pre-treatment.
Patients who received chemo-radiation had utilities that were comparable at pre-treatment baseline (0.86) and 50 months’ post-treatment (0.83), indicating that the toxicity experienced did not have long-term effects on patient-reported HRQoL [37].
Chemotherapy was reported in a single study, based on a regimen of vinorelbine plus cisplatin [41]; after 6 years’ follow-up, patients without symptoms or toxicity (TWiST state) experienced higher utility than those in either the toxicity or relapse state (0.75 vs 0.68 and 0.60, respectively).
3.5 Factors that Impact Utilities
Regression analyses were conducted in 10 studies to identify factors that impact utilities, nine of which considered utility score as the dependent variable (Table 3). The following variables were negatively associated with utility score across the studies: presence of severe AEs; initial treatment with combined radiotherapy and chemotherapy; more advanced disease stage/presence of metastasis; neurocognitive symptoms; female sex; congestive heart failure; chronic obstructive pulmonary disease; and coronary artery disease. Naik et al. [32] reported that although HSUVs were higher in patients who had effectively survived longer, health utility may be driven more by disease site, disease extent, treatment status, and specific treatments received as opposed to time.
3.6 Disutilities
Disutilities associated with resection, Eastern Cooperative Oncology Group (ECOG) score and treatment period were identified. Two studies reported disutilities associated with resection [27, 46]: resection in general was associated with a disutility of − 0.127 2 weeks post-resection compared with baseline, and − 0.016 2 weeks post-resection compared with control subjects at the same timepoint [46]; lobectomy and bilobectomy were associated with a disutility of − 0.059 at 12 months’ post-operatively and − 0.078 at 24 months’ post-operatively [27]. An ECOG score of 4 was associated with a disutility of − 0.024 [43]. Patients treated during the period 1998–2003 and those treated in the period 2006–2011 had equal disutilities of − 0.07 compared with their respective baseline values [47].
3.7 Quality Assessment
Quality assessment of the included studies highlighted several limitations associated with the reported HSUVs (Online Resource 6, see ESM). Absence of information regarding the patient recruitment process, response rates to instruments, and missing data are likely to restrict the usefulness of the studies for informing economic evaluations. Commonly reported limitations across the studies included relatively small sample sizes (total study population ≤ 100 patients in nine studies); limited generalisability of results beyond the study setting (due to the reporting of study populations from a single centre, inclusion of patients with a broad range of disease stages (e.g. IA to IIIB, with no subgroup data reported for the individual stages), and the potentially restrictive inclusion criteria of those studies conducted in a clinical setting); potential over-estimation of HRQoL due to non-responder bias and/or loss to follow up; and lack of external validation of results.
3.8 Assessment of Utilities Identified in the Systematic Literature Review Against HTA Body Reference Cases
An overall summary of the relevance of the identified utilities to each of the HTA body reference cases is provided in Online Resource 7 (see ESM). The assessed HTA bodies prefer patient-derived utilities, however where this is not possible vignettes can be an acceptable alternative. For all included studies, utilities were derived directly from patients, with the exception of Kim et al. [29], which used proxy respondents; adult members of the Korean general public valued a series of vignette health states relating to patients with lung cancer.
Four studies [26, 32, 33, 43] met the requirements of all four HTA bodies, and a further eight studies [24, 28, 30, 38, 39, 42, 46, 48] met the SMC requirements as utilities were derived directly from patients using appropriate instruments to measure HRQoL, and health states were valued using appropriate societal preferences. These studies are likely to be considered the most appropriate for informing economic evaluations across multiple geographical settings. For the remaining studies, they either failed to meet the reference case requirements (n = 18 NICE, n = 6 SMC, n = 6 CADTH, n = 6 PBAC) due to use of inappropriate utility derivation instruments, societal preferences or valuation methods, or it was unclear if they met the requirements (n = 5 NICE, n = 9 SMC, n = 17 CADTH, n = 17 PBAC) due to limited reporting.
4 Discussion
Health status, as perceived by patients or proxy respondents (i.e. the general public), is an important outcome measure when evaluating the impact of disease and treatment on aspects of daily living. Thus, the aim of this SLR was to identify published HSUVs associated with patients with early-stage NSCLC and to present a comprehensive catalogue of available HSUV data for use in future health economic modelling in this indication. Additionally, the SLR aimed to understand the factors impacting on HSUVs in early-stage NSCLC.
