Abstract
Background
For many patients with resected epidermal growth factor receptor mutation-positive (EGFRm) non-small cell lung cancer (NSCLC), current standard of care (SoC) is adjuvant chemotherapy; however, disease recurrence remains high. Based on positive results from ADAURA (NCT02511106), adjuvant osimertinib was approved for treatment of resected stage IB‒IIIA EGFRm NSCLC.
Objective
The aim was to assess the cost-effectiveness of adjuvant osimertinib in patients with resected EGFRm NSCLC.
Methods
A five-health-state, state-transition model with time dependency was developed to estimate lifetime (38 years) costs and survival of resected EGFRm patients treated with adjuvant osimertinib or placebo (active surveillance), with/without prior adjuvant chemotherapy, using a Canadian Public Healthcare perspective. Transitions between health states were modeled using ADAURA and FLAURA (NCT02296125) data, Canadian life tables, and real-world data (CancerLinQ Discovery®). The model used a ‘cure’ assumption: patients remaining disease free for 5 years after treatment completion for resectable disease were deemed ‘cured.’ Health state utility values and healthcare resource usage estimates were derived from Canadian real-world evidence.
Results
In the reference case, adjuvant osimertinib treatment led to a mean 3.20 additional quality-adjusted life-years (QALYs; (11.77 vs 8.57) per patient, versus active surveillance. The modeled median percentage of patients alive at 10 years was 62.5% versus 39.3%, respectively. Osimertinib was associated with mean added costs of Canadian dollars (C$)114,513 per patient and a cost/QALY (incremental cost-effectiveness ratio) of C$35,811 versus active surveillance. Model robustness was demonstrated by scenario analyses.
Conclusions
In this cost-effectiveness assessment, adjuvant osimertinib was cost-effective compared with active surveillance for patients with completely resected stage IB‒IIIA EGFRm NSCLC after SoC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The cost-effectiveness of osimertinib in Canada for the adjuvant treatment of patients with stage IB–IIIA non-small cell lung cancer (NSCLC) was assessed using a state-transition model with time dependency based on data from the ADAURA trial. FLAURA trial and real-world patient population data were also used because of the immaturity of ADAURA’s overall survival data. The model structure and selected data sources were deemed appropriate by several health technology assessment agencies globally. |
Our model estimated that more patients would be alive at 10 years on osimertinib (62.5%) versus active surveillance (39.3%); adjuvant osimertinib was cost-effective in resected epidermal growth factor receptor mutation-positive NSCLC, with an incremental cost-effectiveness ratio of 35,811 Canadian dollars versus active surveillance. |
Results presented here differ markedly from analyses completed by the Canadian Agency for Drugs and Technologies in Health (CADTH). Differences in assumptions regarding cure and long-term disease-free survival recurrence rates drove model result differences. Scenario analyses are presented here to better characterize the heterogeneity in model setup and resulting outcomes. |
1 Introduction
Approximately 30% of patients with non-small cell lung cancer (NSCLC) present with resectable disease [1,2,3], for whom primary treatment is surgical removal of the primary tumor [4]. Platinum-based chemotherapy regimens are recommended as post-operative adjuvant therapy for patients with stage II‒IIIA disease and select patients with stage IB disease [5, 6]. Standard practice in Canada reflects European Society for Medical Oncology (ESMO) and American Society of Clinical Oncology/Cancer Care Ontario (ASCO/CCO) treatment guidelines [5, 6], with most jurisdictions recommending adjuvant chemotherapy for stage II‒IIIA patients. After resection and receiving adjuvant chemotherapy, patients undergo a ‘watch and wait’ or active surveillance period.
Although treatment for patients with stage IB‒IIIA NSCLC is of curative intent, a high proportion of patients have disease recurrence or die. Five-year survival rates range from 36% for stage IIIA NSCLC to 68% for stage IB disease [7]. Disease recurrence rates after adjuvant chemotherapy range from approximately 45% for stage IB to 76% in stage III NSCLC, over a median follow-up of 5.2 years [8]. The risk of dying increases greatly after disease recurrence in all stages of resected NSCLC, so delaying or preventing recurrence is crucial to improving long-term outcomes [9]. As with metastatic epidermal growth factor receptor mutation-positive (EGFRm) NSCLC, targeted therapies in the resectable setting may offer an improvement in survival [10].
Osimertinib is a third-generation, irreversible, central nervous system (CNS)-active epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), and has demonstrated benefit in progression-free survival (PFS) and overall survival (OS) in patients with EGFRm metastatic NSCLC [11,12,13,14,15,16]. The pivotal study supporting osimertinib as an adjuvant therapy is the phase III, double-blind ADAURA trial (NCT02511106) [10], which demonstrated a statistically significant disease-free state (DFS) benefit with osimertinib versus placebo in patients with completely resected stage IB–IIIA EGFRm NSCLC (hazard ratio [HR] 0.20; 99.12% confidence interval [CI] 0.14–0.30, p < 0.001; 11% and 46% maturity for osimertinib and placebo, respectively). OS data were immature (4%) at the time of the unplanned interim exploratory analysis.
