Abstract
Background
Appropriate management of chronic obstructive pulmonary disease (COPD) patients following acute exacerbations can reduce the risk of future exacerbations, improve health status, and lower care costs. While a transition care bundle (TCB) was associated with lower readmissions to hospitals than usual care (UC), it remains unclear whether the TCB was associated with cost savings.
Objective
The aim of this study was to evaluate how this TCB was associated with future Emergency Department (ED)/outpatient visits, hospital readmissions, and costs in Alberta, Canada.
Methods
Patients who were aged 35 years or older, who were admitted to hospital for a COPD exacerbation, and had not been treated with a care bundle received either TCB or UC. Those who received the TCB were then randomized to either TCB alone or TCB enhanced with a care coordinator. Data collected were ED/outpatient visits, hospital admissions and associated resources used for index admissions, and 7-, 30- and 90-day post-index discharge. A decision model with a 90-day time horizon was developed to estimate the cost. A generalized linear regression was conducted to adjust for imbalance in patient characteristics and comorbidities, and a sensitivity analysis was conducted on the proportion of patients’ combined ED/outpatient visits and inpatient admissions as well as the use of a care coordinator.
Results
Differences in length of stay (LOS) and costs between groups were statistically significant, although with some exceptions. Inpatient LOS and costs were 7.1 days (95% confidence interval [CI] 6.9–7.3) and Canadian dollars (CAN$) 13,131 (95% CI CAN$12,969–CAN$13,294) in UC, 6.1 days (95% CI 5.8–6.5) and CAN$7634 (95% CI CAN$7546–CAN$7722) in TCB with a coordinator, and 5.9 days (95% CI 5.6–6.2) and CAN$8080 (95% CI CAN$7975–CAN$8184) in TCB without a coordinator. Decision modelling indicated TCB was less costly than UC, with a mean (standard deviation [SD]) of CAN$10,172 (40) versus CAN$15,588 (85), and TCB with a coordinator was slightly less costly than without a coordinator (CAN$10,109 [49] versus CAN$10,244 [57]).
Conclusion
This study suggests that the use of the TCB, with or without a care coordinator, appears to be an economically attractive intervention compared with UC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A multicentre cohort study with a nested randomized controlled trial assessed the effectiveness of an evidence-based chronic obstructive pulmonary disease (COPD) transition bundle on hospital readmissions and Emergency Department (ED)/outpatient revisits and its influence on care continuity after discharge, compared with usual care. |
Data collected in the study indicated that the transition bundle was associated with shorter inpatient length of stay (LOS) and lower costs than usual care. Decision modelling indicated that total cost per patient was lower under the transition bundle than its comparator. |
Our analyses suggested that the transition bundle was a cost-saving intervention for patients with acute COPD exacerbations. Among the two options evaluated, the transition bundle appears to be the more economically attractive intervention. |
1 Introduction
Chronic obstructive pulmonary disease (COPD) is a common respiratory disease characterized by progressive airflow limitation, and is one of the most common causes of morbidity and mortality both worldwide and in Canada [1,2,3,4,5,6,7]. Individuals living with COPD experience disproportionately higher rates of hospital admissions, longer hospital admissions, and more frequent readmissions that result in increased care costs and significant variability in care provided [8,9,10,11,12,13]. Appropriately managing the complexity of care for individuals with COPD, while being judicious with finite healthcare resources, is an essential part of controlling the burden of COPD on patients, their families, and the healthcare system [14].
Evidence shows that optimization of the management of COPD patients after acute exacerbations can reduce the risk of future exacerbations [15, 16], leading to improved health status and lower care costs, although there is limited information regarding which specific management strategy may be best. Furthermore, there is a need to improve transitions of care for individuals living with COPD across the acute care spectrum and into the community setting to decrease variability in care, as well as to improve coordination and continuity of care. Reduced variability and improved coordination in care may lead to fewer hospital admissions and Emergency Department (ED) visits in COPD patients [17, 18].
Care bundles have been proposed as tools to address unnecessary variability in patient care while simultaneously supporting the translation of evidence-based interventions as well as improving transitions in care and health outcomes [15]. Care bundles are a “structured way of improving the processes of care and patient outcomes: a small, straightforward set of evidence-based practices that, when performed collectively and reliably, have been proven to improve patient outcomes”.Footnote 1 Our COPD transition care bundle (TCB) initiated in the inpatient (IP) care setting was informed by evidence gathered through (1) a meta-analysis of literature on the effectiveness of care bundles [15]; (2) a modified Delphi process with researchers, clinicians (primary and specialty care) and those living with COPD to reach consensus on care bundle elements [19]; and (3) focus groups with patients and clinicians to understand barriers and facilitators to care bundle uptake/use in real-world settings [20].