In total, the SLR identified 27 full publications reporting 217 HSUVs and seven disutilities associated with patients with early NSCLC. The evidence suggests that, for patients with early NSCLC, disease stage may have an impact on patient-reported HRQoL. From the patient perspective, HRQoL was found to decline with increasing disease stage. This trend from patient-elicited values was supported by that observed within a large sample of 515 members of the general public assessed by Kim et al. [29]. Furthermore, the impact of disease stage on utilities is supported by the SLR and meta-analysis conducted by Blom et al. (2020), which summarised reported HSUVs for patients with NSCLC regardless of disease stage [49] and highlighted the importance of the use of stage-specific as well as country-specific utility values for lung cancer. Blom et al. [49] reported the utility value for stage I–II lung cancer as 0.78 (95% CI 0.70–0.86) (based on six studies), which is within the range of values in the current SLR for stage I–II patients. While the Blom et al. review [49] is important in supporting the results of the current SLR, it is important to highlight that they focussed on lung cancer in general whereas the current SLR specifically analyses utilities for resectable early-stage NSCLC. Additionally, the current SLR was necessary as it is more recent and has a less restrictive PICO than that used by Blom et al. [49], thus it has captured additional recently published studies on NSCLC. There were limited data to show that disease progression impacts utilities; only one small, insufficiently powered study was identified [23], and this did not have robust results. This data gap indicates that further robust, well-powered studies on the effect of disease progression on utilities are needed.
There were data to suggest that utility may depend on treatment approach, which could reflect disease stage at presentation. Published guidelines indicate that the treatment approach is selected based on disease stage [3, 4]. This may explain why variability was observed in the utility values reported for patients undergoing different treatment approaches (Fig. 3). Surgery may appear to have marginally higher HSUVs; however, this could be due to the earlier disease stage of these patients compared with, for example, patients undergoing chemotherapy who are likely to have a more advanced disease state. This highlights the importance of considering the impact of both disease stage and treatment approach on HSUVs for use in economic evaluations.
Considering the impact of different treatments on utility over time, most interventions considered in this SLR, including surgery, radiotherapy, and chemo-radiation, were associated with a decline in HSUV in the short-term follow-up after treatment (ranging from 2 to 12 weeks) (Fig. 3). However, after longer follow-up periods, ranging from 26 weeks to 50 months, utility values returned to baseline, with any differences between baseline and final follow-up being marginal. This effect has been confirmed in clinical studies measuring patient-reported HRQoL with non-preference based tools [9, 10]. For chemotherapy, however, the impact on HSUVs depends on the success of the initial treatment and any subsequent regimens administered post-relapse. Those who experience toxicity or relapse post-treatment are likely to experience long-term negative impacts on HRQoL compared with patients who do not experience symptoms or toxicity. Despite these general trends in the treatment-related results, usually only a single study reported utility values for the individual treatment approaches. Therefore, further studies are needed to consolidate these findings. Furthermore, there are currently limited analyses of the HSUVs associated with novel therapies for early NSCLC, including the use of immunotherapies and targeted therapies in the adjuvant setting. Such therapies could be beneficial to patients by slowing disease progression, which may allow patients to maintain a higher level of HRQoL over a longer time.
Despite the identification of a reasonable number of studies (n = 27), the ability to synthesise results for comparable populations was limited, particularly across different treatment approaches, due to study heterogeneity. Substantial variation in estimates was observed across the studies (Figs. 2, 3), likely due to differences in patient characteristics (e.g. age, performance status, resectability status), instruments and tariffs used to derive utilities, and follow-up periods. While a meta-analysis would be useful in facilitating these comparisons, the findings would be subject to large variance due to the substantial heterogeneity across studies, a factor acknowledged by the authors of the previous meta-analysis reported in this indication [49] (e.g. I2 of 92% reported for meta-analysis of patients with stage I–II disease, indicating significant heterogeneity). It is not possible to control for all additional factors across the studies, such as the differences in follow-up time post-treatment, which makes a robust meta-analysis challenging.