Based on ADAURA data, osimertinib was approved by the Food and Drug Administration (FDA) [17], European Medicines Agency (EMA) [18], and other global authorities as adjuvant treatment after tumor resection in patients with EGFRm (ex19 del or L858R) NSCLC. A critical question in all regions is what is the incremental value of the treatment regimen containing adjuvant osimertinib. To estimate incremental value, payers often require estimates of the survival benefits of novel oncology treatments to inform reimbursement decisions; modeling long-term survival benefits of treatments can also be valuable for clinical decision-making. Adjuvant osimertinib was approved by Health Canada on January 18, 2021 [19]; we present the economic evaluation from a Canadian perspective in this setting, which was submitted by the sponsor to support a Canadian health technology assessment (HTA). The objective was to evaluate osimertinib’s cost-effectiveness as an adjuvant treatment for patients with EGFRm NSCLC after complete tumor resection (with or without prior adjuvant chemotherapy), with costs and efficacy associated with subsequent treatments for patients with disease progression or relapse being estimated.
2 Methods
2.1 Model Structure
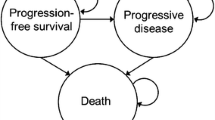
A state transition model with time dependency was developed (Excel®, Microsoft, Washington, USA), using a cycle length of 1 month, to estimate the costs and survival of patients with resectable EGFRm NSCLC based on the ADAURA trial. The model structure comprised five mutually exclusive health states: ‘disease free’ (DF), ‘local/regional recurrence’ (LRR), ‘first-line treatment for distant metastatic NSCLC’ (1L DM), ‘second-line treatment for distant metastatic NSCLC’ (2L DM), and ‘Death’ as the absorbing state (Fig. 1a). Assumptions in the model, model structure and treatment pathway were reviewed and validated with clinical experts (authors PC, BM, and BS, through advisory board and consultation).
Model and treatment pathway. a Five-health-state model structure and b treatment pathway modeled. 1L first-line, 2L second-line, BSC best supportive care, DF disease free, DM distant metastases, LRR local/regional recurrence, PDC platinum doublet chemotherapy, RT radiotherapy, SBRT stereotactic body radiation therapy, TP transition probability. aOf which, 55% of patients received radiotherapy
Patients in the DF state were modeled to transition to distant metastatic disease, local/regional disease (which can be treated with curative intent again), relapse with distant metastatic disease or be cured in stage IB–IIIA and remain DF indefinitely. Distant metastatic disease required two health states as costs and efficacy of drugs in 1L DM (osimertinib) varied markedly from 2L DM (platinum doublet chemotherapy [PDC] or taxanes).
2.2 Treatment Pathway
The modeled treatment pathway compared two different adjuvant treatment arms following complete tumor resection: the osimertinib arm and the active surveillance arm (Fig. 1b). Further details are available in ‘Supplementary Methods’ (see the electronic supplementary material).
Data from ADAURA were used to inform the transitions out of the DF health state. Given the earlier than expected read-out of ADAURA [10], limited long-term follow-up data were available; therefore, data were supplemented with an ‘ADAURA-like’ cohort of patients with resected EGFRm NSCLC who had relapsed into the LRR state. Data for these patients were obtained from the US electronic medical record CancerLinQ Discovery® database [20,21,22] (see ‘Supplementary Methods’), and this modeled population was used to inform transitions out of the LRR health state. The CancerLinQ cohort had comparable patient demographic characteristics to patients in the ADAURA trial, with similar proportions of patients across disease stages (Supplementary Table S1, see the electronic supplementary material). Data from the phase III, double-blind trial FLAURA (NCT02296125) [11, 16], which evaluated first-line osimertinib versus comparator EGFR-TKI (erlotinib or gefitinib) in patients with EGFRm advanced NSCLC, was used to inform transitions in the 1L DM and 2L DM health states. Time to treatment discontinuation was used as a proxy for progression as longer follow-up data were available for this parameter than for PFS. Therefore, time to treatment discontinuation was used to model progression from 1L DM to 2L DM. For patients expected to receive osimertinib on relapse to 1L DM from DFS, data from the osimertinib arm of the FLAURA trial were used (Supplementary Table S1). Patients not expected to receive osimertinib on relapse (see Fig. 1b, patients in the osimertinib arm who progressed within 48 months) were modeled using the comparator EGFR-TKI arm, with an HR [23] applied to correct for the use of PDC instead of comparator EGFR-TKI. The HR for 2L DM to Death is recalibrated to ensure that the combined HR for 1L DM to 2L DM and 2L DM to Death reflects the OS HR from 1L DM to Death in Holleman et al. [23]. Validation of the modeling of the placebo arm of the ADAURA trial versus Canadian real-world data [24] was assessed by calculation of the mean absolute percentage error (MAPE) and correlation coefficient (R2). Key clinical inputs are listed in Supplementary Table S3.
Competing risk events were censored for each transition throughout the model [25]. As an example, for the transition from DF to LRR, DM and death events were censored. Cause-specific hazards for each transition were calculated and applied by state-transition modeling to derive overall state-transition probabilities for each of the five states in the model.
Standard parametric survival modeling was used to model the transitions between health states. For all transitions to ‘death’, the maximum hazard from the selected distribution or general population mortality (GPM) was utilized. Data from each source (ADAURA for DF, CancerLinQ for LRR, and FLAURA for 1L DM and 2L DM) were extrapolated using standard parametric survival modeling to the lifetime horizon and assessed for ‘goodness of fit’ using visual inspection further informed by insights of clinical experts, the Akaike information criterion, Bayesian information criterion, and Schoenfeld residuals test [26, 27]. The generalized gamma distribution was selected as the preferred curve fit for the reference case analysis. Given that the proportional hazards assumption did not hold for all transitions, only individual fits were applied. The parametric models used are provided in Supplementary Table S2, extrapolations are provided in Supplementary Figures S1–S3, and goodness-of-fit information for each transition is supplied in Supplementary Tables S4–S9.
