Abstract
Background
Neonatal respiratory distress syndrome (RDS) is one of the most common problems for preterm infants, and symptoms include tachypnoea, grunting, retractions and cyanosis, which occur immediately after birth. Treatment with surfactants has reduced morbidity and mortality rates associated with neonatal RDS.
Objective
The objective of this review is to describe the treatment costs, healthcare resource utilization (HCRU) and economic evaluations of surfactant use in the treatment of neonates with RDS.
Methods
A systematic literature review (SLR) was performed to identify available economic evaluations and costs associated with neonatal RDS. Electronic searches were conducted in Embase, MEDLINE, MEDLINE In-Process, NHS EED, DARE and HTAD to identify studies published between 2011 and 2021. Supplementary searches of reference lists, conference proceedings, websites of global health technology assessment bodies and other relevant sources were conducted. Publications were screened by two independent reviewers for inclusion and followed the population, interventions, comparators and outcomes framework eligibility criteria. Quality assessment of the identified studies was performed.
Results
Eight publications included in this SLR met all eligibility criteria: three conference abstracts and five peer-reviewed original research articles. Four of these publications evaluated costs/HCRU, and five (three abstracts and two peer-reviewed articles) investigated economic evaluations (two from Russia, and one each from Italy, Spain and England). The main cost drivers and causes of increased HCRU were invasive ventilation, duration of hospitalization and RDS-associated complications. There were no significant differences in neonatal intensive care unit (NICU) length of stay or NICU total costs between infants treated with beractant (Survanta®), calfactant (Infasurf®) or poractant alfa (Curosurf®). However, treatment with poractant alfa was associated with reduced total costs compared with no treatment, continuous positive airway pressure (CPAP) alone or calsurf (Kelisu®), due to shorter duration of hospitalization and fewer complications. Early use of the surfactant after birth was more clinically effective and cost-effective than late intervention in infants with RDS. Poractant alfa was found to be cost-effective and cost-saving compared to beractant for the treatment of neonatal RDS in two Russian studies.
Conclusion
There were no significant differences in NICU length of stay or NICU total costs between surfactants evaluated for treating neonates with RDS. However, early use of surfactant was found to be more clinically effective and cost-effective than late treatment. Treatment with poractant alfa was found to be cost-effective versus beractant and cost-saving compared with CPAP alone or beractant or CPAP in combination with calsurf. Limitations included the small number of studies, the geographic scope of the studies and the retrospective study design of the cost-effectiveness studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There were no significant differences in neonatal intensive care unit (NICU) length of stay or NICU total costs between infants treated with beractant (Survanta®), calfactant (Infasurf®) or poractant alfa (Curosurf®). |
Early use of surfactant in infants with respiratory distress syndrome (RDS) shortens the duration of hospitalization, results in fewer clinical complications and reduces the overall treatment cost of RDS compared with late intervention. |
Poractant alfa (with or without less invasive surfactant administration) is a cost-effective treatment compared with animal-derived surfactant, beractant, and is cost-saving compared with continuous positive airway pressure (CPAP) alone or beractant or CPAP in combination with calsurf. |
1 Introduction
Approximately 11% of all infants are born preterm, and the numbers are rising in many countries internationally [2]. Respiratory insufficiency is one of the most common problems for preterm birth; it manifests as respiratory distress syndrome (RDS), a product of structurally immature lungs and pulmonary surfactant deficiency [2]. In the United States (US), RDS affects 1% of pregnancies and occurs in 20,000–30,000 newborn infants each year [3, 4]. RDS accounts for approximately 860 infant deaths annually in the US [4]. The incidence of RDS is inversely related to gestational age and birth weight [3, 5, 6], and affects 60% of infants with a gestational age of < 28 weeks and 30% of infants with a gestational age between 28 and 34 weeks [3].