The regression analyses reported identified the presence of severe AEs, initial treatment with combined radiotherapy and chemotherapy, more advanced disease stage/presence of metastasis, neurocognitive symptoms, female sex, congestive heart failure, chronic obstructive pulmonary disease, and coronary artery disease as factors that negatively impact HSUVs. As AEs have a major impact on patient-reported HRQoL, even when moderate, the impact of treatment-related AEs on HRQoL is an area that warrants further investigation.
Consideration of the identified studies in line with HTA body reference cases highlighted that few studies align with the requirements. NICE, SMC, CADTH and PBAC prefer patients to estimate utilities through a generic preference-based instrument. The health states should be valued using an appropriate country-specific tariff that reflects societal preferences. Only four studies were identified that met the requirements of all four reference cases [26, 32, 33, 43] (Table 2). These studies are likely to be considered most appropriate for informing economic evaluations. Future economic evaluations in this indication should fully justify the choice of utility inputs, and comprehensive sensitivity analyses should be conducted to thoroughly assess the robustness of the estimates.
The strengths of this SLR include the design of the search strategy and the wide range of data sources searched, which enabled the identification of studies reporting utilities and disutilities associated with early NSCLC. Only full publications were analysed as the limited reporting in conference abstracts suggests a lack of robustness as a data source in comparison with full publications. HSUVs have been described by disease stage and treatment approach, allowing their use in economic modelling. The SLR has also highlighted the studies that conform to NICE, SMC, CADTH and PBAC requirements and thus are likely to be accepted by these HTA bodies.
Despite these strengths, findings from the SLR must also be interpreted with consideration of the individual study caveats and limitations of the overarching evidence base. Quality assessment of the included studies highlighted a number of limitations associated with the utility values reported. In particular, absence of information regarding the patient recruitment process, response rates to instruments, and missing data are likely to restrict the usefulness of the studies for informing economic evaluations. Commonly reported limitations across the studies included small sample sizes (n = 9) [27, 30, 31, 37, 42,43,44, 46, 47]; limited generalisability of results beyond the study setting (n = 9) [25, 28, 32, 35, 37, 43, 46, 48, 50]; potential over-estimation of HRQoL due to non-responder bias and/or loss to follow up (n = 6) [26, 27, 29, 31, 32, 38]; and lack of external validation of results (n = 3) [28, 43, 50]. The small sample sizes (total study population ≤ 100 patients) are a particular issue as this contributes to the limited generalisability of the results to the broader population. Additionally, small sample size is usually reflected in wider variance, which increases the uncertainty around the estimates. Furthermore, several relevant studies had a cross-sectional design which may not capture the full burden of eNSCLC on HSUVs. These limitations must be taken into consideration when interpreting the available evidence and when selecting utilities for use in economic evaluations. Future studies should attempt to address these issues by using prospective study designs enrolling larger cohorts and more generalisable patient populations. Furthermore, although several novel targeted therapies are in development and are recommended as standard of care for some populations (e.g. patients with stage IB–IIIA NSCLC with specific EGFR mutations) [4], there are currently no published HSUVs for these treatments. This indicates a need to use appropriate generic preference-based instruments to gather HSUVs for all treatments available to patients with early NSCLC.
5 Conclusion
This SLR identified published HSUVs associated with patients with early NSCLC. It was found that several factors, including disease stage and treatment approach, may impact patient-reported HRQoL. However, future studies should disentangle the effect of disease stage and treatment approach on HRQoL, in particular for the adjuvant use of novel targeted therapies and immunotherapies, where it will be of interest to confirm whether any improvements in duration of survival are associated with maintenance/improvements in HRQoL versus standard of care. In collecting a comprehensive catalogue of currently available HSUV data, this SLR has highlighted some of the challenges associated with identifying reliable utility value estimates suitable for use in economic evaluations of early NSCLC. The paucity of data conforming to the stringent requirements of HTA bodies has highlighted the need for further studies that comply with HTA body preferences, making them suitable for use in economic evaluations.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
American Cancer Society. What is lung cancer? 2019. https://www.cancer.org/cancer/lung-cancer/about/what-is.html#:~:text=About%2080%25%20to%2085%25%20of,carcinoma%2C%20and%20large%20cell%20carcinoma. Accessed 31 Aug 2022.
Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Supplement 4):iv1–iv21.
Remon J, Soria JC, Peters S. Early and locally advanced non-small-cell lung cancer: an update of the ESMO clinical practice guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. 2021;32(12):1637–42.
Wang X, Janowczyk A, Zhou Y, Thawani R, Fu P, Schalper K, et al. Prediction of recurrence in early stage non-small cell lung cancer using computer extracted nuclear features from digital H&E images. Sci Rep. 2017;7(1):13543.
American Cancer Society. Lung cancer survival rates. 2022. https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/survival-rates.html. Accessed 31 Aug 2022.
Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5):e278S-e313S.
Pisters K, Kris MG, Gaspar LE, Ismaila N. Adjuvant systemic therapy and adjuvant radiation therapy for stage I–IIIA completely resected non-small-cell lung cancer: ASCO guideline rapid recommendation update. J Clin Oncol. 2022;40(10):1127–9.
Bezjak A, Lee CW, Ding K, Brundage M, Winton T, Graham B, et al. Quality-of-life outcomes for adjuvant chemotherapy in early-stage non-small-cell lung cancer: results from a randomized trial, JBR.10. J Clin Oncol. 2008;26(31):5052–9.
Nugent SM, Golden SE, Hooker ER, Sullivan DR, Charles R, Thomas J, Deffebach ME, et al. Longitudinal health-related quality of life among individuals considering treatment for stage I non–small-cell lung cancer. Ann Am Thorac Soc. 2020;17(8):988–97.
Yuan M, Huang LL, Chen JH, Wu J, Xu Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct Target Ther. 2019;4:61.
The National Institute for Health and Care Excellence (NICE). Guide to the methods of technology appraisal, April 2013 [online]. http://www.nice.org.uk/article/PMG9/chapter/Foreword. Accessed 1 Sept 2022.
The National Institute for Health and Care Excellence (NICE). Position statement on use of the EQ-5D-5L value set for England (updated October 2019) [online]. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l#:~:text=We%20do%20not%20recommend%20using,set%20for%20reference%2Dcase%20analyses. Accessed 1 Sept 2022.
Scottish Medicines Consortium (SMC). Guidance to submitting companies for completion of New Product Assessment Form (NPAF), November 2020 [online]. https://www.scottishmedicines.org.uk/media/5599/20200611-guidance-on-npaf.pdf. Accessed 1 Sept 2022.
The Canadian Agency for Drugs and Technologies in Health (CADTH). Guidelines for the economic evaluation of health technologies: Canada 4th edition, March 2017 [online]. https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf. Accessed 1 Sept 2022.
The Pharmaceutical Benefits Advisory Committee (PBAC). Guidelines for preparing a submission to the Pharmaceutical Benefits Advisory Committee Version 5.0, September 2016 [online]. https://pbac.pbs.gov.au/content/information/files/pbac-guidelines-version-5.pdf. Accessed 1 Sept 2022.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Brazier JE, Longworth L. NICE DSU technical support document 8: an introduction to the measurement and valuation of health for NICE submissions. 2011. http://www.nicedsu.org.uk. Accessed 6 Sept 2022.
Papaioannou D, Brazier JE, Paisley S. NICE DCU technical support document 9: the identification, review and synthesis of health state utility values from the literature. 2011. http://www.nicedsu.org.uk. Accessed 6 Sept 2022.
Longworth L RD. NICE DSU technical support document 10: the use of mapping methods to estimate health state utility values. 2011. http://www.nicedsu.org.uk. Accessed 6 Sept 2022.
Hernández Alava M, Wailoo A, Pudney S. Methods for Mapping Between the EQ-5D-5L and the 3L for Technology Appraisal. NICE DSU report. 2017.
Hernández Alava M, Wailoo A, Pudney S. Estimating the relationship between EQ-5D-5L and EQ-5D-3L: results from an English Population Study. 2020.
Andreas S, Chouaid C, Danson S, Siakpere O, Benjamin L, Ehness R, et al. Economic burden of resected (stage IB-IIIA) non-small cell lung cancer in France, Germany and the United Kingdom: a retrospective observational study (LuCaBIS). Lung Cancer. 2018;124:298–309.