2.3 Outcomes
In the model, cost-effectiveness was measured both in costs (total and incremental) and health outcomes: quality-adjusted life-years (QALYs) and life-years (LYs). The incremental cost-effectiveness ratio (ICER) both of incremental cost/QALY and incremental cost/LY were calculated.
2.4 Time Horizon
Patients randomized in ADAURA had a mean age of 62 years, and clinical experts anticipated resection to lead to cure in some patients, so a lifetime was defined as the time required to achieve < 1% survival in both treatment arms, which was estimated to be 100 years of age. Therefore, the model considered a lifetime horizon of 38 years.
2.5 Assumptions
Based on clinical expert feedback, 95% of patients who remained DF for 5 years after the completion of treatment for resectable EGFRm NSCLC were deemed ‘cured.’ For the adjuvant osimertinib arm, this was after 3 years of adjuvant osimertinib treatment plus 5 years of no treatment; for the active surveillance arm, this was 5 years after the start of placebo (‘no’) treatment. Implementation of this assumption was based on long-term DFS results from the POTENT real-world study, a retrospective chart review in patients with resected stage IB‒IIIA EGFRm NSCLC from three Canadian cancer centers [24] and the preference of clinical experts. The POTENT study showed the rate of relapse to markedly decrease at year 4 then to plateau in all treatment groups by year 6. The resulting model implementation is a linear increase from 0% ‘cured’ at year 4 to 95% ‘cured’ at the beginning of year 6 in the active surveillance arm, and a similar transition from 0% ‘cured’ at year 4 to 95% ‘cured’ at the end of year 8 in the osimertinib arm in the DF health state. Patients predicted to be ‘cured’ are assumed to be at no elevated risk of death due to NSCLC.
GPM from Canadian life tables [28] supplemented data sources where extrapolations for the transitions to ‘death’ had lower probability of death from the trial data than the national average (corrected for age and sex), or when patients were assumed to be ‘cured.’ A standardized mortality rate was applied to the GPM hazard to account for additional deaths that may occur due to the higher risk of death among patients in the LRR and DM health states; this was estimated based on excess mortality associated with BRCA mutation in other cancer types [29]. For ‘cured’ patients in the DF health state, GPM without adjustment was considered.
Based on expert opinion, it was assumed that patients who received adjuvant osimertinib could receive retreatment with osimertinib in the 1L DM health state if disease progression took place ≥ 48 months from the start of adjuvant osimertinib treatment. No efficacy data are available for patients retreated with osimertinib in the 1L DM health state, the efficacy is assumed to be the same as for patients receiving osimertinib for the first time. This assumption was validated by clinical experts.
2.6 Perspective
The analysis was conducted from a Canadian Public Healthcare perspective. Model requirements were aligned to Canadian Agency for Drugs and Technologies in Health (CADTH) guidelines [30]. Ontario costs were used in the reference case analysis.
2.7 Health State Utility Values
The reported utility value for osimertinib used in the metastatic setting in patients with stable disease was 0.85 (standard error [SE] 0.027), which had been previously accepted by the Institute National d’Excellence en Santé et en Services Sociaux (INESSS) and the pan-Canadian Oncology Drug Review (pCODR) in the HTA assessment for first-line osimertinib [31]. It was therefore assumed that patients receiving osimertinib in an earlier stage setting must have a utility that is no lower than for the metastatic setting, and thus the utility of 0.85 was used as the reference case value for osimertinib and active surveillance for the DF and LRR states. Disutilities for grade ≥ 3 adverse events (AEs) were applied to the DF state, with no differentiation in AEs assumed in later states. An age-adjustment to the utility in every state was applied [32]. Literature-based health state utility values (HSUVs) for the DF and DM health states were available from Canadian real-world evidence (RWE) [24, 33] and are used as the reference case. Utility data in ADAURA were collected in the Short Form 36 Health Survey Questionnaire (SF-36) and needed to be mapped to the EuroQoL 5-dimensions (EQ-5D) before a Canadian tariff could be applied. Since there was no direct mapping algorithm available from SF-36 to EQ-5D, an intermediate step had to be performed, mapping SF-36 to SF-12. SF-12 was then mapped to EQ-5D-3L answers, on which the Canadian tariff was applied. The many intermediate steps did make the results unreliable and not suited for the reference case. Therefore, the results are only used in scenario analyses. HSUVs are supplied in Supplementary Table S3 (see the electronic supplementary material).
2.8 Healthcare Resource Usage
The model included costs associated with drug acquisition, treatment administration, healthcare resource use, subsequent therapy and AEs. Key costs are shown in Supplementary Table S3 (see the electronic supplementary material). For resource use, in addition to costing inputs by expected utilization and unit costs, the model also allows costing of resource utilization and administration costs through a ‘top-down’ approach using data from a recent Institute for Clinical Evaluative Sciences (ICES) costing analysis of real-world management for patients who were DF, patients with LRR, and metastatic NSCLC in Ontario [31]. In the reference case analysis, costs for drug acquisition (at list price), AE management and EGFR mutation testing were micro-costed, while all other costs were derived from ICES costing studies [34]. Costs and effects were discounted at 1.5% per year, in accordance with CADTH guidelines [30]. Drug costs and other model inputs were obtained from Ontario databases, where possible, and publications. All costs are provided in Canadian dollars (C$). Costs from the past were inflated to 2020 using the Canadian Consumer Price Index.