RDS symptoms include tachypnoea, grunting, retractions and cyanosis, and occur immediately after birth [4]. Chest radiography and blood gas analysis provide confirmation of a diagnosis of RDS; a diffuse ground-glass appearance with air bronchograms and hypoexpansion on a chest radiograph, and hypoxaemia and acidosis on blood gas analysis, are strongly indicative of RDS [4]. Symptoms typically progress in the first 12–24 h after birth [4], and infants often require invasive and non‑invasive respiratory support, supplementary oxygen and treatment with surfactant [2]. Although management has evolved gradually over the years (resulting in improved survival for the smallest infants), rates of bronchopulmonary dysplasia are high [7].
The aim of RDS treatment is to provide interventions that maximize survival whilst minimizing potential adverse events (AEs), including bronchopulmonary dysplasia [7]. Randomized clinical trials have provided evidence that prophylactic continuous positive airway pressure (CPAP), with or without surfactant, for RDS management reduces bronchopulmonary dysplasia incidence versus invasive mechanical ventilation (IMV) [8]. Therefore, early use of CPAP after birth with selective administration of surfactant is now recommended by the European Consensus and the American Academy of Pediatrics in preterm neonates with RDS [7, 9].
As the number of infants being born preterm is rising, and overall survival is improving, optimal early management of these infants is likely to confer lifelong health benefits [2]. Although routine prophylactic administration of surfactant in preterm newborns who do not show clinical signs of RDS is not recommended by international guidelines, surfactant administration using an early rescue approach is recommended for infants with RDS receiving CPAP who continue to clinically progress [7, 9, 10]. Observational studies report that surfactant should be administered to infants with RDS receiving CPAP when the fraction of inspired oxygen (FiO2) is > 0.30 [7]; this threshold has been established as a good predictor of CPAP failure [7].
The morbidity and mortality associated with RDS in premature infants have been greatly reduced by the administration of exogenous, animal-derived surfactants [11,12,13,14]. Commonly used and licensed surfactant preparations for the treatment of RDS include beractant (Survanta®), bovactant (Alveofact®), calfactant (Infasurf®), calsurf (Kelisu®) and poractant alfa (Curosurf®). Of the surfactants licensed in Europe, bovactant is recommended at an initial dose of 50 mg/kg [7] and beractant is indicated at an initial dose of 100 mg/kg for rescue therapy [15]. Surfactants approved elsewhere for the treatment of infants with RDS include calfactant, which is recommended at an initial dose of 100 mg/kg in the US [16] and other countries, and calsurf, which is commonly used in China at an initial dose of 40–100 mg/kg (average 70 mg/kg) [17]. While all other surfactants are bovine-derived, poractant alfa is the only porcine lung extract, and is recommended at a dose of 100–200 mg/kg [18]; however, an initial dose of 200 mg/kg is recommended for optimal respiratory outcomes [7, 19].
Economic evaluations are essential in making informed treatment decisions because of the limitations in economic resource use and the increasing cost of novel treatments. To our knowledge, no systematic review has been published that has discussed the treatment costs, healthcare resource utilization (HCRU) and economic evaluations of surfactant use in neonates with RDS.
1.1 Objective
The objective of this review is to describe the treatment costs, HCRU and economic evaluations of surfactant use in the treatment of neonates with RDS.
2 Methods
2.1 Search Strategy and Selection Criteria
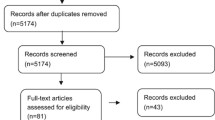
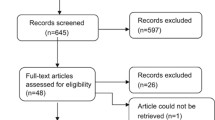
A systematic literature review (SLR) was performed using a pre-written protocol to identify publications detailing total costs and economic evaluations of surfactants in the treatment of neonates with RDS. The Preferred Reporting Items for Systematic reviews and Meta-Analyses literature search extension (PRISMA-S) 2021 guidelines [20] were followed to identify and screen the literature and extract the data (Fig. 1).