A multicentre study of a TCB for people with COPD was conducted with a nested randomized controlled trial (RCT) evaluating the addition of a care coordinator. The study compared patients exposed to the TCB and those who were not exposed, i.e. the usual care (UC) group, to determine whether the TCB could optimize patient outcomes. Full results are reported elsewhere [21]. While it was observed that the TCB was associated with lower patient readmissions to hospital and increased care continuity between acute and primary care, a question remaining to address is whether TCB was also associated with cost savings. Alongside the analysis of the clinical outcomes, we conducted an economic evaluation of the care bundles based on the results of the study. The aim of our economic analysis was to assess how the TCB intervention may impact future ED/outpatient visits, hospital readmissions, and health services use among individuals discharged after receiving care for an acute exacerbation of COPD. Therefore, we now report on the estimated hospital resource utilization and costs. This economic analysis, when considered alongside the clinical study results, may provide broader insight into decision making on health care resource allocation.
2 Methods
2.1 Study Design, Participants and Transition Bundle
Patients with any severity of COPD were recruited between February 2017 and June 2019 from five hospitals with full-service emergency care facilities for treating acute COPD exacerbations in Alberta, Canada. We included patients aged 35 years and older with a primary diagnosis of COPD or a secondary diagnosis of COPD if the first diagnosis was respiratory related (e.g., pneumonia, etc.) where it is plausible that the COPD likely contributed to their admission [22, 23]. The inclusion of patients aged 35 years and older was based on the validated methodology and case definition by Gershon et al. [24]. COPD and asthma can present with similar symptoms, but only COPD develops after years of smoking. To avoid misdiagnosis with asthma cases, age 35 years was used as the cut-off in the current trial [25]. Primary outcomes in the clinical trial were hospital admissions and ED/outpatient visits for the index admissions and 7, 30 and 90 days post-index discharge.
At the time of implementation, there was no standardized COPD TCB in Alberta, therefore patients at all sites received UC during the initial (pre-implementation) phase. The COPD TCB elements were integrated into a COPD admission order set so that discharge planning could commence at admission. The TCB has seven core elements [15, 19].
-
1.
Ensure the patient has demonstrated an adequate inhaler technique.
-
2.
Send the discharge summary to the family physician and arrange follow-up.
-
3.
Optimize respiratory medications.
-
4.
Assess patient and caregiver comprehension of discharge instructions and provide a written management plan.
-
5.
Refer to pulmonary rehabilitation.
-
6.
Screen for frailty and any comorbid conditions.
-
7.
Refer to a smoking cessation programme, if needed.
Prior to patient discharge, the in-hospital care team initiated the COPD bundle elements and provided the patient’s primary care provider (PCP) notification of care items completed (or not), so that the PCP knew what had been done and what needed disease-specific follow-up.
The TCB was further studied with the addition of a care coordinator (known as an enhanced TCB [ETCB]), who was either a registered nurse (RN) or registered respiratory therapist (RRT). Those patients who received the TCB were randomized to receive either only the TCB or the ETCB. The care coordinator contacted the patients by phone for follow-up at 48–72 h, and between 7 and 10 days after discharge, and asked for information on any follow-up with the family physician, pulmonary rehabilitation, or smoking cessation programmes. If these referrals/appointments had not been completed/booked, the care coordinator helped to ensure these were completed.
The trial was registered with the US National Library of Medicine Clinical Trials database (identifier: NCT03358771; https://clinicaltrials.gov/ct2/show/NCT03358771). Ethics approval was obtained from the University of Alberta Research Ethics Board, and a waiver of consent was granted by the Ethics Board. The project was conducted in accordance with the ethical standards as laid down in the Declaration of Helsinki and its subsequent amendments.
2.2 Data Collection
We performed the analysis from the public payer’s perspective in Alberta where we benefit from a single publicly funded health system. Collected data included patient characteristics such as age, sex, Charlson Comorbidity Index (CCI), and healthcare utilization associated with IP admissions, ED/outpatient visits, and physician visits. Costs of IP admission and ED/outpatient visits were estimated using a resource intensity weighting (RIW) approach that multiplies the RIW score by the cost of a standard hospital stay (CSHS), where CSHS represents the hospital’s average full cost of treating the average acute inpatient [26]. Physician costs were actual dollar amounts paid to physicians.
Data were obtained from health administrative databases that provide individual patient information. The Discharge Abstract Database (DAD) provides information on patients with an IP stay, and the National Ambulatory Care Reporting System (NACRS) provides information on patients with an ED/outpatient visit. DAD and NACRS costs cover all activities other than physician services in these settings. Examples of the DAD and NACRS costs include salaries for non-physician staff, drugs, medical and surgical supplies, administration, and support services. Information on physician visits were obtained from the Practitioner Claims database. These data included all activities performed by physicians in primary care, ED/outpatient and IP care settings. Community pharmacy costs were not included in the analysis owing to limitations in the data related to how prescriptions dispensed in community settings are procured.