Bendixen M, Kronborg C, Jorgensen OD, Andersen C, Licht PB. Cost-utility analysis of minimally invasive surgery for lung cancer: a randomized controlled trial. Eur J Cardiothorac Surg. 2019;56(4):754–61.
Black WC, Gareen IF, Soneji SS, Sicks JD, Keeler EB, Aberle DR, et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med. 2014;371(19):1793–802.
Grutters JP, Joore MA, Wiegman EM, Langendijk JA, de Ruysscher D, Hochstenbag M, et al. Health-related quality of life in patients surviving non-small cell lung cancer. Thorax. 2010;65(10):903–7.
Ilonen IK, Rasanen JV, Knuuttila A, Sihvo EI, Sintonen H, Sovijarvi ARA, et al. Quality of life following lobectomy or bilobectomy for non-small cell lung cancer, a two-year prospective follow-up study. Lung Cancer. 2010;70(3):347–51.
Jang RW, Isogai PK, Mittmann N, Bradbury PA, Shepherd FA, Feld R, et al. Derivation of utility values from European Organization for Research and Treatment of Cancer Quality of Life-Core 30 questionnaire values in lung cancer. J Thorac Oncol. 2010;5(12):1953–7.
Kim EJ, Ock M, Kim KP, Jung NH, Lee HJ, Kim SH, et al. Disease severity-based evaluation of utility weights for lung cancer-related health states in Korea. BMC Cancer. 2018;18(1):1081.
Koide R, Kikuchi A, Miyajima M, Mishina T, Takahashi Y, Okawa M, et al. Quality assessment using EQ-5D-5L after lung surgery for non-small cell lung cancer (NSCLC) patients. Gen Thorac Cardiovasc Surg. 2019;67(12):1056–61.
Manser RL, Wright G, Byrnes G, Hart D, Conron M, Carter R, et al. Validity of the Assessment of Quality of Life (AQoL) utility instrument in patients with operable and inoperable lung cancer. Lung Cancer. 2006;53(2):217–29.
Naik H, Howell D, Su S, Qiu X, Brown MC, Vennettilli A, et al. EQ-5D health utility scores: data from a comprehensive Canadian Cancer Centre. Patient. 2017;10(1):105–15.
Sharples LD, Jackson C, Wheaton E, Griffith G, Annema JT, Dooms C, et al. Clinical effectiveness and cost-effectiveness of endobronchial and endoscopic ultrasound relative to surgical staging in potentially resectable lung cancer: results from the ASTER randomised controlled trial. Health Technol Assess. 2012;16(18):1–81.
Sigel K, Yin Kong C, Leiter A, Kale M, Mhango G, Huang B, et al. Assessment of treatment strategies for stage I non-small cell lung cancer in patients with comorbidities. Lung Cancer. 2022;170:34–40.
Swan JS, Lennes IT, Stump NN, Temel JS, Wang D, Keller L, et al. A patient-centered utility index for non-small cell lung cancer in the United States. MDM Policy Pract. 2018;3(2):2381468318801565.
Tramontano AC, Schrag DL, Malin JK, Miller MC, Weeks JC, Swan JS, et al. Catalog and comparison of societal preferences (utilities) for lung cancer health states: results from the Cancer Care Outcomes Research and Surveillance (CanCORS) study. Med Decis Mak. 2015;35(3):371–87.
Vogel J, Wang X, Troxel AB, Simone CB, Rengan R, Lin LL. Prospective assessment of demographic characteristics associated with worse health related quality of life measures following definitive chemoradiation in patients with locally advanced non-small cell lung cancer. Transl Lung Cancer Res. 2019;8(4):332–9.
Witlox WJA, Ramaekers BLT, Joore MA, Dingemans AMC, Praag J, Belderbos J, et al. Health-related quality of life after prophylactic cranial irradiation for stage III non-small cell lung cancer patients: results from the NVALT-11/DLCRG-02 phase III study. Radiother Oncol. 2020;144:65–71.