PRISMA flow diagram of the selection process that identified eight publications reporting costs and economic evaluations in RDS in neonates. EU European Union, HCRU healthcare resource utilization, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RDS respiratory distress syndrome, SLR systematic literature review, US United States
The following databases were searched: Cochrane and NHS EED (22 September 2021), Embase, MEDLINE, MEDLINE In-Process (12 October 2021), DARE and HTAD (Tables 1–4 in the Electronic Supplementary Material). In addition, manual searches of select conference proceedings (search period 2018–2021) were performed to capture the most recent economic data associated with the use of surfactant treatment in infants with RDS. Data from congress abstracts were used to supplement the SLR with relevant, novel findings that were not yet published in full-text articles (refer to Table 5 in the Electronic Supplementary Material for a list of conference proceedings included in the search). Searches of citations/reference lists of identified publications, narrative reviews, systematic reviews (bibliographic search) and health technology assessment agency websites were conducted (search period 15–19 November 2021) to identify missing publications that may be relevant to the SLR (Table 5 in the Electronic Supplementary Material). Our search strategy utilized a combination of Emtree subject headings (Embase®), MeSH (medical subject headings, PubMed®) and free-text terms to retrieve all the relevant publications. The following entry terms/keywords were used in literature searches along with any possible synonyms of these terms: neonates and respiratory distress syndrome, cost-effectiveness, cost-utility, cost-benefit, cost-minimization, budget impact, cost consequences, and exclusionary terms such as letters, notes, editorials, comments, addresses, books, chapters and studies in animals.
Eligibility criteria were determined using the population, intervention, comparator, outcomes (PICOS) approach [21] to identify relevant patient populations; eligible patients included neonates with a diagnosis of RDS receiving surfactant treatment. Outcomes by cost categories are included in Table 7 of the Electronic Supplementary Material. Searches were not restricted by surfactant or any other intervention to have a high degree of sensitivity. The relevant publications on surfactants were manually screened and identified. Due to changing practice in the management of neonates with RDS and evolving treatment patterns, searches were restricted to studies that were published between 2011 and 2021 (2018–2021 for congress website searches) and included relevant studies with full texts published in the English language. Only studies conducted in developed and pharmerging countries were included. Pharmerging countries were those who had a per capita income of < US$30,000 in 2020 and forecasted a 5-year aggregate pharma sale growth of > US$1 billion (absolute or rounded) in at least two forecasts [22]. Treatment patterns, clinical practice and economic valuations may differ in low-income countries due to inadequate and resource-limited neonatal care. Low-income countries were therefore excluded from the study. EndNote was used to handle the references in the SLR. Selection of the relevant studies was performed by the reviewers and strictly followed the eligibility criteria (Table 1).
2.2 Screening, Data Extraction and Quality Assessment
Citation screening, data extraction and quality assessment were performed by an independent reviewer. A second reviewer (unblinded from the decisions taken by the first reviewer) checked the screening, extractions and quality assessment. Any discrepancies were discussed with a third reviewer or resolved by consensus. The citations were first screened based on titles and abstracts, followed by full-text screening. Data for the following variables were extracted into tables (data extraction tools) in Microsoft Word format: country, study design, study population, data source, population characteristics, age, intervention, cost-related data and economic evaluation data. The included studies were critically appraised using the adapted Drummond’s checklist [23] (Table 6 in the Electronic Supplementary Material).
3 Results
A total of 1346 citations were identified after a systematic search in public databases (Fig. 1). These were subject to a screening process using the predefined PICOS criteria. Additional exclusions were applied to align with the objective of this article. The full texts of 107 publications were reviewed, leading to a selection of six studies. None of the selected studies were from the DARE or NHS EED database or conference searches. Two additional studies were identified from bibliographic searches. The eight publications included in this SLR comprised three conference abstracts and five peer reviewed original research articles.
