Abstract
Objective
The aim of this study was to perform a systematic review, meta-analysis and cost-consequence analysis of the single-use bronchoscope, Ambu aScopeTM 5 Broncho, in relation to reusable flexible bronchoscopes (RFB) available within three high procedure volume university hospitals and academic institutions in the USA.
Methods
The primary outcome was incremental cost and the secondary outcome was incremental cross-infection risk of use for both the single-use flexible bronchoscope (SUFB) and RFBs. Cost estimates included capital, repair, and reprocessing costs derived from a prospective observational micro-costing approach within three large university hospitals and academic institutions. All costs were valued in 2022 US dollars (USD). A meta-analysis based on literature covering cross-contamination and infection from 2010 to 2020 investigated cross-infection risk following bronchoscopy procedures with RFBs. Capital costs were discounted at 3% over 5–8 years. All parameters were evaluated using both univariate deterministic and probabilistic sensitivity analyses.
Results
In high-volume hospitals, RFBs were cost minimizing compared to SUFBs. Probabilistic sensitivity analysis showed that RFBs were cost saving in 88% of iterations. Univariate analyses illustrated sensitivity of the base-case result to the procedure volume. Data from sensitivity analyses suggest that the two interventions are cost neutral at a break-even point of 756 procedures per year or 46 procedures per bronchoscope per year.
Conclusion
Assuming equivalent clinical performance, single-use flexible bronchoscopes are not cost minimizing when including the costs associated with cross-infection in high-volume US university hospitals and academic institutions. Overall, the benefits of conversion from RFBs to SUFBs are dependent on the annual procedure volume of individual hospitals, expected cross-infection risk, and purchase price of the aScope 5 Broncho.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
When deciding between RFBs and SUFBs, it is important to consider factors such as annual procedure volume, expected cross-infection risk, acquisition cost of each option, and their environmental and organizational impacts. |
In bronchoscopy suites performing less than 756 bronchoscopies per year, SUFBs may be more cost minimizing than RFBs. |
The findings of this study estimated a cross-infection risk of 0.22%. In addition, it was observed that the transportation and reprocessing of RFBs require 23 min of hands-on time in average. |
1 Introduction
Various single-use flexible bronchoscopes (SUFB) have been introduced, but are yet to gain widespread acceptance within the bronchoscopy suite [1]. SUFBs have been proven to perform on par and, in certain scenarios, are preferred over reusable flexible bronchoscopes (RFB) [1,2,3]. Before, but also during the COVID-19 pandemic, single-use endoscopy attracted more focus from multiple national institutions, highlighting and recommending the sterility, availability, improved maneuverability, and cost effectiveness in relation to RFBs [3,4,5,6,7]. Additionally in 2021 the US Food and Drug Administration (FDA) issued a safety communication focusing on single-use endoscopy in circumstances of increased risk of infection, treating immunocompromised patients, or in situations where the endoscopes cannot be immediately reprocessed.
Overall, ~ 500,000 bronchoscopy procedures are currently performed within the USA with either RFBs or SUFBs. However, SUFBs are not considered adequate for advanced procedures in the bronchoscopy suite since no comparative studies have demonstrated equivalent performance between SUFBs and RFBs yet [1, 8]. The Danish Medical company Ambu A/S launched the aScopeTM 5 Broncho (aS5 Broncho) in 2022, with the aim of facilitating utilization of SUFBs for more complex bronchoscopy procedures within the bronchoscopy suite. Documented sterility of the novel SUFB instrument, together with assumed utility for advanced procedures, should enable the maintenance of high-quality diagnostic and therapeutic procedures in the bronchoscopy suite. The assumed diminished risk of cross-infection may provide better clinical value for patients admitted to pulmonology wards in the USA. Diminished risk is based on endoscopes presently being classified as semi-critical devices, which means they do not strictly need to be sterilized. In accordance with Centers for Disease Control and Prevention (CDC), their recommendation for reprocessing in the USA is to follow the instructions-for-use (IFU) provided by the manufacturer. The IFU requires at least a high-level disinfection (HLD) or sterilization. For both reprocessing methods, a precleaning and visual inspection are required [9]. Studies have previously documented the costs in intensive care units, operating rooms, or other clinical settings [6, 7, 10]. No study has yet documented the costs related to the use of RFBs in high-volume US bronchoscopy suites and estimated the break-even point indicating the cost neutrality of reusable and single-use flexible bronchoscopes.
The main objective of this study was to examine costs and cross-infection risk of the novel aScopeTM 5 Broncho compared with those of conventional RFBs from a US Hospital sector perspective by using a micro-costing approach. The objective was analyzed within only high-volume US university hospitals and academic institutions.