2.2.1 Estimating the Unit Cost of Transition Care Bundle Delivery
The care team cost of implementing TCB and ETCB was also included. We obtained data on the cost of providing TCB directly from participating hospitals. TCB resource use varies by patient and changes with each patients’ ability to understand the information provided. There is also considerable variation in the category of care professionals who provide patient and caregiver education, as well as the time spent providing this education. Of the care professionals providing education, most were RNs, RRTs or clinical pharmacists. Time spent providing patient education ranged from 15 to 60 min. In the analysis, we assume 40 min was used per patient when estimating the average cost of patient education. The unit cost of the patient education session was estimated based on the hourly remuneration paid to RNs. The Alberta Careers, Learning and Employment Information System (ALIS)Footnote 2 indicates that the hourly remuneration for RNs range from Canadian dollarsFootnote 3 (CAN$) 36.48 to CAN$53.60, with an average remuneration of CAN$45.4. Unit costs for care coordinators were collected from the actual payments made to the coordinators. Based on the financial data from the study, the cost of providing care coordinators for 320 patients was CAN$98,500, or CAN$308 per patient. Note that we used RNs to represent the educational cost as most time spent on the education was on RNs and the difference in unit cost between RNs and RRTs was small (CAN$45.4 vs. CAN$46.01 based on ALIS data).
2.3 Decision Model
We conducted our analysis using data collected as part of the clinical trial [21], supplemented by administrative data as described above, to estimate the cost implication of TCB compared with UC within a 90-day time horizon. In COPD clinical trials, the 90-day period was considered long enough to assess the effects of interventions being tested and chosen by subsequent our clinical study [21]. Analysis of the study economic data was conducted per the study analysis plan. On identifying a large difference between UC and TCB patients receiving combined ED/outpatient and inpatient care, we additionally developed a simple decision model that was not part of the planned analysis, to enable sensitivity analysis related to the difference. We selected UC as the comparator since it is existing practice in Alberta. A decision tree was developed to capture the expected differences in care path and resource use of the study patients. During the analysis, we identified a large difference in patients receiving combined ED and inpatient care—64% of patients in the UC group compared with 82% in the transition bundle group. The model was developed to allow us to investigate the impact of this difference and to undertake sensitivity analysis of changing these values.
2.3.1 Decision Model Structure
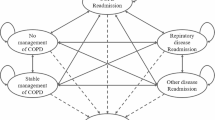
We developed a decision tree model to estimate the cost implication of TCB compared with UC. The decision tree was developed to capture the expected differences in care path and resource use of the study patients. In addition, the cost of implementing the TCB and care coordinator were evaluated in the decision tree model. Analysis of patient flow indicated that the proportion of patients receiving IP care only or combined IP and ED/outpatient care is different between study groups and the model was developed to accommodate this. The model started from patients who met the inclusion criteria for the TCB study. These patients were then split into two groups, with one group receiving IP care only and the other receiving combined IP and ED/outpatient care. Patients exposed to TCB were further split into ‘with’ and ‘without’ care coordinator cohorts. The decision model is presented in Fig. 1a. The model was programmed and implemented in R (The R Foundation for Statistical Computing, Vienna, Austria) and the model code is available on request.
a Decision tree model. Patients were split into two groups, with one group receiving IP care only and the other receiving combined IP and ED/outpatient care. Patients under TCB are further split into with and without a care coordinator. ED Emergency department, IP inpatient, TCB transition care bundle, UC usual care, w/ with, w/o without. b Decision tree model in sensitivity analysis by coordinator presence. Three are three decision options including TCB with coordinator, TCB without coordinator and UC. Note that UC has no coordinator involved. Patients under each decision option were split into two groups, with one group receiving IP care only and the other receiving combined IP and ED/outpatient care. ED Emergency department, IP inpatient, TCB transition care bundle, UC usual care, w/ with, w/o without
It is important to note that while presence of the coordinator should typically be treated as a decision node in an analysis of this type, in this analysis it was treated as a chance node. In the clinical study on which this analysis was based, the primary aim is to compare TCB with or without coordinators versus UC (under which no patients had a coordinator). Furthermore, in the primary trial, the coordinator was found to have no independent effect on outcomes and therefore it was determined that the economic analysis should focus on the TCB component alone. Instead, in our base-case analysis, patients under TCB were treated as a mixed group, some of whom received coordinators and others did not. In a sensitivity analysis, we treated the coordinator presence as a decision node, as shown in Fig. 1b.
2.3.2 Decision Model Inputs
Several input parameters used in the decision model were extracted from the clinical trial, including the proportion of patients receiving IP care only and combined IP and ED/outpatient care, proportion of patients receiving care coordinator support, and costs of inpatient stay, ED/outpatient visit, physician visit, and TCB delivery. Data on the inputs are reported in Table 1.