Yang SC, Lai WW, Chang HY, Su WC, Chen HH, Wang JD. Estimation of loss of quality-adjusted life expectancy (QALE) for patients with operable versus inoperable lung cancer: adjusting quality-of-life and lead-time bias for utility of surgery. Lung Cancer. 2014;86(1):96–101.
Rauma V, Andersson S, Robinson EM, Rasanen JV, Sintonen H, Salo JA, et al. Thoracotomy and VATS surgery in local non-small-cell lung cancer: differences in long-term health-related quality of life. Clin Lung Cancer. 2019;20(5):378–83.
Jang RW, Le Maitre A, Ding K, Winton T, Bezjak A, Seymour L, et al. Quality-adjusted time without symptoms or toxicity analysis of adjuvant chemotherapy in non-small-cell lung cancer: an analysis of the National Cancer Institute of Canada Clinical Trials Group. JBR10 trial. J Clin Oncol. 2009;27(26):4268–73.
Jeppesen SS, Matzen LE, Brink C, Bliucukiene R, Kasch S, Schytte T, et al. Impact of comprehensive geriatric assessment on quality of life, overall survival, and unplanned admission in patients with non-small cell lung cancer treated with stereotactic body radiotherapy. J Geriatr Oncol. 2018;9(6):575–82.
Khan I, Morris S, Pashayan N, Matata B, Bashir Z, Maguirre J. Comparing the mapping between EQ-5D-5L, EQ-5D-3L and the EORTC-QLQ-C30 in non-small cell lung cancer patients. Health Qual Life Outcomes. 2016;14:60.
Trippoli S, Vaiani M, Lucioni C, Messori A. Quality of life and utility in patients with non-small cell lung cancer. Quality-of-life Study Group of the Master 2 Project in Pharmacoeconomics. Pharmacoeconomics. 2001;19(8):855–63.
Wolff HB, Alberts L, Kastelijn EA, Lissenberg-Witte BI, Twisk JW, Lagerwaard FJ, et al. Differences in longitudinal health utility between stereotactic body radiation therapy and surgery in stage I non-small cell lung cancer. J Thorac Oncol. 2018;13(5):689–98.
Brocki BC, Andreasen JJ, Westerdahl E. Inspiratory muscle training in high-risk patients following lung resection may prevent a postoperative decline in physical activity level. Integr Cancer Ther. 2018;17(4):1095–102.
Mahal AR, Cramer LD, Wang EH, Wang S, Davidoff AJ, Gross CP, et al. Did quality of life for older cancer survivors improve with the turn of the century in the United States? J Geriatr Oncol. 2021;12(1):102–5.
Yang SC, Kuo CW, Lai WW, Lin CC, Su WC, Chang SM, et al. Dynamic changes of health utility in lung cancer patients receiving different treatments: a 7-year follow-up. J Thorac Oncol. 2019;14(11):1892–900.
Blom EF, Haaf K, de Koning HJ. Systematic review and meta-analysis of community- and choice-based health state utility values for lung cancer. Pharmacoeconomics. 2020;38(11):1187–200.
Andreas S, Chouaid C, Danson S, Siakpere O, Benjamin L, Ehness R, et al. Economic burden of resected (stage IB-IIIA) non-small cell lung cancer in France, Germany and the United Kingdom: a retrospective observational study (LuCaBIS). Lung Cancer (Amsterdam, Netherlands). 2018;124:298–309.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The research was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Conflict if interest
NJ, SA, DDM, and RB are employees of F. Hoffmann-La Roche Ltd, Basel, Switzerland. PF, SB, and LGJ were paid consultants to F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Availability of data and material
The manuscript makes use of publicly available data from published studies. All data generated during this study are included in this article and its online supplementary material files.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
NJ, SA, DDM and RB assisted with the SLR design, data analysis and manuscript preparation. PH designed the SLR and collected and analysed the data. SB collected and analysed the data. LGJ collected and analysed the data and conducted draft manuscript preparation. All authors read and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jovanoski, N., Abogunrin, S., Di Maio, D. et al. Health State Utility Values in Early-Stage Non-small Cell Lung Cancer: A Systematic Literature Review. PharmacoEconomics Open 7, 723–738 (2023). https://doi.org/10.1007/s41669-023-00423-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-023-00423-0