Abstract
Objective
The aim of this study was to evaluate the attributable patient cost of antimicrobial resistance (AMR) in Ghana to provide empirical evidence to make a case for improved AMR preventive strategies in hospitals and the general population.
Methods
A prospective parallel cohort design in which participants were enrolled at the time of hospital admission and remained until 30 days after the diagnosis of bacteraemia or discharge from the hospital/death. Patients were matched on age group (± 5 years the age of AMR patients), treatment ward, sex, and bacteraemia type. The AMR cohort included all inpatients with a positive blood culture of Escherichia coli or Klebsiella spp., resistant to third-generation cephalosporins (3GC), or methicillin-resistant Staphylococcus aureus (MRSA). We matched the AMR cohort (n = 404) with two control arms, i.e., patients with the same bacterial infections susceptible to 3GC or S. aureus that was methicillin-susceptible (susceptible cohort; n = 152), and uninfected patients (uninfected cohort; n = 404). Settings were Korle-Bu and Komfo Anokye Teaching Hospitals, Ghana. The outcome measures were the length of hospital stay (LOS) and the associated patient costs. Outcomes were evaluated from the patient perspective.
Results
From a total of 5752 blood cultures screened, 1836 participants had growth in blood culture, of which, based on our inclusion criteria, 426 were enrolled into the AMR cohort; however, only 404 completed the follow-up and were matched with participants in the two control cohorts. Patients in the AMR cohort stayed approximately 5 more days (95% confidence interval [CI] 4.0–6.0) and 8 more days (95% CI 7.2–8.6) compared with the susceptible and uninfected cohorts, respectively. The mean extra patient cost due to AMR relative to the susceptible cohort was US$1300 (95% CI 1018–1370), of which about 30% resulted from productivity loss due to presenteeism and absenteeism from work. Overall, the estimated annual patient cost due to AMR translates to about US$1 million and US$1.4 million when compared with the susceptible and uninfected cohorts, respectively.
Conclusion
We have shown that AMR is associated with a significant excess LOS and patient costs in Ghana using prospective data from two public tertiary hospitals. This calls for infection prevention and control strategies aimed at mitigating the prevalence of AMR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Antimicrobial resistance (AMR) associates significantly with patients’ length of hospital stay and costs in Ghana. |
The extra patient cost due to AMR was equivalent to patients’ mean household consumption expenditure, about 70% of which was related to the cost of hospitalization and treatment. |
The result provides a good case for investment in AMR interventions by the government and others interested in reversing the spread and economic impact of AMR. |
1 Introduction
Antimicrobial resistance (AMR) is a growing phenomenon that has been declared by the World Health Organization (WHO) as one of the top 10 global public health threats facing humanity [1]. It results from the ability of microbial pathogens to withstand the effects of antimicrobial drugs used to treat infections in humans and animals [2, 3]. Studies show that the emergence and spread of AMR know no boundaries, but notable disparities in the associated morbidity and mortality exist across regions [4,5,6,7]. Recent AMR surveillance in Europe shows that the number of AMR cases in the region is high [8]. However, recent modelling of the global impact of AMR suggests that low- and middle-income countries (LMICs) and West Africa, in particular, risk suffering AMR’s largest health and economic impact [9]. Thus, AMR has the potential to drive economic inequality among populations. Compared with high-income countries (HICs), the case fatality rate of bloodstream infections (BSIs) and the associated AMR in LMIC settings is up to 15 times higher among hospitalized patients but may be underestimated due to data limitations and methodological inaccuracies [5, 9,10,11,12].
Beyond the worrying health implication of AMR is the direct and indirect economic burden it imposes on patients, healthcare providers, and society, such as the extra cost of care due to the associated prolonged length of hospital stay (LOS) [13, 14]. Patients infected with resistant microorganisms may need more expensive treatment [15, 16]. The direct costs may include laboratory tests, medicine, medical consultation, and post-discharge reviews, which, depending on the healthcare system, may be more or less co-financed by the patient [17]. In HICs such as Europe, where clearer estimates of the societal burden of AMR exist, the annual direct patient cost of AMR is thought to amount to €900 million [18, 19]. Depending on the causative bacteria, study perspective, geographic location, and the cost items included, the direct patient cost of AMR may vary significantly [19,20,21]. For example, the estimated excess direct medical cost per AMR patient at a University Hospital in South Carolina was US$229, corresponding to an extra 5 days LOS [22]. In a similar study in the US, the mean extra direct patient cost associated with methicillin-resistant Staphylococcus aureus (MRSA) BSIs was US$1275 compared with a susceptible cohort [23].
Adding to the direct patient costs, are the indirect patient costs of AMR, measured as the cost of reduced productivity at work, mainly due to absenteeism or lost working days associated with the LOS, and also from presenteeism defined as attending to work at reduced working capacity. The latter implies that even though a person may be able to resume work post-discharge, they may not be able to work as effectively as when in full health. A 2015 study on BSI caused by MRSA and third-generation cephalosporin (3GC)-resistant Escherichia coli that involved 1293 hospitals from 31 countries in the European region found a total of 375,748 excess bed days translating into lost productive days [15]. Again, a global assessment by Prestinaci and colleagues [19] reports that up to 40% of an estimated €1.5 billion and 64% of US$55 billion of the patient cost of AMR in Europe and the US, respectively, are due to productivity losses resulting from deaths and absence from work by patients and their caregivers.