2 Methods
2.1 Systematic Review
Two meta-analyses were performed, one for cross-contamination and one for subsequent infection, to identify the overall risk of cross-infection. To screen for studies encompassing these two areas, a systematic literature search was performed in PubMed and Embase databases with adherence to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [11]. An overview of the PRISMA and CHEERS checklist can be found in supplementary information (SI 1). Identification of the relevant literature was based on the population, intervention, comparison, and outcome model (PICO model), which covered outbreaks, pseudo-outbreaks, infections, and cross-contamination related to RFBs. The population and comparator domains were excluded from the PICO model, owing to no requirement for specific populations or comparators. All studies on children (aged < 18 years) were excluded. The following search terms were utilized in PubMed: bronchoscope, bronchoscopy, equipment reuse and bronchoscop*, in addition to equipment contamination, disease outbreaks, infection, cross-infection, outbreak, pseudo-outbreak, and cross-contamination for the outcome column. In Embase, the following search terms were included: bronchoscope, bronchoscopy, bronchoscop*, medical device contamination, contamination, cross infection, outbreak, pseudo-outbreak, and cross-contamination.
Eligibility criteria were set up for both cross-contamination and infection studies. For inclusion, cross-contamination studies had to explicitly describe the number of bronchoscope samples investigated and positive samples detected. Reports of positive samples were assessed using the guidelines of the European Society of Gastrointestinal Endoscopy and European Society of Gastrointestinal Endoscopy Nurse and Associates (ESGE-ESGENA) to define the contamination threshold. All studies reporting less than ten samples were excluded. The eligibility criteria for infection studies were as follows: information on the number of contaminated patients, together with how many clinically diagnosed patients were identified with an infection, in addition to the analytical techniques employed, such as pulsed-field gel electrophoresis (PFGE) or polymerase chain reaction (PCR). Studies on both cross-contamination and infection were published within the period 2010–2022 due to modified reprocessing guidelines published by the CDC and recommendations from the Healthcare Infection Control Practices Advisory Committee (HICPAC) and FDA. Cross-contamination and subsequent infection probabilities were estimated via two random-effects meta-analyses performed in R studio to calculate the weighted effect size [12]. Weighted effect sizes were subsequently converted from rate to probability using the formula:
The rate was equal to the weighted effect size and time (t) was equal to one, due to no longer than one procedure being investigated. To date, no studies have reported on the risk of cross-contamination with SUFB. However, to acknowledge the possibility of its occurrence, a minor cross-contamination risk of 1% was assumed for the SUFB treatment arm.
2.2 Study demographics
The time horizon was one procedure with a US hospital sector perspective. Cost data were collected from high-volume hospitals and academic institutions in Milwaukee (Wisconsin), Chicago (Illinois), and Cleveland (Ohio), on the basis of an annual performance of > 1000 bronchoscopy procedures. Additionally, all three hospitals served as teaching hospitals with various fellow programs. No patient demographics were explored in the economic model, as it was assumed to be independent from patient demographics and actual procedure costs. Instead, it only included the cost of items used to diagnose or treat the patient and the cost of cleaning the bronchoscopes after the procedure. Table 1 provides a simple overview of the number of procedures, the number of bronchoscopes used, the type of hospital settings, and a brief note on patient demographics.
A total of six hospitals were asked if they were interested in partaking in a cost analysis of their respective hospitals; three out of the six hospitals responded. Patient population and characteristics were not analyzed, although it is further assumed that difficultness of procedures was equally distributed within all examined university hospitals and the academic center. Cost data were obtained via an observational micro-costing approach in the bronchoscopy suites and reprocessing areas of the hospitals, followed by retrospective assessment of hospital purchasing records to estimate item and repair costs. Capital acquisitions, repairs, costs of water, and technician salaries were estimated using a retrospective approach, while time used to reprocess RFBs, personnel protective equipment (PPE) consumption, detergent, alcohol, and water usage were examined prospectively. Information on all repair costs from 1 January 2021 to 31 March 2022 was obtained. In the supplementary information (SI 1) section, cost data collected using the observational micro-costing approach and retrospective assessment are presented in detail with a follow-up summary. The data sheet incorporates bronchoscope and reprocessing acquisition costs, repair costs, number of repairs, reprocessing costs (including PPE, allocation keys, water consumption, and labor), together with time measurements of each reprocessing procedure. For SUFBs, the capital required for acquisition of the bronchoscope and processing/displaying unit, Ambu aBox 2, was provided by Ambu A/S; both costs are indicated as market prices. Within the USA, Axess Vision, Boston Scientific, Vathin, and Verathon also operate as single-use endoscope manufacturing companies, although none of above-mentioned companies are known today for having fully penetrated the bronchoscopy suite. Reviewing their product brochures, all companies’ products differentiate in specifications. No paper has yet compared the performance of each product, which makes it difficult to estimate if all products can be assumed to be equal in terms of clinical performance. Before any clinical performance studies are published it is difficult to estimate their impact related to cost comparison, performance deviations, and clinical usage.