2.9 Reference Case
The reference case was conducted as a probabilistic analysis to account for uncertainty of the underlying parameter estimates [30]. The choice of distribution was selected based on recommendations of Briggs et al. [35].
When possible, the reported SEs from the data sources, or alternatively standard deviation (SD) or 95% CIs used to calculate an SE, were used to define parameter uncertainty. Otherwise, when not reported, the SE was estimated as 10% of the default value. The probabilistic analyses used 1500 iterations, as this gave less than 1% deviation in the mean ICER.
2.10 Sensitivity Analyses and Scenario Analyses
One-way deterministic sensitivity analysis was performed to identify key model drivers. Parameters were varied one at a time between their upper and lower 95% CIs, which were determined using SEs when available (e.g., for utilities), or using SEs estimated as ± 10% the mean where measures of variance were not available.
Probabilistic scenario analyses were conducted as for the reference case to explore parameter uncertainty and test model robustness.
3 Results
3.1 Reference Case Analysis
The model predicted that patients with completely resected stage IB‒IIIA EGFRm NSCLC who received adjuvant osimertinib treatment would have an increased life expectancy compared with patients with active surveillance. Patients who received adjuvant osimertinib were predicted to spend longer in the DF health state than those in the active surveillance arm, and this was estimated to result in a longer OS. Patients who received placebo and underwent active surveillance were more likely to transition directly to the 1L DM health state, thus leading to a shorter OS compared with patients who received adjuvant osimertinib.
In the reference case, adjuvant osimertinib treatment was predicted to lead to a clinically meaningful gain in QALYs of a mean 11.77 (95% CI 8.58–14.95) QALYs per patient compared with 8.57 (95% CI 7.24–10.05) for active surveillance, that is 3.20 mean additional QALYs per patient (Table 1). Adjuvant osimertinib treatment was predicted to lead to a mean 14.02 (95% CI 10.38–17.65) LYs compared with 10.32 (95% CI 8.78–12.03) for active surveillance. Collectively, adjuvant osimertinib treatment was predicted to nearly double the QALYs and LYs accrued in the DF state. In the DF state, mean 10.31 (95% CI 6.71–14.37) versus 5.97 (95% CI 4.14–7.95) QALYs and mean 12.13 (95% CI 7.89–16.91) versus 7.03 (95% CI 4.88–9.36) LYs were estimated in the osimertinib and the active surveillance arms, respectively. This predicted increase in the proportion of time spent DF led to a modeled median percentage of patients alive at 5 years of 84.1% (95% CI 65.1‒94.6) for osimertinib versus 69.8% (95% CI 61.7‒77.4) for active surveillance. There was an estimated 23.2% absolute improvement in patients alive at 10 years: the modeled median percentage of patients alive at 10 years was 62.5% (95% CI 42.3‒79.3) for osimertinib versus 39.3% (95% CI 30.5‒49.2) for active surveillance. Adjuvant osimertinib was associated with mean added costs of C$114,513 per patient (Table 1). Supplementary Table S10 shows a breakdown per category (see the electronic supplementary material). The resulting probabilistic ICER (incremental cost per QALY) for adjuvant osimertinib versus the active surveillance arm was C$35,811, with 95% CI of 59,565–164,779 for the incremental cost and 95% CI of − 0.28 to 6.79 for the QALY. The deterministic sensitivity analysis showed a similar ICER of C$37,028, indicating the robustness of the model. The importance of the parameters in the model on the ICER are summarized in Fig. 2a.
a Tornado plot of the effect of one-way sensitivity analyses on model parameter uncertainty. b Probabilistic scatterplot of incremental cost-effectiveness ratio between treatment with adjuvant osimertinib and active surveillance. The broken line indicates the willingness-to-pay threshold of C$50,000/QALY. 1L first-line, C$ Canadian dollars, DFS disease-free state, DM distant metastases, QALY quality-adjusted life-year, RDI relative dose intensity
The robustness of the model is also highlighted when comparing modeled DFS and OS rates with RWE; the model-derived DFS and OS rates were validated by real-world DFS and OS rates in patients with EGFRm NSCLC in Ontario (Fig. 3). Modeled placebo arm survival aligned well to real-world data, with an MAPE of 22.5% and an R2 of 0.98. This validation also supports the lifetime horizon as applied within the model. At a willingness-to-pay threshold of C$50,000 per QALY gained [36], adjuvant osimertinib was cost-effective in 66.3% of iterations (Fig. 2b).
3.2 Scenario Analyses
Probabilistic scenario analyses were conducted to explore uncertainty of the model parameters (Table 2). For all scenarios explored, ICERs ranged from C$22,396 to C$64,911. A scenario analysis shortening the time horizon to 15 years resulted in the highest ICER (C$64,911). Allowing no retreatment with osimertinib resulted in the lowest ICER of C$22,396. The ICER was minimally affected by using mapped EQ-5D utilities derived from the ADAURA trial data. Changing the ‘cure’ assumption in the osimertinib arm so that the model transitioned patients without recurrence to ‘cured’ over a shorter time (from year 4 to end of year 7, instead of end of year 8) resulted in a 18% smaller ICER. A scenario without cure (which clinical experts advised was an implausible scenario) led to an ICER of C$61,977, corresponding to a 73% increase. In another ‘cure’ scenario, the standardized mortality ratio was applied to the transitions from DF and LRR to ‘Death,’ leading to an ICER increase of 16%. Scenarios included alternatives for parametric distribution modeling of the transitions between health states. Choosing loglogistic as an alternative curve fit for the transition probability (TP) from DF to LRR (DF → LRR) resulted in an 11% greater ICER; choosing lognormal as an alternative curve fit for DF → LRR resulted in a 7% smaller ICER. Choosing an alternative curve fit for 2L DM → Death minimally impacted the ICER (~ 0.1% lower). Scenario analyses demonstrated that the conclusions from the reference case analysis remained robust in terms of the cost-effectiveness of osimertinib versus active surveillance.