2.3.3 Statistical Analysis
We estimated the value of parameters for use in the decision model using the study data, and included the number of IP admissions and ED/outpatient visits per patient, associated length of stay (LOS) and costs for the TCB and UC groups. As shown in Table 2, patients in the UC arm were both older and had more comorbid conditions. To adjust for this imbalance, we selected a generalized linear model (GLM). The GLM extends the linear modelling approach to outcomes that are not normally distributed, as is typical of resource use data [27]. The predictors of hospital cost and physician cost in IP care were age, sex, case-mix group and CCI. In our data for patients in ED/outpatient care, there was no information on case-mix group and CCI, and thus they were not included in the GLM model for the cost prediction in ED/outpatient care. In the analysis, we fitted the model using a Gamma distribution and log link, as appropriate when analysing positively skewed cost data [28, 29]. The exception to this was when estimating physician costs in ED/outpatient care, where the Gamma distribution did not converge. As an alternative, we estimated the model using a Gaussian distribution with identity link function that implies a linear relation between the cost and predictors. The predicted values from the GLM were used to represent the IP admissions and ED/outpatient visits per patient, associated LOS, and costs for each group. The differences between the TCB and UC groups were tested using a Wilcoxon non-parametric test, which is more suitable for mean difference testing of data that are not normally distributed [30].
Data investigation indicated unusually high ED/outpatient visits, IP admissions, and costs for some patients compared with the average cost for each group. Although these outliers represent the actual healthcare expenditure of individual patients, they skewed average costs and are unlikely to represent the true average expenditure for healthcare services [31, 32]. It is widely accepted that estimating the population mean cost is in the statistical interest of health policy makers [33]. We therefore trimmed the outliers in each group. We used a traditional univariate boxplot method to deal with the outliers in the per-patient ED/outpatient visits, IP admissions, LOS, and costs, with the data more than 1.5 times the interquartile range (IQR) below the first quartile or above the third quartile being replaced with the mean values [34]. We also present the estimates without the mean replacement, which represented data deviated from the main trial evaluation without trimming outliers, in electronic supplementary material (ESM) Table A.1.
2.3.4 Uncertainty
Differences in means were assessed using Monte Carlo simulation, a mathematical technique commonly used to capture estimates of uncertainty in the results. Cost data were repeatedly modelled for 10,000 iterations based on predetermined probability distributions [35,36,37,38]. The statistical analysis was performed using R version 3.6.0 and the decision-analytic model was performed using TreeAge Pro 2015 (TreeAge Software, Inc., Williamstown, MA, USA).
Inpatient hospital admissions are typically more expensive than ED/outpatient visits. It is unclear to what extent the proportion of patients with combined ED/outpatient visits and IP admissions would have on overall costs. We conducted a sensitivity analysis to test the impact of varying this proportion. In addition, the base-case analysis assessed the cost implication of TCB where only a proportion of patients received care coordinator support. Whether including the support is a matter of choice or not is debatable. We conducted a sensitivity analysis to compare the cost implications under three decision strategies, i.e. TCB with care coordinator support, TCB without care coordinator support, and UC.
This model followed the guidelines for cost-effectiveness analysis (CEA) alongside clinical trials by the Professional Society for Health Economics and Outcomes Research (ISPOR) Good Research Practices Task Force [35, 39] and the 2022 Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement [40]. No discount rate was applied to costs, as the time horizon of the study was less than 1 year. All costs were in CAN$ and were adjusted to a standard price year of 2019, using the Alberta Consumer Price Index (CPI).
3 Results
3.1 Patient Characteristics
Overall, 3776 patients were discharged from the participating hospitals during the study period. Of these patients, 66 died in hospitals and communities and the remaining 3710 patients were eligible for study inclusion—3106 in the UC group and 604 in the TCB group. Of the 604 patients in the TCB group, 320 were randomized to the ETCB group and 284 had no care coordinator follow-up (see Fig. 2 for a patient flow diagram). Note that all 66 patients who died were in the UC group, i.e. 2% of UC patients.
Patient flow diagram. There were about ~ 20 + different reasons that we just clumped into "other" for sake of clarity and viewability of the figure. For instance, unable to trace the patient in our administrative databases using the information provided on the transition bundle, patient does not exist/entry errors, duplicative records, etc. COPD: Chronic obstructive pulmonary disease
3.2 Length of Stay and Cost
Estimates of LOS and costs per patient are presented in Tables 1 and 3, respectively. The differences in LOS and costs between pairs of groups (e.g., UC vs. TCB with a care coordinator, TCB with a care coordinator vs. TCB without a care coordinator, etc.) were statistically significant, although with some exceptions. The analysis of clinical trial data indicated that inpatient LOS was longer for patients in UC than patients in TCB: 7.1 days (95% CI 6.9–7.3) in UC versus 6.1 (95% CI 5.8–6.5) in TCB with a care coordinator and 5.9 (95% CI 5.6–6.2) in TCB without a care coordinator. Consistent with the increased LOS, the inpatient cost was higher for patients in UC than patients in TCB with and without a care coordinator, namely CAN$13,131 (95% CI CAN$12,969–CAN$13,294), CAN$7634 (95% CI CAN$7546–CAN$7722) and CAN$8080 (95% CI CAN$7975–CAN$8184), respectively. We also examined the number of ED/outpatient visits and inpatient admissions. The mean number of visits was approximately 1 and 1.28 in patients under TCB and UC, respectively. Detailed results of descriptive statistics are presented in ESM Table A.2.