The identified articles consisted of intervention costs, HCRU and economic evaluation from China, England, Italy, Spain, Russia and the US [3, 24,25,26,27,28,29,30]. The interventions or comparators identified in the studies were surfactants such as poractant alfa, beractant, calsurf and calfactant [3, 24,25,26,27,28,29,30]. Four of the studies in the SLR reporting on economic evaluations included a healthcare sector perspective [24,25,26,27]. The perspective taken by Krasnova et al. was not reported [28]. The sources of clinical and cost data were derived from real-world settings [3, 28], pharmacy information systems, electronic medical records [30], a prior clinical study, hospital data [24, 29] and a meta-analysis [27].
The overall assessment for the articles comprised minor limitations or potentially serious limitations. Three of the eight studies were published as conference abstracts with inadequate information, which is the main limitation for quality assessment. None of the studies provided information on discounting and incremental analysis. However, Dani et al. [24] provided justification that discounting is not required for a short time period (< 1 year), and an incremental cost-effectiveness ratio (ICER) is not required, as early rescue treatment was dominant in terms of both efficacy and costs when compared to late rescue treatment. Five studies provided sufficient details about the competing alternatives and measured the costs and consequences in appropriate physical units. All the studies had some limitations in most of the domains. Further details are provided in Table 6 of the Electronic Supplementary Material.
3.1 Costs and HCRU
Four of these studies (all peer-reviewed articles) evaluated the costs of interventions used during the treatment of RDS in neonates (Tables 2, 3). Brown et al. reported higher average medication costs (US$1756.44 vs. US$1329.78) but lower hospital charges (US$258,083 vs. US$290,158) for poractant alfa compared with beractant [30]. An additional US study found no significant differences between beractant, calfactant and poractant alfa in adjusted neonatal intensive care unit (NICU) length of stay (26.7 vs. 27.8 vs. 26.2 days, respectively, all p > 0.05) or NICU total costs (US$50,929 vs. US$50,785 vs. US$50,212, respectively, all p > 0.05) [3]. A further study reported that treatment costs were significantly lower in neonates treated with poractant alfa and CPAP versus calsurf and CPAP (p = 0.041) during the period of 2014–2017; however, the physical units of cost were not provided [29]. Although there were few efficacy differences between the two groups, treatment with poractant alfa was more advantageous in terms of safety, length of hospitalization and treatment costs [29].
A study conducted in Italy [24] analysed the impact of early versus late surfactant treatment in preterm infants with RDS. Infants < 30 weeks gestational age were administered 200 mg/kg poractant alfa, and a second dose of 100 mg/kg was administered if required. The overall average cost for infants treated with the early strategy was moderately lower than for infants treated with the late strategy (€4901.70 vs. €4960.07). Early treatment reduced the need for mechanical ventilation (MV) within the first 7 days of life versus late treatment, leading to a moderate reduction in financial burden.
3.2 Economic Evaluations
Five studies (three abstracts and two peer-reviewed articles) investigated the cost‑effectiveness/budget impact of surfactant administration, including two from Russia and one each from Italy, Spain and England (Tables 4, 5).
A study conducted in Italy [24] reported that early poractant alfa treatment in preterm infants with RDS that are of a gestational age of < 30 weeks is more clinically effective and cost-effective than late treatment (Table 5). Despite slightly higher initial costs of surfactant in the early versus late treatment group (€458.49 vs. €311.74), the late group displayed higher treatment costs due to MV (€108.85 [early] vs. €259.25 [late]).
In a Spanish study [26], early rescue with less invasive surfactant administration and CPAP therapy in preterm infants that were of a gestational age of 25–28 weeks and with FiO2 ≥ 0.3 resulted in cost savings (–€1,812,203; probability of cost saving, 59%), compared with CPAP alone. While less invasive surfactant administration plus CPAP therapy resulted in a reduction in costs versus CPAP alone in infants of a gestational age of 29–32 weeks with FiO2 ≥ 0.3, the cost saving was not as significant as that reported in infants of younger gestational age (–€206,813; probability of cost saving, 48%). Overall, in preterm infants of a gestational age of 25–32 weeks with FiO2 ≥ 0.3, the study reported that €1,605,390 was expected to be saved with early rescue using less invasive surfactant administration.