4 Discussion
As in patients unselected for EGFR mutations [8], the rates of disease recurrence remain high in completely resected patients with EGFRm NSCLC following surgery with curative intent [37], and survival outcomes are poor [7, 38]. There has been little innovation in this treatment setting in the past 15 years; chemotherapy was the only adjuvant treatment option to improve DFS after surgery, and its incremental benefit is low [8, 39, 40]. There is a clear unmet need for a targeted, efficacious, and well-tolerated treatment option for patients with EGFRm NSCLC following complete tumor resection. Following the positive DFS findings from the ADAURA trial, osimertinib has been approved as an adjuvant treatment in this disease setting [10, 17, 18]. This economic evaluation based on the Canadian HTA submission indicated that adjuvant osimertinib is predicted to increase life expectancy versus placebo for stage IB–IIIA EGFRm NSCLC, with patients predicted to spend a substantial proportion of time DF, resulting in an estimated 23.2% absolute improvement in OS at 10 years and a mean 3.20 additional QALYs per patient. Additional data cuts with more mature data from the ADAURA trial will inform long-term survival outcomes in the future.
Adjuvant osimertinib treatment was associated with a probabilistic ICER of C$35,811 versus active surveillance. The ICER was most sensitive to clinical assumptions pertaining to the percentage of patients that received treatment upon relapse to metastatic disease and the quality of life of patients in the DF and DM health states. At a willingness-to-pay threshold of C$50,000 per QALY gained, osimertinib was cost-effective in 66.3% of iterations.
In this analysis, the model used five health states to cover the possible clinical outcomes observed after complete resection and adjuvant treatment in EGFRm NSCLC. A key assumption is that patients in the DF state can transition to LRR or distant metastases, with the former state being treated in a curative manner. Another consideration is that the lifetime horizon used was 38 years. CADTH requires a time horizon of lifetime, often defined as the time until < 1% of patients are alive. Shorter time horizons were included in the scenario analyses; the time horizon of 15 years may have resulted in a larger ICER than in the reference case because the full benefits of cure could not be realized within this shortened time horizon.
At the unplanned interim analysis for ADAURA, maturity of the OS data was 4%, so a non-direct method was applied, using CancerLinQ and FLAURA data to extrapolate outcomes to model a lifetime horizon. Methodological best practices were followed for extrapolation and for choosing the most clinically valid distributions. In addition, clinical experts provided validation to confirm that the predicted estimates were plausible and clinically relevant. Survival in the modeled active surveillance arm was well-aligned with real-world data [24]. Model goodness of fit increases as MAPE approaches 0 and R2 approaches 1. A MAPE of 22.5% compared well with established, respected health economic models in other fields [41]. There are challenges around using real-world data rather than rigorously collected data from clinical trials; however, the CancerLinQ cohort had comparable patient demographics to the ADAURA trial, including the proportion of patients at each disease stage, with a limitation regarding the index date being diagnosis compared with post-surgery within the ADAURA trial. Besides OS data, there remains uncertainty regarding the current DFS data, more specifically on the impact of treatment cessation on DFS. Several scenario analyses were conducted with alternative parametric survival functions to account for the uncertainty regarding extrapolation of survival outcomes. The results of those scenario analyses showed that adjuvant osimertinib treatment consistently provided increased QALYs and LYs versus active surveillance following complete resection. A scenario with the risk of relapse for the osimertinib arm was set equal to the risk of relapse for the placebo arm (treatment arm) after year 3, but had no significant impact on the ICER. Collectively, scenario analyses have highlighted the model's robustness and demonstrated that osimertinib remained cost-effective across most scenarios. As well as the need to use other sources to extrapolate OS data, health-related quality of life assessment in the ADAURA trial did not readily support the creation of HSUVs for Canada. This was because the trial took place in many other countries; thus, HSUVs were assumed from real-world data from Canada [33, 42].
Another limitation is the validation of the cure assumption, as the long-term cure rate for both trial arms remains uncertain. The definition of cure in resected EGFRm NSCLC, is as yet unclear. While patients were deemed ‘cured’ after being DF for 5 years after the end of treatment, thus 8 years in the osimertinib arm compared with 5 years in the active surveillance arm, by beginning to transition patients in both arms to cure at 4 years, we ensured that a proportion of patients in the model were cured at 5 years. The time it took to transition patients to ‘cure’ was based on RWE that showed DFS plateauing in resected EGFRm NSCLC retrospective cohorts, and validated by the clinical experts, thus minimizing this limitation.