Medicare severity diagnosis related group (MS–DRG) codes were further used to identify costs related to pulmonary infection due to contaminated bronchoscopy-related transmission. All codes, costs, calculations, and discharges are presented in supplementary information (SI 1). To avoid the potential overestimation of costs related to a single infection, the infection cost was weighted on the basis of the number of discharges of every MS–DRG code. Finally, costs related to repair of processing units and automated endoscope reprocessors (AER) were not included in this analysis due to the lack of retrospective documentation by the university hospitals or academic institutions on costs or repairs for this equipment category. All costs were adjusted to March 2022 in US dollars ($), using the consumer price index (CPI) including all items in the CPI index. RFBs were annualized across a 5-year period, which is in accordance with the Drummond et al. reference case [13]. Capital acquisitions, such as processors, light sources, and washing machines were annualized across an 8-year period [14]. All capital costs were discounted by 3%, according to the reference case of the Institute for Clinical and Economic Reviews. To compare the purchases that link to using reusable equipment, the upfront payment for reusable bronchoscopes and equipment are converted to an annual expense and thereafter divided by the annual procedure volume that each hospital is performing, see following equation:
To transform this annual expense to a per procedure cost, the annual expense is divided by the respective procedure volume:
The lifetime expenses of the capital equipment were defined as A, yearly expense defined as y, the discount rate as r, and lastly the annualizing period n, which was a period determined in years. A decision tree was generated to modulate the incremental costs for both SUFBs and RFBs. The transition state diagram is illustrated in Fig. 1. Analysis of the cost consequence was performed using Microsoft Excel 365 (version 2022). The constructed model was assessed for fulfillment of the principles of good practice for decision analytic modeling in healthcare evaluation [15].
2.3 Parameters
In Table 2, all weighted mean values, standard errors of the mean, and distributions are illustrated. The infection cost per procedure was calculated on the basis of risk of a patient acquiring an infection multiplied by weighted infection cost extracted from the MS–DRG system. All cost parameters were imputed into the Monte Carlo simulation to modulate the cost consequence. The weighted mean values were calculated by weighing each hospital based on how many annual procedures they were performing. Additionally, the standard error of the mean (SEM) is calculated on the basis of the following formula:
2.4 Basecase and Sensitivity Analyses
The base-case calculation was estimated based on included parameters and incremental cost expressed as:
The base-case results were further subjected to deterministic sensitivity analysis (DSA) and probabilistic sensitivity analysis (PSA). All cost and clinical effect parameters deviated (by ± 20% of the mean) in univariate analyses. Additionally, the discount rate was tested in two scenarios (0% and 5%). Scenario analyses further examined the effects of a fixed bronchoscope fleet of 10 and 20 RFBs and varying procedure volumes (between 500 and 4000 procedures per year) with the current setup. All univariate analyses were presented as a Tornado diagram. Additionally, the incremental cost is expected to have a relationship to the procedure volume. This relationship is explored by using a logarithmic regression model in Microsoft Excel 365 (version 2202). The dependent variable that is explored is the incremental per procedure cost, where the independent variable used to explore the dependent variable is the procedure volume. The relationship is further illustrated in a scatter plot. To examine the logarithmic relationship the following formula is used:
The incremental per procedure cost is defined as Y, yearly procedure volume as X, whereas a and b define two regression coefficients.
The procedure dependent scenario analysis is further used to estimate the break-even point, where the procedure dependent incremental cost line intersects $0 on the Y-axis of the (X, Y) scatterplot. To generalize the findings from the three hospitals examined in this study, data on volume splits of US bronchoscopy procedures were collected from Definitive Healthcare [16]. The distribution of US hospitals was divided into 4 ranged, below 500 annual procedures, between 500 and 1499 annual procedures, between 1500 and 2500 annual procedures, and lastly above 2500 annual bronchoscopy procedures. Data from a total of 2388 hospitals was collected from definitive healthcare. In Table 3 the procedure split with belonging percentages are illustrated:
Probabilistic sensitivity analysis (PSA) was conducted as a Monte Carlo simulation with 10,000 iterations of incremental costs and presented as an incremental cost scatterplot with number of iterations on the X-axis and incremental cost on the Y-axis.
3 Results
3.1 Meta-analyses
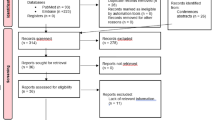
A total of 926 records were identified after duplicates were removed through PubMed, Embase and free text searches. The included studies were subsequently screened for titles and abstracts, while 881 studies were excluded due to having different focuses, such as ventilator-associated infection or endoscopic procedures in the gastroenterology department. A total of 45 full-text articles in the English language were assessed for eligibility. All studies from 2010 until 2022 that fulfilled the eligibility criteria were included due to modified reprocessing guidelines published by the Centers for Disease Control and Prevention (CDC) and recommendations from the Healthcare Infection Control Practices Advisory Committee (HICPAC) and FDA. A total of eight cross-contamination studies [17,18,19,20,21,22,23,24] and nine infection studies [25,26,27,28,29,30,31,32,33] were included for analysis. A PRISMA diagram visualizing the study selection process is depicted in Fig. 2.