In our analysis, small differences in LOS are associated with relatively large differences in costs. We estimated the cost in hospital using an RIW approach that weighted the resources used on the basis of demographic characteristics, comorbidity level and surgical events of an individual patient. That is, patients from some demographic categories and/or with greater comorbidity scores would be associated with higher costs, for a given LOS. As shown in Table 2, patients under UC were both older and had more comorbid conditions than those under TCB, which may contribute to higher costs in the former, although we conducted regression analyses to adjust the imbalance in baseline characteristics.
3.3 Base-Case Analysis and Sensitivity Analyses
The mean cost and standard deviation (SD) are reported in Table 4. TCB was associated with lower total costs [CAN$10,172 (SD 40)] per patient than UC [CAN$15,588 (SD 85)], a difference of CAN$5416 per patient.
We performed a sensitivity analysis to test the robustness of our findings to changes in model inputs. In the scenario where we increased the proportion of patients’ combined ED/outpatient visits and IP admissions in the UC group from 64 to 82% as seen in the TCB group, the total cost of UC increased slightly by CAN$242, from CAN$15,588 to CAN$15,830 (Table 4). The expanding patient population from receiving IP care only to combined care implies an increase in the number of ED/outpatient visits, and thus the expansion would have implications on ED-related costs only. Findings from the sensitivity analysis was in agreement with the observation that ED/outpatient costs were approximately only one-tenth of the costs of hospitalization stay. The analysis is likely to be in favour of access to combined care, with limited implications in overall cost. To assess the impact of the care coordinator in the TCB group, we evaluated the cost implications under the decision strategies of TCB with and without care coordinator support, as well as UC. Note that the results under UC were the same as those in the base-case analysis (Table 4). The analysis indicated that patient care, which included care coordinator follow-up, was associated with slightly lower costs of CAN$135 than inpatients without a care coordinator (CAN$10,109 vs. CAN$10,244). The results suggest that the economic effect of the care coordinators was mostly offset by the costs of hiring them.
4 Discussion
Using data from the COPD TCB clinical trial [21], our analyses indicated that use of the TCB was less costly than UC. The main cost driver in each arm was inpatient admissions, and the main difference in costs is due to reductions in subsequent hospital readmissions. Our study observed statistically significant lower hospital and physician costs during hospital admissions in patients under TCB than those under UC. There were no statistically significant differences in hospital costs or physician costs between the groups during subsequent ED/outpatient visits.
The TCB was associated with reduced 7- and 30-day inpatient readmissions and patients under TCB were 2.4 times more likely to revisit the ED/outpatient department within 30 days, as reported in our previous study [21]. Published studies reported that inpatient stay was the major cost driving factor for COPD management [41,42,43]. In Canada, in agreement with our data, studies reported that inpatient care is approximately 10 times more expensive than ED/outpatient care [7, 44]. Given that, we believe that the cost savings in patients under TCB is largely attributable to the effect of TCB on inpatient cost. TCB patients used less of a more expensive inpatient resource and more of a less expensive ED/outpatient resource, resulting in overall lower costs.
The TCB was initiated within the latter days of the index hospitalization and there was concern that the additional activities associated with the intervention may impact LOS and cost during the index hospitalization (e.g., by entering someone into the TCB arm, they would have a longer index admission than otherwise). To address this concern, we included the costs and LOS of the index admission to measure the cost and LOS of TCB in ED/outpatient revisits and inpatient readmissions.
In addition to the analyses of the trial data, we modelled hospital costs and considered the combination of ED/outpatient and inpatient services. Since an inpatient stay is much more expensive than an ED/outpatient visit, an increase in patients attending the ED/outpatient department may not be a significant driving factor of our cost findings. Our sensitivity analysis confirmed this.
Our study observed that the TCB group had higher use of combined treatment (82% of ED + IP), compared with 64% in the UC group. One possible explanation is that by being in the TCB arm, patients may have had heightened awareness of their symptoms as a result of the education component of the TCB, and thereby (re)visited the ED/outpatient department more frequently. As a result, they may attend earlier when symptoms are less severe and do not require an IP admission. This would be advantageous from a cost savings perspective because patients are accessing lower-cost care at a more appropriate time. The ideal setting for such care would be a primary care physician or urgent care centre, but this study was not designed to explore reasons why patients attended the ED/outpatient department as opposed to a primary care setting. Similar findings were found in the study by Aboumatar et al. [45], where a transition bundle was associated with greater COPD-related hospitalizations and ED visits.