It should be noted that in the Canadian HTA submission, CADTH made several changes to the model and reanalyzed the data. The reanalysis resulted in a substantially higher ICER of C$328,026 for adjuvant osimertinib versus active surveillance [36]. The key difference between the CADTH reanalysis and our model was the survival distribution used to model TPs for DF → LRR and DF → 1L DM for osimertinib: Gompertz by CADTH [36] and generalized gamma by our model. TPs for DF → LRR and DF → 1L DM are critical parameters of the model; selection of the Gompertz curve assumes near complete loss of benefit within 2 years of completing adjuvant treatment (Supplementary Figures S1A and S2A, see the electronic supplementary material). While data post-discontinuation of adjuvant osimertinib remain immature, the Gompertz curve in the CADTH reanalysis assumed a 15-times increase in rate of relapse after year 3, which is not supported by current evidence from the clinical trial. CADTH opted not to model indefinite benefit in the reference case. CADTH assumed that there was no DFS benefit from osimertinib by year 5; the updated ADAURA data with 2 years additional follow-up from the data published in 2020 highlighted a sustained DFS benefit, as the DFS HR was 0.27 (95% CI 0.21–0.34) for patients with stage IB–IIIA EGFRm NSCLC [43]. The model used for the CADTH submission has been submitted in England, Scotland, the Netherlands, and Canada to support HTAs by the National Institute for Health and Care Excellence, Scottish Medicines Consortium, Zorginstituut Nederland, and INESSS, respectively, for whom the selected TPs aligned more closely with the reference case TPs [36, 44,45,46]. In addition, ADAURA data have been in other independent cost-effectiveness models of adjuvant osimertinib, published in a peer-reviewed manuscript [47] as well as presented at congress [48]. The models produced different ICERs due to different structures without the potential for cure, shorter time horizons, different retreatment assumptions, and extrapolation of OS data assuming 5% benefit. The methods employed in our analysis are robust and aligned to HTA and academic expectations, and as noted above, the modeled OS data aligned well with real-world data.
Osimertinib is a highly efficacious and well-tolerated treatment that can play a role in an adjuvant treatment regimen with a potentially curative intent [10]. Osimertinib for patients with completely resected EGFRm NSCLC represents a paradigm shift to patients and healthcare providers in a disease area with significant unmet needs. Further to the important clinical benefits of osimertinib to patients, adjuvant osimertinib was found to be a cost-effective treatment when compared with established clinical management, reporting an ICER of C$35,811 per QALY versus active surveillance, with a 95% CI of 59,565–164,779 for the incremental cost and a 95% CI of − 0.28 to 6.79 for the QALY.
References
Cagle PT, Allen TC, Olsen RJ. Lung cancer biomarkers: present status and future developments. Arch Pathol Lab Med. 2013;137(9):1191–8.
Datta D, Lahiri B. Preoperative evaluation of patients undergoing lung resection surgery. Chest. 2003;123(6):2096–103.
Le Chevalier T. Adjuvant chemotherapy for resectable non-small-cell lung cancer: where is it going? Ann Oncol. 2010;21(suppl 7):vii196–8.
Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early-stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical practice guidelines. Ann Oncol. 2017;28(suppl_4):iv1–21.
Kris MG, Gaspar LE, Chaft JE, Kennedy EB, Azzoli CG, Ellis PM, et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline update. J Clin Oncol. 2017;35(25):2960–74.
Remon J, Soria J-C, Peters S, Committee obotEG. Early and locally advanced non-small-cell lung cancer: an update of the ESMO clinical practice guidelines focusing on diagnosis, staging, systemic and local therapy. 2021 01 September [cited 2021 7 September]. https://www.esmo.org/guidelines/lung-and-chest-tumours/early-stage-and-locally-advanced-non-metastatic-non-small-cell-lung-cancer/eupdate-early-and-locally-advanced-non-small-cell-lung-cancer-nsclc-treatment-recommendations2. Accessed 7 Sep 2021.
Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51.
Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–9.
Consonni D, Pierobon M, Gail MH, Rubagotti M, Rotunno M, Goldstein A, et al. Lung cancer prognosis before and after recurrence in a population-based setting. J Natl Cancer Inst. 2015;107(6):djv059.
Wu Y-L, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383(18):1711–23.
Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50.
Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(33):3290–7.
Wu YL, Ahn MJ, Garassino MC, Han JY, Katakami N, Kim HR, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol. 2018;36(26):2702–9.
Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046–61.
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–40.
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–25.
U.S. Food and Drug Administration. TAGRISSO® (osimertinib). Highlights of Prescribing Information. 2020 01 December [cited 2022 18 May]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf. Accessed 18 May 2022.
European Medicines Agency. TAGRISSO® (osimertinib). Summary of Product Characteristics. 2021 01 July [cited 2021 08 October]. https://www.ema.europa.eu/en/documents/product-information/tagrisso-epar-product-information_en.pdf. Accessed 8 Oct 2021.
AstraZeneca Canada Inc. TAGRISSO® (osimertinib). 40 mg and 80 mg osimertinib as osimertinib mesylate. Product Monograph. 2021 15 January [cited 2021 08 October]. https://pdf.hres.ca/dpd_pm/00059644.PDF. Accessed 8 Oct 2021.
Potter D, Brothers R, Kolacevski A, Koskimaki JE, McNutt A, Miller RS, et al. Development of CancerLinQ, a health information learning platform from multiple electronic health record systems to support improved quality of care. JCO Clin Cancer Inform. 2020;4:929–37.
ClinicalTrials.gov. NCT03653546. 08 July 2022 [cited 2022 11 February]; AZD3759 versus gefitinib and erlotinib, phase 3 trial]. https://clinicaltrials.gov/ct2/show/NCT03653546. Accessed 11 Feb 2022.
American Society of Clinical Oncology. The CancerLinQ database. 2021 [cited 2020 08 October]. www.cancerlinq.org/. Accessed 8 Oct 2021.