The eight contamination studies examined a total of 2169 bronchoscopes for pathogens after high-level disinfection. Our results showed pathological growth within 189 bronchoscopes after high-level disinfection (HLD). Overall, nine infection studies included 426 contaminated patients. In two studies [25, 29], prophylactic antibiotic treatment was initiated, and no patients were included for the infection meta-analysis to limit the possibility of overestimating the cross-infection risk by assuming they would have acquired an infection if no prophylactic antibiotic treatment was initiated. A total of ten patients experienced an infection after treatment with a contaminated bronchoscope. All contamination and infection studies are illustrated in Tables 4 and 5, respectively. For each paper a grading score is provided, all grading is conducted by following the GRADE Handbook [34]. Since all studies are observational retrospective or prospective studies that are predefined as low grading, no paper is ranked superior to others. An overview of the grading scheme can be found in SI 1.
In the supplementary information section (SI 2), meta-analyses of contamination and subsequent infection are presented as forest plots, funnel plots and Egger’s regression tests. The effect size for cross-contamination was estimated as 12.0%, while that for infection was 2% when filtering out prophylactic antibiotic treated patients. The forest plot for contamination illustrated a large degree of heterogeneity: \(\left({I}^{2}=84\%\right)\) and p value < 0.05. Publication bias was present and Egger’s regression test yielded a Z value > 1.96 and limit estimate equal to −1.56, indicating funnel plot asymmetry. The forest plot for infection studies illustrated a low degree of heterogeneity: \(({I}^{2}=28\%; p=0.20)\). No publication bias was indicated in the funnel plot or Egger’s regression test.
3.2 Parameters
The average volume of bronchoscopy procedures within the bronchoscopy suites of the three facilities was estimated as 2200 per year cf. Table 1. The weighted average capital cost per procedure with RFBs was $68.8 (SEM $32), whereas the weighted average cost per reprocessing was $101.0 (SEM $76), weighted repair cost per procedure was $69.3 (SEM $28), and accumulated risk of cross-infection was calculated as 0.22%, resulting in an infection cost per procedure of $35. The total per procedure cost for RFB was $274, while for SUFB, the per procedure cost was estimated as $403. The incremental value was finally calculated as an additional cost of $129.4 per procedure with SUFBs and a decreased infection risk of 0.2%.
DSAs were varied by ± 20% for all costs. The decision illustrates that it is only sensitive to the per procedure cost for aScopeTM 5 Broncho, when reducing cost. If it is hypothesized that all patients who were treated with prophylactic antibiotic treatment would have developed an infection if they were not treated, the subsequent infection risk for RFBs increased from 2% to 17% and the total cross-infection risk from 0.22% to 2.0%, leading to a change in incremental cost from $129.4 to −$204. Utilizing 500 and 4000 procedures as the fixed lower and upper procedure volumes, incremental per procedure costs of −$90 and $142 were obtained, respectively. Both scenarios were sensitive to the change in the incremental value. Finally, the logarithmic regression analysis illustrated a significant relationship between the yearly procedure volume and incremental cost per procedure (p < 0.05). The correlation coefficient was measured to be 0.92. All parameters included in univariate sensitivity analyses are summarized in Table 6 and presented in Fig. 3.
Tornado diagram illustrating the ten parameters investigated. Four parameters, with deviations of ± 20%, and six scenarios were analyzed. The red lines illustrate the upper limit and how this affects the base-case, and vice versa the blue lines illustrate the lower limit and the derivative outcome to the base-case
In Fig. 4, the procedure volume scenario is further analyzed. The logarithmic regression analysis illustrated a significant relationship between the procedure volume and the incremental cost per procedure. The break-even point, where RFB and SUFB are cost neutral, is found at 756 procedures per site per year or 46 procedures per bronchoscope per year.
Univariate analysis illustrating the procedure volume in conjunction with the incremental cost per procedure. Two lines are illustrated in the plot. The red line illustrates the logarithmic regression model. The blue line illustrates the incremental cost per procedure, differing in relation to the procedure volume
Additionally, the logarithmic regression analysis was used to predict incremental per procedure cost for the four procedure ranges presented in Table 3. For hospitals performing < 500 procedures annually, a mean incremental per procedure cost was found to be −$119 with a 95% confidence interval (95% CI −268, 30), and for hospitals performing between 500 and 1499 annual bronchoscopy procedures, the mean was $45 (95% CI −104, 193). For hospitals performing between 1500 and 2500 annual procedures, the mean value was $121 (95% CI −28.1, 269), and lastly, for hospitals performing above 2500 annual procedures, the mean value was $191 (95% CI 42, 339).