The TCB is not expected to modify direct acute patient care for the index COPD hospitalization. It is a transition coordination tool between acute care and primary/community care so that the patient understands the next steps in their care and supports them to understand what care to expect. It empowers the patient to take the lead on their health and care where possible. While it might be expected that TCB is not directly associated with LOS, it is an important outcome with respect to both patient experience and healthcare resource use and therefore is included in this current study. Not all changes in LOS can be attributed to TCB, as LOS is likely to be a function of both patient care during the hospital encounter, as well as adherence and effectiveness of TCB (e.g., effectiveness of education materials, smoking cessation counselling, pulmonary rehabilitation). Understanding the relationship between increased LOS and TCB warrants further investigation.
There are several limitations to our study. First, while quality-adjusted life-years (QALYs) are a commonly used effectiveness measure in economic evaluations [46], these data were not captured during the study. We conducted a literature search for relevant studies to identify data that would have an enabled estimate of quality of life to be used in our modelling. The only identified studies on COPD and quality of life were based on disease severity [47,48,49]. Because no disease severity data were captured within the trial, we were not able to link the evidence from the literature to the study data. We therefore suggest further work should explore collecting data on disease severity to allow researchers to link disease state and quality of life to estimate QALYs. Having data on patient-reported health-related quality of life could lead to a more robust analysis of the cost effectiveness of the COPD TCB in the future.
Second, another shortcoming of this analysis relates to the study’s time horizon, which was less than 1 year. Although this time horizon is in line with many other clinical trials and their associated economic evaluations, decision makers might still be interested in results over a longer time horizon as COPD is a long-term chronic illness.
Third, the economic model should be interpreted with caveats regarding its generalizability. We conducted the analysis along with one clinical study from a single publicly funded health system for the whole province, with coordinated hospital and post-hospital care and a vertically integrated strategic clinical network. Future research might need to extend the TCB to other health system structures. In addition, the cost to society and the cost of medications prescribed and dispensed in community settings were not included in our model. Inclusion of these costs would influence the economic consequences of the intervention.
Fourth, we did not undertake an evaluation of the distributional effects of the programme according to population-specific variables such as socioeconomic status, ethnicity, and geographical location, although these are frequently important for decision makers concerned about equity in access to care [50]. Given the prevalence of COPD across different segments of the population, further research exploring the equity related implications of TCB may be warranted. In particular, given the large population dispersion in Alberta, a spatial analysis considering discrepancy in geographical location would be informative.
Future studies may want to stratify based on global obstructive lung disease (GOLD) groups (I–IV) or classes (A–D). Currently, we were not able to stratify based on GOLD I–IV or A–D because we did not have lung function or dyspnea/CAT information. These data are required for GOLD categorization, but none of these variables are routinely measured at the time of an acute COPD ED/hospital admission—true not only in Canada but also throughout the world—and therefore our approach reflects the ‘real world’ approach to clinical patient assessment and management.
5 Conclusion
Our results suggest that with respect to inpatient admissions, the COPD TCB was associated with lower resource use than UC but slightly greater resources used in ED/outpatient visits. Given that an inpatient admission is associated with a higher cost than an ED/outpatient attendance, introducing the TCB to the healthcare system would result in lower total overall costs. This study suggests that among the two options evaluated, the TCB, with or without a care coordinator, appears to be the most economically attractive intervention.
Notes
Cited from the Institute for Healthcare Improvement: http://www.ihi.org/Topics/Bundles/Pages/default.aspx.
We accessed the ALIS website on 30 May 2020 for the data (https://alis.alberta.ca/occinfo/occupations-in-alberta/occupation-profiles/registered-nurse/).
1 Canadian dollar = 0.75 US dollars, based on the Bank of Canada website. Access on 1 February 2023: https://www.bankofcanada.ca/.
References
Halbert R, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–32.
WHO, World Health Organization. Global health estimates 2016: deaths by cause, age, sex, by country and by region, 2000–2016. Geneva: World Health Organization; 2018.
Soriano JB, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Respir Med. 2017;5(9):691–706.
Khakban A, et al. Ten-year trends in direct costs of COPD: a population-based study. Chest. 2015;148(3):640–6.
Canadian Institute for Health Information. Inpatient hospitalizations, surgeries and newborn indicators. 2016–2017: https://secure.cihi.ca/free_products/hospch-hosp-2016-2017-snapshot_en.pdf.
Halpin DM, Miravitlles M. Chronic obstructive pulmonary disease: the disease and its burden to society. Proc Am Thorac Soc. 2006;3(7):619–23.