Holleman MS, van Tinteren H, Groen HJ, Al MJ, Uyl-de Groot CA. First-line tyrosine kinase inhibitors in EGFR mutation-positive non-small-cell lung cancer: a network meta-analysis. Onco Targets Ther. 2019;12:1413–21.
Kuruvilla MS, Liu G, Syed I, Gwadry-Sridhar F, Sheffield BS, Sachdeva R, et al. EGFR mutation prevalence, real-world treatment patterns, and outcomes among patients with resected, early-stage, non-small cell lung cancer in Canada. Lung Cancer. 2022;173:58–66.
Williams C, Lewsey JD, Mackay DF, Briggs AH. Estimation of survival probabilities for use in cost-effectiveness analyses: a comparison of a multi-state modeling survival analysis approach with partitioned survival and Markov decision-analytic modeling. Med Decis Mak Int J Soc Med Decis Mak. 2017;37(4):427–39.
Latimer N. NICE DSU Technical Support Document 14: survival analysis for economic evaluations alongside clinical trials—extrapolation with patient-level data: NICE Decision Support Unit; 2013. March 2013. Report No.: TSD 14.
Rutherford MJ, Lambert PC, Sweeting MJ, Pennington B, Crowther MJ, Abrams KR, et al. NICE DSU technical support document 21: flexible methods for survival analysis: NICE Decision Support Unit; 2020. January 2020. Report No.: TSD 21.
Statistics Canada. Life Tables, Canada, Provinces and Territories, 1980/1982 to 2016/2018. Stat Can: https://www150.statcan.gc.ca/n1/en/catalogue/84-537-X2019002; 2020 28 January 2020. Report No.: 2019002 (Issue Number) and Tables: 84-537-X2019002.
Mai PL, Chatterjee N, Hartge P, Tucker M, Brody L, Struewing JP, et al. Potential excess mortality in BRCA1/2 mutation carriers beyond breast, ovarian, prostate, and pancreatic cancers, and melanoma. PLoS ONE. 2009;4(3): e4812.
The Canadian Agency for Drugs and Technologies in Health (CADTH). Guidelines for the economic evaluation of health technologies: Canada. 2017 July [cited 2021 08 October]. https://cadth.ca/guidelines-economic-evaluation-health-technologies-canada. Accessed 8 Oct 2021.
Pan-Canadian Oncology Drug Review. Osimertinib Reviewer Report. Canada's Drug and Health Technology Agency.
Ara R, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health J Int Soc Pharmacoecon Outcomes Res. 2011;14(4):539–45.
Jiang SX, Walton RN, Hueniken K, Baek J, McCartney A, Labbé C, et al. Real-world health utility scores and toxicities to tyrosine kinase inhibitors in epidermal growth factor receptor mutated advanced non-small cell lung cancer. Cancer Med. 2019;8(18):7542–55.
Seung SJ, Hurry M, Hassan S, Walton RN, Evans WK. Cost-of-illness study for non-small-cell lung cancer using real-world data. Curr Oncol. 2019;26(2):102–7.
Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. New York: Oxford University Press; 2006.
Canadian Journal of Health Technologies (CADTH). CADTH reimbursement review osimertinib (TAGRISSO®). 2022 04 March [cited 2022 21 March] . https://www.cadth.ca/sites/default/files/DRR/2022/PC0246-Tagrisso.pdf. Accessed 21 Mar 2022.
Zhong WZ, Wang Q, Mao WM, Xu ST, Wu L, Shen Y, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1–N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19(1):139–48.
Zhong WZ, Wang Q, Mao WM, Xu ST, Wu L, Wei YC, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1–N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 phase III trial. J Clin Oncol. 2021;39(7):713–22.
Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le Chevalier T, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010;375(9722):1267–77.
Artal Cortes A, Calera Urquizu L, Hernando CJ. Adjuvant chemotherapy in non-small cell lung cancer: state-of-the-art. Transl Lung Cancer Res. 2015;4(2):191–7.
McEwan P, Ward T, Bennett H, Bergenheim K. Validation of the UKPDS 82 risk equations within the Cardiff diabetes model. Cost Eff Resour Alloc. 2015;13(1):12.
Hueniken K, Hurry M, Jiang S, Labbe C, Brown MC, Eng L, et al. PRO-CTCAE toxicities in advanced NSCLC patients with EGFR mutations: a real world assessment. In: World lung cancer conference; 2018; Toronto, Canada: J Thorac Oncol; 2018. p. S586.
Herbst RS, Wu YL, John T, Grohe C, Majem M, Wang J, et al. Adjuvant osimertinib for resected EGFR-mutated stage IB-IIIA non-small-cell lung cancer: updated results from the phase III randomized ADAURA trial. J Clin Oncol. 2023;JCO2202186. https://doi.org/10.1200/JCO.22.02186.
National Institute for Health and Care Excellence. Osimertinib for adjuvant treatment of EGFR mutation-positive non-small-cell lung cancer after complete tumour resection. Technology appraisal guidance [TA761]. 2022 19 January [cited 2022 9 June]. https://www.nice.org.uk/guidance/ta761. Accessed 9 Jun 2022.
Scottish Medicines Consortium. Osimertinib 40 mg and 80 mg film-coated tablets (TAGRISSO®). 2021 8 November [cited 2022 9 June]. https://www.scottishmedicines.org.uk/medicines-advice/osimertinib-tagrisso-full-smc2383/. Accessed 9 Jun 2022.