An additional study investigating less invasive surfactant administration and CPAP in England [25] reported that there was a cost saving with early rescue less invasive surfactant administration compared with CPAP alone. The study estimated savings per case treated of 5146 British Pounds (GBP£) for preterm infants of a gestational age of 25–28 weeks and GBP£176 for preterm infants of a gestational age of 29–32 weeks.
Two studies from Russia [27, 28] analysed beractant and poractant alfa for treatment of RDS by using decision-tree models. Yagudina et al. reported that the cost-effectiveness ratios per life saved with beractant and poractant alfa were €5087 and €4585, respectively. Krasnova et al [28] compared the cost-effectiveness of poractant alfa 100 mg/kg versus poractant alfa 200 mg/kg or beractant 100 mg/kg for the treatment of RDS; the investigators used a cost-effectiveness ratio based on the ‘prevention of death’ of the number needed to treat. On the 28th day of treatment, the cost‑effectiveness ratios for poractant alfa 100 mg/kg and 200 mg/kg were $11,681 and $11,822, respectively, and the cost-effectiveness ratio was $12,197 for beractant 100 mg/kg (Table 5).
4 Discussion
Eight studies analysing the costs, economic evaluations/budget impact and cost-effectiveness of surfactant administration in infants with RDS were identified in this SLR. The main cost drivers and causes of increased HCRU were found to be the use of invasive ventilation [24, 25], duration of hospitalization [29, 30] and RDS-associated complications [25, 26].
The publications identified in this SLR support the notion that early use of surfactants after birth in infants with RDS shortens the duration of hospitalization [25, 29, 30], results in fewer clinical complications [24] and reduces the overall treatment cost of RDS, compared with late intervention [24]. In addition, poractant alfa (with or without less invasive surfactant administration) was found to be cost-effective compared with beractant or CPAP alone [25, 26].
The results presented are in line with a large retrospective study conducted in the US, which reported that invasive ventilation is associated with a higher HCRU burden, compared with non‑invasive ventilation [31]. Despite this, new evidence from worldwide institutions regarding the assessment of cost and HCRU associated with surfactant administration is currently lacking. The most recent study addressing this topic emerged from a single-country study in England (published in 2022 [25]) and may not be applicable to global healthcare settings.
One US study that met the eligibility criteria of the current SLR was presented at the Pediatric Academic Societies congress in 2021 [32], and was identified during an ad-hoc search of neonatology congresses after closure of the SLR. Yao et al. developed a cost–consequence model to assess MV from a healthcare-delivery perspective and evaluate the impact of selective early surfactant administration via INtubate-SURfactant-Extubate (IN-SUR-E) or less invasive surfactant administration versus standard surfactant administration via endotracheal intubation. Patients were infants with RDS and an FiO2 of 0.3; 2020 was the drug-cost year used. The results of the model showed an early rescue approach resulted in a 50% decrease in mortality and overall savings of US$3453 for each infant with RDS. Total annual surfactant costs were higher with selective early surfactant administration (US$77,278) versus standard surfactant administration (US$47,981); however, higher surfactant costs were offset by savings in total hospital (US$15,797) and complication (US$358,771) costs. These data support the results presented in this SLR, which suggest that early surfactant treatment reduces the overall treatment cost of RDS.
A further study that was published after the SLR was conducted investigated outcomes and costs of less invasive surfactant administration in infants treated with poractant alfa 200 mg/kg in the delivery suite [33]. The study determined that, compared with historical controls, less invasive surfactant administration was associated with a reduction in the need for MV within 72 h after birth (20.2% vs. 56.6%, p < 0.001), a reduced incidence of moderate-to-severe bronchopulmonary dysplasia (8.2% vs. 20.2%, p = 0.02) and a decrease in the median costs of NICU stay (GBP£1218 vs. GBP£2436, p = 0.03) and total neonatal unit stay (GBP£12,888 vs. GBP£17,240, p = 0.04). As the study assessed bronchopulmonary dysplasia in infants, it did not meet the eligibility criteria of this SLR, but the data reflect those reported in this review, with earlier treatment resulting in increased upfront costs that are offset by reduced costs later in the treatment process.