The probabilistic Monte Carlo simulation used to investigate stochastic uncertainty showed that RFBs were cost minimizing in 88% of the iterations among the high-volume university hospitals and academic institutions examined. The Monte Carlo simulation is illustrated in Fig. 5. The mean and confidence interval for all cost iterations is: $135 (95% CI −132.6, 137.2). In addition, the SI 1 document includes an incremental cost-effectiveness scatter plot.
4 Discussion
Our cost-consequence analysis, systematic review, and meta-analysis indicated that SUFB may be more cost minimizing than currently used RFBs in low/mid volume settings, but not in high volume US university hospitals or academic institutions. However, the analysis was subject to uncertainty due to the sensitivity of two parameters. For example, the inclusion of all patients who received prophylactic antibiotic treatment, which were excluded from the main analysis but included in the univariate sensitivity analyses, led to an incremental cost per procedure saving of $200 by using SUFB. This finding was uncertain, as the inclusion or exclusion of prophylactic antibiotic treated patients could impact the result significantly.
Extending the scenario to include prophylactic treated patients, assuming that 50% or 25% of them developed an infection, revealed that including 50% made the two interventions nearly cost neutral, while including 25% resulted in RFB being the dominant decision. The difference in cross-infection risk between excluding or including all prophylactic-treated patients ranged from 0.2% to 1.9%. However, the results should be interpreted with caution due to the uncertainty surrounding the inclusion or exclusion of prophylactic treated patients, and the available data not providing a robust estimate of the risk of infection. Further research, such as a large cohort study or randomized trial, is needed to obtain a more reliable estimate of the real cross-infection risk.
The second parameter to which the findings were sensitive was the procedure volume. This parameter varied from 500 to 4000 annual procedures. In the observational micro-costing analysis, facility 3 that performed 4000 annual procedures indicated a substantial reduction in their capital costs compared with the university hospitals investigated.
We hypothesized that incremental per procedure cost is associated with the procedure volume at local facilities. During the observational micro-costing exercise at the facilities under investigation, the procedure volume ranged from 1200 to 4000 procedures annually, the only difference being that the hospital where 4000 procedures were undertaken had an extra procedure room, one bronchoscope more, and access to more staff. The efficient setup and greater number of local reprocessing staff and personnel to run high-volume facilities clearly indicates that facilities can reduce the per procedure cost of RFB at sites dealing with a high volume of patients on an annual basis. In view of the correlation between procedure volume and incremental cost, institutions should estimate their annual procedure volume before considering either RFB or SUFB in their bronchoscopy suites. This study estimated an average cost per procedure for RFB in well-established US bronchoscopy suites as $274, in keeping with a recent systematic review and meta-analysis by the Andersen group [35], who calculated an average RFB procedure cost of $306. In addition, Andersen et al. [35] reported that the per procedure cost for RFB was significantly dependent on the annual procedure volume per facility, consistent with our findings. Moreover, the group calculated the break-even point between SUFB and RFB. The base-case of their study was estimated as 306 procedures per site annually and 39 procedures annually per RFB. Based on their sensitivity analysis (including abstracts, magazines, and articles), a break-even point of 713 procedures per site and 61 procedures annually per RFB was obtained, comparable to our results, which show a break-even point at 756 procedures per site annually or 46 procedures annually per RFB.
The main strength of this study is that the prospective observational micro-costing approach provided adequate inputs for cost components included in the simulation process [36]. However, the issue of whether the cost of disposing of waste, such as PPE or SUFBs, after use differs remains to be established. Single-use interventions may mitigate the need for larger reprocessing facilities and complex adherence to reprocessing guidelines, and the need to improve hygiene in the hospital setting [22, 37]. Ofstead et al. [38] described the challenges in achieving effective high-level disinfection in endoscope reprocessing. In their study, \(70\%\) of the respondents felt pressured to reprocess faster and did not have sufficient time to deal with the large workloads. Additionally, the study conducted a systematic review and two meta-analyses that collected all available evidence in the literature. However, workloads, complex reprocessing guidelines, and lack of institutional monitoring of microbiology outbreaks may have led to a risk of under reporting infections and pseudo-infections [39, 40]. The current investigation included eight reports covering cross-contamination and nine with a focus on infections. More studies had relatively small sample sizes that, even when combined, provide a rather uncertain foundation to draw an evidence-based conclusion [13, 18, 21, 22, 24, 27, 29]. This heterogeneity in the published literature can be avoided by establishing uniform and standardized surveillance of microbiology techniques [40]. The studies used in the analysis were designed as observational retrospective or prospective study designs with no comparators. According to the evidence hierarchy and the GRADE Handbook [34], observational studies with no special strengths provide low quality evidence. Due to the resulting low quality of evidence and the large heterogeneity observed between studies, it is evident that uncertainty exists regarding this parameter.