Tran D, et al. Current and future direct healthcare cost burden of chronic obstructive pulmonary disease in Alberta, Canada. Can J Respir Crit Care Sleep Med. 2020;4(1):39–47.
Iheanacho I, et al. Economic burden of chronic obstructive pulmonary disease (COPD): a systematic literature review. Int J Chron Obstruct Pulmon Dis. 2020;15:439.
McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796–803.
Press VG, Konetzka RT, White SR. Insights about the economic impact of chronic obstructive pulmonary disease readmissions post implementation of the hospital readmission reduction program. Curr Opin Pulm Med. 2018;24(2):138–46.
Mittmann N, et al. The cost of moderate and severe COPD exacerbations to the Canadian healthcare system. Respir Med. 2008;102(3):413–21.
Mulpuru S, et al. Factors contributing to high-cost hospital care for patients with COPD. Int J Chron Obstr Pulmon Dis. 2017;12:989.
Alshabanat A, et al. Impact of a COPD comprehensive case management program on hospital length of stay and readmission rates. Int J Chron Obstruct Pulmon Dis. 2017;12:961.
López-Campos JL, Soler-Cataluña JJ, Miravitlles M. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2019 report: future challenges. Arch Bronchopneumol. 2020;56(2):65–7.
Ospina M, et al. A systematic review of the effectiveness of discharge care bundles for patients with COPD. Thorax. 2017;72(1):31–9.
Resar R, et al. Using care bundles to improve health care quality. IHI innovation series white paper. Cambridge: Institute for Healthcare Improvement; 2012.
Yang F, et al. Continuity of care to prevent readmissions for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD J Chronic Obstruct Pulm Dis. 2017;14(2):251–61.
Ridwan ES, et al. Effects of transitional care on hospital readmission and mortality rate in subjects with COPD: a systematic review and meta-analysis. Respir Care. 2019;64(9):1146–56.
Ospina MB, et al. Development of a patient-centred, evidence-based and consensus-based discharge care bundle for patients with acute exacerbation of chronic obstructive pulmonary disease. BMJ Open Respir Res. 2018;5(1).
Michas M, Deuchar L, Leigh R. Factors influencing the implementation and uptake of a discharge care bundle for patients with acute exacerbation of chronic obstructive pulmonary disease: a qualitative focus group study. Implement Sci Commun. 2020;1:3.
Atwood CE, et al. Optimizing COPD acute care patient outcomes using a standardized transition bundle and care coordinator: a randomized clinical trial. Chest. 2022;162(2):321–30.
Calverley PM, et al. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir Med. 2018;6(5):337–44.
Rabe KF, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48.
Gershon A, et al. Identifying individuals with physician diagnosed COPD in health administrative databases. COPD J Chronic Obstr Pulm Dis. 2009;6(5):388–94.
O’donnell DE, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(Suppl B):5B-32B.
Glussich A. Estimating costs of a hospital stay. Ed 2015. Canadian Institute for Health Information; 2020.
Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ. 2005;24(3):465–88.
Deber RB, KCK Lam. Handling the high spenders: implications of the distribution of health expenditures for financing health care. APSA 2009 Toronto meeting paper.
Gruber J. Financing Health Care Delivery. Annu Rev Financ Econ. 2022;14:209–229.
Dalgaard P. Introductory statistics with R. Berlin: Springer; 2008.
Newhouse JP. Health economics and econometrics. Am Econ Rev. 1987;77(2):269–74.
Basu A, Manning WG. Issues for the next generation of health care cost analyses. Med Care. 2009;47(7 Suppl 1):S109–14.
Arrow K, Lind R. Uncertainty and the evaluation of public investment decisions. Am Econ Rev. 1970;60(3):364–78.
Tukey JW. Exploratory data analysis. Environmental engineering from Brandenburg. Reading: Addisson. Wesley Publishing Company; 1977. p. 254.
Ramsey SD, et al. Cost-effectiveness analysis alongside clinical trials II—an ISPOR good research practices task force report. Value Health. 2015;18(2):161–72.
Glick HA, et al. Economic evaluation in clinical trials. Oxford: Oxford University Press; 2014.
Briggs AH, Mooney CZ, Wonderling DE. Constructing confidence intervals for cost-effectiveness ratios: an evaluation of parametric and non-parametric techniques using Monte Carlo simulation. Stat Med. 1999;18(23):3245–62.
O’Brien BJ, et al. In search of power and significance: issues in the design and analysis of stochastic cost-effectiveness studies in health care. Med Care. 1994;32:150–63.
Wolowacz SE, et al. Estimating health-state utility for economic models in clinical studies: an ISPOR good research practices task force report. Value Health. 2016;19(6):704–19.