Among the studies reporting data for cost-effectiveness, it was evident that early use of surfactant is both more clinically effective and cost-effective than late treatment [24]. Additionally, the data indicate that surfactant administration via less invasive surfactant administration (with or without CPAP) is a cost‑effective alternative compared with no surfactant (CPAP only) for preterm infants with RDS [25, 26]. Additionally, evidence included in this SLR indicates that poractant alfa has a better cost‑effectiveness ratio compared with beractant [27]. Studies implementing decision-tree modelling to investigate the pharmacoeconomic impact of surfactant therapy in infants with RDS reported that poractant alfa was cost-effective and cost-saving compared with beractant at both the 100 mg/kg and 200 mg/kg doses [27, 28]. While these studies were conducted in Russia and may not be generalizable to healthcare institutions worldwide, further global investigations exploring the impact of surfactant use on economic burden are needed to establish how infants with RDS may be treated safely and cost-effectively. Economic models identifying the most cost-effective, approved surfactants will, ultimately, reduce the economic burden of RDS.
4.1 Limitations
As this SLR identified a very small number of studies, and a proportion of these studies were congress abstracts with limited or inadequate information, the results should be interpreted with caution. Further analyses with more studies and larger patient samples are required to make an accurate assessment of HCRU with different surfactant regimens. Limiting the literature search to studies in developed and pharmerging countries may affect the generalizability of the conclusions. Additionally, given that our review was more descriptive and exploratory and less inclined towards data synthesis, we did not follow the Synthesis Without Meta-analysis (SWiM) guidelines. Moreover, we cannot rule out the introduction of unknown bias in the decisions taken by the reviewers for screening, data extraction and quality assessment due to preconceived ideas, overemphasis on statistically significant results, or lack of transparency (no disclosure of biases). Other limitations of the study were that all economic evaluations included were retrospective studies, and one study reported an unlicensed dose of poractant alfa (70 mg/kg) [29].
5 Conclusion
This SLR has provided a comprehensive review of the studies published in the last 10 years (2011 to 2021) reporting the costs, HCRU and economic evaluations of surfactants in the treatment of neonatal RDS. One study reported that there are no significant differences in NICU length of stay or NICU total costs between infants treated with beractant, calfactant or poractant alfa. Two studies reported lower hospital charges and treatment costs with poractant alfa alone versus beractant (n = 1 study) and poractant alfa with CPAP versus calsurf with CPAP (n = 1 study), respectively. While there was a limited number of studies identified in this SLR, one study assessing the comparative cost-effectiveness of different surfactants indicated that poractant alfa is cost-effective compared with beractant in the treatment of infants with RDS.
One study reported that early use of the surfactant after birth was found to be more clinically effective and cost-effective than late intervention in infants with RDS. Two studies reported that compared with CPAP alone, early surfactant administration with CPAP was associated with fewer complications and a lower overall treatment cost, resulting in an overall cost saving. Two Russian studies reported better cost-effectiveness ratios per life saved (n = 1 study) or prevention of death (n = 1 study) for poractant alfa compared to beractant. Despite the findings of these studies, further global investigations identifying the most cost-effective approved surfactants are required to reduce the economic burden of RDS.
References
DeTora LM, Toroser D, Sykes A, et al. Good publication practice (GPP) guidelines for company-sponsored biomedical research: 2022 update. Ann Intern Med. 2022;175(9):1298–304.
Course C, Chakraborty M. Management of respiratory distress syndrome in preterm infants in Wales: a full audit cycle of a quality improvement project. Sci Rep. 2020;10(1):3536.