In resource-limited settings such as Ghana, the patient cost of AMR may be catastrophic, especially because patients tend to pay up to two-thirds or more of the direct cost of care, even if they have valid national health insurance [24]. Furthermore, the capacity of hospitals to admit patients for emergency and critical care may be compromised due to prolonged LOS caused by AMR [14, 22]. Evidence exists that AMR is a problem in Ghana, and this may be driven in part by inappropriate antibiotic use. A recent global study named Ghana as one of five countries where misuse of antibiotics was at risk of a disproportionate increase if no action is taken to reverse inappropriate antibiotic use [25]. As income levels in Ghana grow, it may stimulate increased use of health services, including wider use of medications such as antibiotics, leading to a further rise in the development and spread of AMR. These concerns coupled with evidence of hospital-acquired BSI and the associated AMR impacts in Ghana [26, 27] motivated us to undertake this study.
There is an increasing need to evaluate the patient cost of AMR, especially in LMICs where little data exist to drive policy and investment into AMR prevention and control [2]. Therefore, we aimed to evaluate the patient cost of AMR in Ghana using prospective data derived from AMR surveillance. From established knowledge [14, 15], we aimed to answer two research questions: (1) Do inpatients with infections caused by AMR organisms stay longer in hospitals compared with patients with infections caused by susceptible organisms and similar but uninfected patients? (2) Is there a significant difference in direct and indirect patient costs between AMR patients and patients with susceptible AMR infections and uninfected patients?
Our study focused on BSI caused by MRSA and 3GC-resistant E. coli or Klebsiella spp. These are important indicator-resistance phenotypes and 3GC are among the WATCH and RESERVED group of antibiotics that, according to WHO, are not supposed to be easily accessible but are nevertheless commonly used [28,29,30]. In Ghana, the empirical treatment initiated in patients suspected of BSI is not effective against these organisms and treatment has to be changed in response to the results of culture and susceptibility testing that are usually available after 3 days. Adding to this, appropriate treatment may be further delayed as patients or relatives will need to raise funds to afford effective treatment that is not covered by national health insurance.
2 Methodology
2.1 Design
We conducted a prospective, parallel cohort study and undertook a cost evaluation to assess AMR-attributable costs from the patient perspective. Participants entered the cohort at hospital admission and remained until 30 days after a positive blood culture or discharge from the hospital/death, whichever was sooner. The study received ethics approval from the Institutional Review Boards of the Komfo Anokye Teaching Hospital (KATH) and the Korle-Bu Teaching Hospital (KBTH) with reference numbers KATH-IRB/AP/030/21 and KBTH/MD/93/21, respectively. For quality reporting and methodological transparency, we report according to the relevant sections of the Consolidated Health Economic Evaluation Reporting Standard (CHEERS) checklist [31]. Again, we crosschecked our design and sampling with the Cochrane tool for assessing the risk of bias in cohort studies [32].
2.2 Setting
This study was conducted at the KATH and the KBTH in Ghana, a lower- to middle-income nation in West Africa. KATH is a 1200-bed capacity tertiary hospital located in the Ashanti Regional capital in Kumasi, while KBTH is a 2000-bed capacity hospital located in the Greater Accra Region, the national capital. Both hospitals have central microbiology laboratories capable of processing an average of 4000 blood cultures yearly, with a daily inpatient admission of 110 at KATH and 250 at KBTH [33, 34].
2.3 Population and Sampling
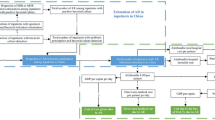
Our study source population included inpatients of all ages with suspected BSI who had blood cultures performed at the central microbiology laboratories of the participating hospitals during the participant recruitment period of June–December 2021 (N = 5752). A subgroup of the study population with positive blood cultures of E. coli or Klebsiella spp., resistant to 3GC, or with MRSA, constituted the AMR cohort (n = 426). Excluded from the analysis were patients lost to follow-up with incomplete data or who declined participation, thus bringing the total sample of AMR patients studied to 404. We determined a required sample size of 400 (95% confidence interval [CI] 322–478) for the AMR cohort based on a 50% population proportion at a 5% error margin and 95% CI (z-score 1.96). For comparison of cost, we matched the 404 participants in the AMR cohort with two control arms, i.e., patients with the same bacterial infections non-resistant to 3GC or S. aureus that were methicillin-susceptible (susceptible cohort) and uninfected patients (uninfected cohort). Figure 1 summarizes the sample selection and matching process. Briefly, for each new AMR patient included, we sought out one susceptible and one uninfected patient based on the same age group (±5 years), same admission ward, same sex, and same bacteraemia type for susceptible AMR patients.