A significant limitation to this study is that only three hospitals chose to be enrolled. To try generalize the results to other US settings, the authors chose to collect registry data from definitive healthcare [16] to compare the procedure volumes investigated at the two university hospitals and the academic center with other settings. The collected data from definitive healthcare reflected that the investigated hospitals were positioned in the top 19% of hospitals in terms of bronchoscopy procedures, annually. Facility three that performed 4000 procedures a year was even positioned in top 1% of US hospitals. Due to economy of scale, these hospitals might represent settings that are able to lower their total costs compared with settings with less annual procedures. Another limitation that occurred during the observational micro-costing was that no repairs for automated endoscope reprocessors, video processors, and light sources were able to be collected. All equipment that is used on a daily basis accumulates wear and tear, which means that maintenance would be necessary during the assumed 8-year lifespan. Therefore, it is expected that the real cost associated with all this diverse equipment might be slightly higher than what was observed in this study. The last mentionable limitation was that the study did not explore the patient demographics. It can be argued that hospitals with a larger annual cohort of advanced patients, such as immunocompromised patients, patients with prion disease, and lung transplant patient might incur increased costs due to more strict reprocessing, more repairs of bronchoscopes, and a larger fleet of bronchoscopes to maintain an efficient workflow. To clarify if patient demographics are a significant factor, a study focusing on whether the impact of handling patients with severe lung diseases significantly differs from hospitals diagnosing and treating patients with a wider range of severity is needed.
Finally, there is considerable debate on the application of single-use endoscopy due to the requirement for disposal after use [41]. Studies both in favor of and against single-use technologies have been published, but to our knowledge, none have compared the entire developmental process and supplementary equipment involved in the production, usage, and disposal of single-use and reusable endoscopes [41, 42]. Further research is warranted for comprehensive evaluation of both product types, rather than only focusing on small areas of waste management, to determine the real environmental impact.
5 Conclusion
The outcomes of this cost-consequence analysis between aScopeTM 5 Broncho and reusable flexible bronchoscopes within the US bronchoscopy suite setting indicates that the single-use technology is not cost minimizing among high-volume US university hospitals and academic institutions. A direct change in aScopeTM 5 Broncho usage in these hospitals would incur an additional cost of $129 per procedure, but decrease cross-infection risk by 0.22%. For hospitals performing less than 756 procedures a year, it may be beneficial to switch to single-use technology, which may provide a more cost-neutral approach.
References
Ho E, Wagh A, Hogarth K, Murgu S. Single-use and reusable flexible bronchoscopes in pulmonary and critical care medicine. Diagnostics. 2022;12(1):1–14. https://doi.org/10.3390/diagnostics12010174.
Barron SP, Kennedy MP. Single-use (disposable) flexible bronchoscopes: the future of bronchoscopy? Adv Ther. 2020;37(11):4538–48. https://doi.org/10.1007/s12325-020-01495-8LK.
Zaidi S, et al. Research bal using single use disposable bronchoscope. Thorax. 2016;71:A108–9. https://doi.org/10.1136/thoraxjnl-2016-209333.191LK.
Mankikian J, et al. An evaluation of a new single-use flexible bronchoscope with a large suction channel: reliability of bronchoalveolar lavage in ventilated piglets and initial clinical experience. Anaesthesia. 2014;69(7):701–6. https://doi.org/10.1111/anae.12641.
Marshall DC, et al. Experience with the use of single-use disposable bronchoscope in the ICU in a tertiary referral center of Singapore. J Bronchol Interv Pulmonol. 2017;24(2):136–43. https://doi.org/10.1097/LBR.0000000000000335.
Mouritsen JM, Ehlers L, Kovaleva J, Ahmad I, El-Boghdadly K. A systematic review and cost effectiveness analysis of reusable vs. single-use flexible bronchoscopes. Anaesthesia. 2020;75(4):529–40. https://doi.org/10.1111/anae.14891.
Mærkedahl A. Cost-utility analysis of the Ambu® aScope TM 4 broncho single-use flexible video bronchoscope compared to reusable flexible video bronchoscopes. J Basic Clin Pharma. 2020;11:1–6.
Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital and Health Statistics, Series 13: Data on Health Resources Utilization, vol 13, no 139. 1998.
Healthcare Infection Control Practices Advisory Committee. Essential Elements of a Reprocessing Program for Flexible Endoscopes–The Recommendations of the Healthcare Infection Control Practices Advisory Committee (HICPAC). 2016.