Sekar K, Fuentes D, Krukas-Hampel MR, et al. Health economics and outcomes of surfactant treatments for respiratory distress syndrome among preterm infants in US level III/IV Neonatal Intensive Care Units. J Pediatr Pharmacol Ther. 2019;24(2):117–27.
Hermansen CL, Mahajan A. Newborn respiratory distress. Am Fam Physician. 2015;92(11):994–1002.
Condo V, Cipriani S, Colnaghi M, et al. Neonatal respiratory distress syndrome: are risk factors the same in preterm and term infants? J Matern Fetal Neonatal Med. 2017;30(11):1267–72.
Muñoz-García M, Santiago-Gutiérrez C, Martínez-Padilla MC, et al. Survival and factors predicting mortality in preterms infants. J Perinat Med. 2015;43:P-0549. https://doi.org/10.1515/jpm-2015-2003.
Sweet DG, Carnielli V, Greisen G, et al. European consensus guidelines on the management of respiratory distress syndrome—2019 update. Neonatology. 2019;115(4):432–50.
Subramaniam P, Ho JJ, Davis PG. Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database Syst Rev. 2016;(6):Cd001243.
Polin RA, Carlo WA, Committee on Fetus and Newborn, et al. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014;133(1):156–63.
National Health Service. Surfactant replacement therapy for neonates with respiratory distress. https://www.eoeneonatalpccsicnetwork.nhs.uk/wp-content/uploads/2022/02/Surfactant-administration_ODN_v2_150418.pdf. Accessed 15 July 2022.
Sardesai S, Biniwale M, Wertheimer F, et al. Evolution of surfactant therapy for respiratory distress syndrome: past, present, and future. Pediatr Res. 2017;81(1–2):240–8.
Engle WA, American Academy of Pediatrics Committee on Fetus and Newborn. Surfactant-replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics. 2008;121(2):419–32.
Halliday HL. Surfactants: past, present and future. J Perinatol. 2008;28(Suppl 1):S47-56.
Ramanathan R, Bhatia JJ, Sekar K, et al. Mortality in preterm infants with respiratory distress syndrome treated with poractant alfa, calfactant or beractant: a retrospective study. J Perinatol. 2013;33(2):119–25.
Survanta® (beractant). Summary of product characteristics. https://mhraproducts4853.blob.core.windows.net/docs/6a704ce803670e6511a6c607e26ab87b557a3983. Accessed 17 May 2022.
Infasurf® (calfactant). Prescribing information. https://infasurf.com/prescribing-information/. Accessed 20 June 2022.
Guo X, Luo S, Amidani D, et al. In vitro characterization and in vivo comparison of the pulmonary outcomes of poractant alfa and Calsurf in ventilated preterm rabbits. PLoS ONE. 2020;15(3):e0230229.
Curosurf® (poractant alfa). Summary of product characteristics. https://www.medicines.org.uk/emc/medicine/21421. Accessed 9 May 2022.
Singh N, Halliday HL, Stevens TP, et al. Comparison of animal-derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev. 2015;(12):CD010249.
Rethlefsen ML, Kirtley S, Waffenschmidt S, et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10(1):39.
Richardson WS, Wilson MC, Nishikawa J, et al. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123(3):A12–3.
The IQVIA Institute. The Global Use of Medicines 2022: Outlook to 2026. https://www.iqvia.com/insights/the-iqvia-institute/reports/the-global-use-of-medicines-2022. Accessed 11 November 2022.
Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ. 1996;313(7052):275–83.
Dani C, Ravasio R, Fioravanti L, et al. Analysis of the cost-effectiveness of surfactant treatment (Curosurf®) in respiratory distress syndrome therapy in preterm infants: early treatment compared to late treatment. Ital J Pediatr. 2014;40(1):40.
Federici C, Fornaro G, Roehr CC. Cost-saving effect of early less invasive surfactant administration versus continuous positive airway pressure therapy alone for preterm infants with respiratory distress syndrome. Eur J Hosp Pharm. 2022;29(6):346–52.