Zorginstituut Nederland. Osimertinib (TAGRISSO®) als adjuvante behandeling na volledige tumorresectie bij volwassenen met stadium IB-IIIA NSCLC met EGFR-mutaties. 2021 15 November [cited 2022 9 June]. https://www.zorginstituutnederland.nl/binaries/zinl/documenten/adviezen/2021/11/15/pakketadvies-osimertinib-tagrisso-bij-niet-kleincellige-longkanker-nsclc/Brief+aan+staatssecretaris+van+Volksgezondheid+Welzijn+en+Sport+betreft+pakketadvies+osimertinib+%28Tagrisso%29.pdf. Accessed 9 Jun 2022.
Lemmon CA, Zabor EC, Pennell NA. Modeling the cost-effectiveness of adjuvant osimertinib for patients with Resected EGFR-mutant non-small cell lung cancer. Oncologist. 2022;27(5):407–13.
Choi B, Alrawashdh N, McBride A, Abraham I. Cost evaluation of adjunctive osimertinib use in resected epidermal growth factor receptor-positive non-small cell lung cancer. In: ASCO annual meeting 2021; 2021; Virtual: J Clin Oncol. p. 8525.
Acknowledgements
Thanks to all the patients and their families. The study was funded by AstraZeneca. The authors would like to acknowledge Matthew Dyer, formerly of AstraZeneca, for his contribution to the analysis. They would also like to acknowledge Sally Cotterill, PhD, CMPP, of Ashfield MedComms, Macclesfield, UK, an Inizio Company, for technical editing support that was funded by AstraZeneca in accordance with Good Publications Practice (GPP) guidelines (https://www.ismpp.org/gpp-2022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was funded by AstraZeneca. The sponsor was involved in the study design; in the collection, analysis, and interpretation of data; and in the writing of the report. Draft 1 was written by the first and last authors. Technical editing support was funded by AstraZeneca in accordance with Good Publications Practice (GPP) guidelines (https://www.ismpp.org/gpp-2022).
Conflict of interest
Andre Verhoek reports funding from AstraZeneca to his employers (Cytel) for development of the model for the submitted work. Parneet Cheema reports consulting fees from AstraZeneca and Roche; honoraria from AstraZeneca, Bristol Myers Squibb, and Merck; advisory boards or committees for Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Janssen, Merck, Novartis, Pfizer, Roche, Sanofi, and Takeda; invited speaker activities for AstraZeneca, Bristol Myers Squibb, and Merck; and advisory roles for Amgen, AstraZeneca, Bristol Myers Squibb, EMD Serono, Merck, Novartis, Pfizer, and Roche. Barbara Melosky declares no competing interests. Benoit Samson reports advisory boards or committees for AstraZeneca, Bristol Myers Squibb, Merck, Pfizer, and Takeda. Frances A. Shepherd reports stock options from AstraZeneca. Filippo de Marinis reports consulting fees from AstraZeneca, Bristol Myers Squibb, MSD, and Roche AG, and advisory boards or committees for AstraZeneca, Bristol Myers Squibb, MSD, and Roche AG. Thomas John reports consulting fees from AstraZeneca, Bristol Myers Squibb, Ignyta, Merck & Co., MSD, Pfizer Inc., Roche AG, Specialised Therapeutics, and Takeda Pharmaceutical, and advisory boards and committees for AstraZeneca, Bristol Myers Squibb, Ignyta, Merck & Co., MSD, Pfizer Inc., Roche AG, Specialised Therapeutics, and Takeda Pharmaceutical, outside the submitted work. Yi-Long Wu reports grants or contracts from AstraZeneca, Beigen, Boehringer Ingelheim, Bristol Myers Squibb, Hengrui, and Roche; advisory boards or committees for AstraZeneca, Boehringer Ingelheim, Novartis, and Takeda; and honoraria received from AstraZeneca, Beigen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, MSD, Pfizer, Roche, and Sanofi, outside the submitted work. Bart Heeg reports funding from AstraZeneca to his employers (Cytel) for development of the model for the submitted work. Nadia Van Dalfsen reports funding from AstraZeneca to her employers (Cytel) for development of the model for the submitted work. Benjamin Bracke reports employment and holds stock options from AstraZeneca. Miguel Miranda reports employment and holds stock from AstraZeneca, outside the submitted work. Simon Shaw reports employment and holds stocks from AstraZeneca. Daniel Moldaver reports employment from AstraZeneca and holds stock/stock options from AstraZeneca, outside the submitted work.
Availability of data and material
Provision of standard data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. The code for the model is not available publicly.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
CRediT authorship contribution statement
Conceptualization: AV, BM, FdM, Y-LW, BH, NVD, SS, DM. Methodology: AV, BH, NVD, BB, MM, SS, DM. Software: AV, NVD, DM. Validation: PC, BS, BH, BB, MM, DM. Formal analysis: AV, BM, FdM, BH, NVD, BB, DM. Investigation: BM, DM. Resources: FAS, DM. Data curation: FdM, BH, DM. Writing—original draft: AV, DM. Writing—reviewing and editing: AV, PC, BM, BS, FAS, FdM, TJ, Y-LW, BH, NVD, BB, MM, DM. Visualization: AV, BM, DM. Supervision: BM, TJ, BH, BB, SS, DM. Project administration: SS. Funding acquisition: SS.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Verhoek, A., Cheema, P., Melosky, B. et al. Evaluation of Cost-Effectiveness of Adjuvant Osimertinib in Patients with Resected EGFR Mutation-Positive Non-small Cell Lung Cancer. PharmacoEconomics Open 7, 455–467 (2023). https://doi.org/10.1007/s41669-023-00396-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-023-00396-0