Terjesen CL, Kovaleva J, Ehlers L. Early assessment of the likely cost effectiveness of single-use flexible video bronchoscopes. Pharmacoecon Open. 2017;1(2):133–41. https://doi.org/10.1007/s41669-017-0012-9LK.
Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2020. https://doi.org/10.1136/bmj.n71.
Lee YH. An overview of meta-analysis for clinicians. Korean J Intern Med. 2018;33(2):277–83. https://doi.org/10.3904/kjim.2016.195.
Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes, Second Edition. Am J Prev Med. 1998;14(3):243. https://doi.org/10.1016/S0749-3797(97)00069-X.
Ofstead CL, Quick MR, Eiland JE, Adams SJ. A glimpse at the true cost of reprocessing endoscopes: results of a pilot project. 2017 [Online]. https://www.bostonscientific.com/content/dam/bostonscientific/uro-wh/portfolio-group/LithoVue/pdfs/Sterilization-Resource-Handout.pdf.
Weinstein MC, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices-Modeling Studies. Value Health. 2003;6(1):9–17. https://doi.org/10.1046/j.1524-4733.2003.00234.x.
Definitive Healthcare: Procedure volume in bronchoscopy suites. 2022. https://www.definitivehc.com/. Accessed 6 Dec 2022.
Cosgrove SE, et al. Caveat emptor: the role of suboptimal bronchoscope repair practices by a third-party vendor in a pseudo-outbreak of pseudomonas in bronchoalveolar lavage specimens. Infect Control Hosp Epidemiol. 2012;33(3):224–9. https://doi.org/10.1086/664051.
Marino M, et al. Is reprocessing after disuse a safety procedure for bronchoscopy?: a cross-sectional study in a teaching hospital in Rome. Gastroenterol Nurs. 2012;35(5):324–30. https://doi.org/10.1097/SGA.0b013e3182608fd5.
Vincenti S, et al. Non-fermentative Gram-negative bacteria in hospital tap water and water used for haemodialysis and bronchoscope flushing: prevalence and distribution of antibiotic resistant strains. Sci Total Environ. 2014;499:47–54. https://doi.org/10.1016/j.scitotenv.2014.08.041.
Gavaldà L, et al. Microbiological monitoring of flexible bronchoscopes after high-level disinfection and flushing channels with alcohol: results and costs. Respir Med. 2015;109(8):1079–85. https://doi.org/10.1016/j.rmed.2015.04.015.
Batailler P, Saviuc P, Picot-gueraud R, Bosson J, Mallaret M. Usefulness of adenosinetriphosphate bioluminescence assay (ATPmetry) for monitoring the reprocessing of endoscopes. Infect Control Hosp Epidemiol. 2015. https://doi.org/10.1017/ice.2015.212.
Ofstead CL, et al. Effectiveness of reprocessing for flexible bronchoscopes and endobronchial ultrasound bronchoscopes. Chest. 2018;154(5):1024–34. https://doi.org/10.1016/j.chest.2018.04.045.
Troiano G, et al. Microbiological surveillance of flexible bronchoscopes after a high-level disinfection with peracetic acid: preliminary results from an Italian teaching hospital. Ann Ig. 2019;31(1):13–20. https://doi.org/10.7416/ai.2019.2254.
Cottarelli A, et al. Microbiological surveillance of endoscopes and implications for current reprocessing procedures adopted by an Italian teaching hospital. Ann Ig. 2020;32(2):166–77. https://doi.org/10.7416/ai.2020.2340.
Guy M, et al. Outbreak of pulmonary Pseudomonas aeruginosa and Stenotrophomonas maltophilia infections related to contaminated bronchoscope suction valves, Lyon, France, 2014. Eurosurveillance. 2016. https://doi.org/10.2807/1560-7917.ES.2016.21.28.30286LK.
Abdolrasouli A, et al. A pseudo-outbreak of Rhinocladiella similis in a bronchoscopy unit of a tertiary care teaching hospital in London, United Kingdom. Mycoses. 2021;64(4):394–404. https://doi.org/10.1111/myc.13227.
Zhang Y, Zhou H, Jiang Q, Wang Q, Li S, Huang Y. Bronchoscope-related Pseudomonas aeruginosa pseudo-outbreak attributed to contaminated rinse water. Am J Infect Control. 2020;48(1):26–32. https://doi.org/10.1016/j.ajic.2019.06.013LK.
Campos-Gutiérrez S, Ramos-Real MJ, Abreu R, Jiménez MS, Lecuona M. Pseudo-outbreak of Mycobacterium fortuitum in a hospital bronchoscopy unit. Am J Infect Control. 2020;48(7):765–9. https://doi.org/10.1016/j.ajic.2019.11.019.
Botana-Rial M, et al. A pseudo-outbreak of Pseudomonas putida and Stenotrophomonas maltophilia in a bronchoscopy unit. Respiration. 2016;92(4):274–8. https://doi.org/10.1159/000449137LK.