Solozabal M, López-Sanromà M, Pérez I, et al. PRS8 budget IMPACT analysis of less invasive surfactant administration (LISA) technique as early rescue strategy for preterm infants with respiratory distress syndrome in Spain. Value Health. 2020;23:S717.
Yagudina R, Kulikov A, Serpik V. Prs50 - Cost-effectiveness analysis of surfactant therapy for the treatment of respiratory distress syndrome newborn in the Russian Federation. Value Health. 2018;21:S412.
Krasnova L, Vorobiev P, Tyurina I. Clinical and economic analysis of two surfactants for the prevention and treatment of respiratory distress syndrome. Value Health. 2016;19(7):A403.
Yuan G, Wu Z, Chen X, et al. Comparison of the efficacy and safety of bovine lung phospholipid and poractant alfa injections in the treatment of neonatal hyaline membrane disease with continuous positive airway pressure. Int J Clin Exp. 2019;12(3):3007–13.
Brown S, Hurren J, Sartori H. Poractant alfa versus beractant for neonatal respiratory distress syndrome: a retrospective cost analysis. J Pediatr Pharmacol Ther. 2018;23(5):367–71.
Kugelman A, Riskin A, Said W, et al. A randomized pilot study comparing heated humidified high-flow nasal cannulae with NIPPV for RDS. Pediatr Pulmonol. 2015;50(6):576–83.
Yao W, Jensen I, Claussen M, et al. Cost consequence analysis of using selective early surfactant administration vs. Standard surfactant administration via endotracheal intubation and mechanical ventilation. Presented at: annual meeting of the Pediatric Academic Societies (PAS); 30 April–4 May, 2021; Virtual. EP-152.10612021.
Arattu Thodika FMS, Ambulkar H, Williams E, et al. Outcomes following less-invasive-surfactant-administration in the delivery-room. Early Hum Dev. 2022;167:105562.
Verder H, Albertsen P, Ebbesen F, et al. Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks’ gestation. Pediatrics. 1999;103:e24.
Dargaville PA, Aiyappan A, De Paoli AG, et al. Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed. 2013;98:F122–6.
Göpel W, Kribs A, Ziegler A, et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open label, randomised, controlled trial. Lancet. 2011;378:1627–34.
Isayama T, Iwami H, McDonald S, et al. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA. 2016;316:611–24.
Acknowledgements
Medical writing support was provided by Sarah Cocklin, Ph.D., and Laura Graham, Ph.D., of Parexel International and was funded by Chiesi Farmaceutici S.p.A.. Writing support was conducted in accordance with Good Publication Practice (GPP 2022) guidelines [1]. The authors would like to thank Alessia Colucciello (employee of Chiesi Farmaceutici S.p.A.) for her critical review and insightful comments on the manuscript revisions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Chiesi Farmaceutici S.p.A.
Conflict of interest
Tiziana Magni, Natalia Meshchenkova, Martina Orlovic, Nicola Pelizzi and Chiara Ragni are employees of Chiesi Farmaceutici S.p.A.. Jyothsna Nathani, Lucia Perez-Kempner, Sheetal Sharma and Erika Turkstra were commissioned by Chiesi Farmaceutici S.p.A. and received a consulting fee to conduct the systematic review presented in the article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Code availability
Not applicable.
Author contributions
TM, CR, NP, MO, NM (employees of Chiesi Farmaceutici S.p.A.), LP-K and ET contributed to the study conception and design; SS and JN contributed to the collection and assembly of data, data analysis, interpretation and preparation of the manuscript. All authors commented on the manuscript drafts and read and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Magni, T., Ragni, C., Pelizzi, N. et al. Health Economic Studies of Surfactant Replacement Therapy in Neonates with Respiratory Distress Syndrome: A Systematic Literature Review. PharmacoEconomics Open 7, 359–371 (2023). https://doi.org/10.1007/s41669-023-00399-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-023-00399-x