Husereau D, et al. Consolidated health economic evaluation reporting standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II good practices task force. Value Health. 2022;25(1):10–31.
ur Rehman A, et al. The economic burden of chronic obstructive pulmonary disease (COPD) in the USA, Europe, and Asia: results from a systematic review of the literature. Expert Rev Pharmacoecon Outcomes Res. 2020;20(6):661–72.
Løkke A, et al. Economic burden of COPD by disease severity—a nationwide cohort study in Denmark. Int J Chronic Obst Pulm Dis. 2021;16:603.
Kirsch F, et al. Direct and indirect costs of COPD progression and its comorbidities in a structured disease management program: results from the LQ-DMP study. Respir Res. 2019;20(1):1–15.
Poder TG, et al. Eosinophil counts in first COPD hospitalizations: a 1-year cost analysis in Quebec, Canada. Int J Chronic Obst Pulm Dis. 2018;13:3065.
Aboumatar H, et al. Effect of a hospital-initiated program combining transitional care and long-term self-management support on outcomes of patients hospitalized with chronic obstructive pulmonary disease: a randomized clinical trial. JAMA. 2019;322(14):1371–80.
Drummond MF, et al. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2015.
Jordan RE, Majothi S, Heneghan NR. Supported self-management for patients with moderate to severe chronic obstructive pulmonary disease (COPD): an evidence synthesis and economic analysis. Health Technol Assess. 2015;19(36):1–516.
Sin DD, Golmohammadi K, Jacobs P. Cost-effectiveness of inhaled corticosteroids for chronic obstructive pulmonary disease according to disease severity. Am J Med. 2004;116(5):325–31.
Moayeri F, Hseuh YS, Clarke P, Dunt D. Do model-based studies in chronic obstructive pulmonary disease measure correct values of utility? A meta-analysis. Value Health. 2016;19(4):363–73.
Cookson R, et al. Distributional cost-effectiveness analysis: quantifying health equity impacts and trade-offs. Oxford: Oxford University Press; 2020.
Acknowledgements
This work was made possible through collaboration with clinical and operational leadership, front-line and local improvement teams at the five hospitals (Foothills Medical Centre, Red Deer Regional Hospital Centre, Rockyview General Hospital, Royal Alexandra Hospital and University of Alberta Hospital), as well as with the support of the Medicine Strategic Clinical Network™, Alberta Health Services.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors acknowledge funding from the Canadian Institute of Health Research (CIHR; Grant no. 138884) and Alberta Innovates Health Solutions PRIHS II (Grant no. 201400390).
Conflicts of interest
Charles Yan, Jeff Round, Ilke Akpinar, Chantal E. Atwood, Lesly Deuchar, Mohit Bhutani, Richard Leigh, and Michael K. Stickland declare that they have no conflicts of interest.
Ethics approval
Ethics approval was obtained from the University of Alberta Research Ethics Board (PRO00065003), and adheres to ethical standards as outlined in the Declaration of Helsinki and its subsequent amendments, as well as other comparable ethical standards.
Consent to participate
A waiver of consent was granted by the Ethics Board.
Consent for publication
Not applicable.
Availability of data and material
The data supporting the findings of the analysis are publicly available within the article, its supplementary materials and the articles referenced in this manuscript.
Code availability
Codes of the analysis are available from the author upon request.
Author contributions
CY contributed to the study conception and design, modelling, data analysis and interpretation, statistical analysis, and revision of the manuscript for critical content/manuscript preparation. JR contributed to the study conception and design and revision of the manuscript for critical content/manuscript preparation. IA contributed to study conception and design, economic expert review of the literature, and revision of the manuscript for critical content/manuscript preparation. CA contributed to the development, data collection, implementation, and analysis of the original COPD TCB study. In the current study, she contributed to the study design, data collection and interpretation, and revision of the manuscript for subject matter/content. LD contributed to the conception, development and implementation of the original COPD TCB study, as well as revision of the current manuscript for subject matter/content. MB was co-principal investigator on the initial COPD TCB study and thereby contributed to the conception, development and analysis of that study, which generated the data informing the current study. In the current study, he contributed to the interpretation of data and revision of the manuscript for subject matter/content. RL was co-principal investigator on the initial COPD TCB study and thereby contributed to the conception, development and analysis of that study, which generated the data informing the current study. In the current study, he contributed to the interpretation of data, and revision of the manuscript for subject matter/content. MS was principal investigator on the initial COPD TCB study and thereby contributed to the conception, development and analysis of that study, which generated the data informing the current study. In the current study, he contributed to the interpretation of data and revision of the manuscript for subject matter/content. All authors approved the final version for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yan, C., Round, J., Akpinar, I. et al. Cost Analysis of a Transition Care Bundle Compared with Usual Care for COPD Patients Being Discharged from Hospital: Evaluation of a Randomized Controlled Trial. PharmacoEconomics Open 7, 493–505 (2023). https://doi.org/10.1007/s41669-023-00400-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-023-00400-7