Rosengarten D, et al. Cluster of pseudoinfections with Burkholderia cepacia associated with a contaminated washer-disinfector in a bronchoscopy unit. Infect Control Hosp Epidemiol. 2010;31(7):769–71. https://doi.org/10.1086/653611.
Waite TD, Georgiou A, Abrishami M, Beck CR. Pseudo-outbreaks of Stenotrophomonas maltophilia on an intensive care unit in England. J Hosp Infect. 2016;92(4):392–6. https://doi.org/10.1016/j.jhin.2015.12.014.
Tschudin-Sutter S, et al. Emergence of glutaraldehyde-resistant Pseudomonas aeruginosa. Infect Control Hosp Epidemiol. 2011;32(12):1173–8. https://doi.org/10.1086/662624.
de Carvalho NFG, et al. Hospital bronchoscopy-related pseudo-outbreak caused by a circulating Mycobacterium abscessus subsp. massiliense. J Hosp Infect. 2018;100(3):e138–41. https://doi.org/10.1016/j.jhin.2018.07.043.
Schünemann OAH, Brożek J, Guyatt G. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. 2013. guidelinedevelopment.org/handbook. Accessed 6 Dec 2022.
Andersen CØ, Travis H, Dehlholm-Lambertsen E, Russel R, Jørgensen EP. The cost of flexible bronchoscopes: a systematic review and meta-analysis. Pharmacoecon Open. 2022. https://doi.org/10.1007/s41669-022-00356-0.
Xu X, Nardini HKG, Ruger JP. Micro-costing studies in the health and medical literature: protocol for a systematic review. Syst Rev. 2014;3(1):1–7. https://doi.org/10.1186/2046-4053-3-47.
Kovaleva J, Peters FTM, van der Mei HC, Degener JE. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin Microbiol Rev. 2013;26(2):231–54. https://doi.org/10.1128/CMR.00085-12.
Ofstead CL, Hopkins KM, Buro BL, Eiland JE, Wetzler HP. Challenges in achieving effective high-level disinfection in endoscope reprocessing. Am J Infect Control. 2020;48(3):309–15. https://doi.org/10.1016/j.ajic.2019.09.013.
Mughal MM, Minai OA, Culver DA, Mehta AC. Reprocessing the bronchoscope: the challenges. Semin Respir Crit Care Med. 2004;25(4):443–9. https://doi.org/10.1055/s-2004-832717.
Culver DA, Gordon SM, Mehta AC. Infection control in the bronchoscopy suite: a review of outbreaks and guidelines for prevention. Am J Respir Crit Care Med. 2003;167(8):1050–6. https://doi.org/10.1164/rccm.200208-797CC.
Agrawal D, Tang Z. Sustainability of single-use endoscopes. Tech Innov Gastrointest Endosc. 2021;23(4):353–62. https://doi.org/10.1016/j.tige.2021.06.001.
Lilholt-Sørensen B. Comparative study on environmental impacts of reusable and single-use bronchoscopes. Am J Environ Prot. 2018;7(4):55. https://doi.org/10.11648/j.ajep.20180704.11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The corresponding author, Anders Engelbrecht Kristensen, was a masters student with an internship at Ambu A/S, Ballerup during the preparation of the paper. During this collaboration, he received a monthly payment of 1000 DKK. Additionally, he had his expenses covered during the data collection period in the USA. All remaining authors did not receive any specific funding for this research from public, commercial, or not-for-profit sectors.
Conflicts of interest
Anders Engelbrecht Kristensen is currently employed by Ambu A/S, Ballerup Denmark. Jonathan S. Kurman is a consultant for Ambu A/S and Boston Scientific. Douglas Kyle Hogarth is a consultant for Ambu A/S, Boston Scientific, Endovision, and Olympus A/S. Sonali Sethi is a consultant for Ambu A/S. Sabrina Storgaard Sørensen has no conflicts of interest that are directly relevant to the contents of this manuscript.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Ethics approval
Not applicable.
Availability of data and material
All data collected and analyzed during the study period are included in the published paper and supplementary information files.
Code availability
Statistical coding is available upon request to the corresponding author.
Author contributions
Initiation of the study, data collection, data analysis, and manuscript writing were performed by A.E.K. Access to facilities was permitted by J.S.K., D.K.H., and S.S. S.S.S. supervised completion of the manuscript. Revision of the manuscript for important intellectual content was performed by all authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kristensen, A.E., Kurman, J.S., Hogarth, D.K. et al. Systematic Review and Cost-Consequence Analysis of Ambu aScope 5 Broncho Compared with Reusable Flexible Bronchoscopes: Insights from Two US University Hospitals and an Academic Institution. PharmacoEconomics Open 7, 665–678 (2023). https://doi.org/10.1007/s41669-023-00417-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-023-00417-y