Flow chart of sample selection for the study. For standard operation procedure, we screened patients in the AMR cohort using third-generation cephalosporins (3GC) to identify resistance in Klebsiella spp. and E. coli bacterial infection. The 3GC used for the screening includes cefotaxime, ceftriaxone, and ceftazidime. For Staphylococcus aureus bacterial infections, Cefoxitin was used to screen for methicillin-resistant Staphylococcus aureus (MRSA)
2.3.1 Data collection
Data were collected from eligible patients after they provided written informed consent. If the patient was aged < 18 years, we collected the data from their parents or guardians responsible for paying their hospital bills. Patients included in the study were followed up on days 3, 12, 21, and 30, while post-discharge follow-up was through a phone call. Missing and incomplete data that resulted from AMR and susceptible patients lost to follow-up were excluded from the cost analysis. Among uninfected cohorts, no patient was lost to follow-up.
Data were collected in person by trained hospital staff (intern nurses) using a computer-assisted personal interviewing (CAPI) tool embedded with a validated data collection protocol design with CS Pro version 7.6.0 software. The data collection protocol comprised 46 closed and open-ended questions divided into two modules. Module one elicited data on participants’ demographic characteristics (age and sex), residential location, primary admission diagnosis, ward of admission, comorbidity/severity of illness, LOS, and hospital resource use, which included staff time consumed by inpatients available in the patient hospital folder, measured in minutes. Data on the severity of underlying illness was collected using the McCabe score (0 = normal life expectancy; 1 = ultimately fatal life expectancy < 5 years; and 2 = rapidly fatal life expectancy < 1 year). Thus, a score of 1 or 2 meant a patient had a severe underlying illness. Module two includes questions on employment and productivity loss, health insurance, source of funds for payment of patient hospital bills, household consumption expenditure, and direct and indirect medical expenses before and after discharge. Data on direct medical costs were derived from itemized official patient invoices and receipts at discharge. The unit cost of drugs was confirmed by the Head of the Pharmacy at the participating hospitals. As we limited the analysis to the patient perspective, productivity loss included that of the patient, or that of caregivers if the patient was a minor or below age 18 years, requiring the presence of an adult caregiver (parent or relative) throughout the duration of hospital admission.
2.4 Outcomes
The primary outcomes were the extra LOS and the associated patient costs due to AMR; thus, outcomes were evaluated from the patient perspective.
2.4.1 Measurement of Outcomes
We measured LOS as the sum of inpatient days, adjusted for a hospital stay before a diagnosis of bacteraemia to avoid lead-time bias that may contribute to the overestimation of LOS [35,36,37]. For direct patient cost and productivity loss, we used a standard approach for a precise estimate of all patient-related expenses [38,39,40], which involves an activity-based micro-costing known as the precision costing method. The direct patient cost for each patient comprised pharmaceuticals, medical consultation, laboratory and diagnostic tests, accommodation, and feeding. For each direct cost component, we measured the quantities used and multiplied by the unit costs (Eq. 1). Productivity losses (indirect patient cost) for each patient were measured as the sum of the cost of reduced productivity due to absenteeism from work and presenteeism at work (Eq. 2)
where items consumed are constituted by the list of resources used per patient, including pharmaceuticals, laboratory tests, medical consultation, accommodation and feeding.
where GDInc represents gross daily income estimated as the reported gross monthly income divided by the number of working days in a month for the ith patient, L equals the number of lost working days in the last 30 days, CRP was calculated as the absolute monetary value of daily reduced productivity at work for the ith patient, and N is the number of days worked by the ith patient within the last 30 days, which is the duration of the follow-up. We limited the cost horizon to 30 days because epidemiological data on the study population and other studies suggest the LOS for patients with BSI plus AMR is within 30 days [38, 41,42,43].
2.5 Statistical Analysis
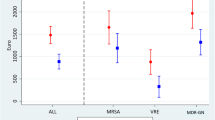
We obtained and analysed descriptive statistical profiles on 13 sociodemographic variables characterizing participants in each cohort. Second, we evaluated the hypothesis of a significant difference in LOS between cohorts using t-test statistics accompanied by 95% CIs of the mean estimates, preceded by an assumption of normality check presented in a graph. Again, we performed a negative binomial regression (NBR) analysis to evaluate whether AMR significantly associates with LOS and a marginal effect estimate of the extra days spent by AMR cohorts considering the assumption of overdispersion in the dataset [44, 45].
For the direct patient costs, we performed a disaggregated analysis of the direct and indirect medical expenses for each cohort and presented the estimated mean and 95% CI for each category. To characterize heterogeneity and distributional effects, we stratified the direct patient cost by bacteraemia type and study site. As established elsewhere [46, 47], we assume comorbidity and the associated severity of illness among patients with BSI and AMR may affect study outcomes. We therefore adjusted for the severity of an underlying disease by stratifying the extra direct patient cost due to AMR for patients without the severity of illness. The same approach was used to analyse the cost of productivity loss between cohorts. Additionally, a generalized linear model (GLM) with NBR as a link function was used to analyse the association between patient background characteristics and the extra costs due to AMR. We calculated all the costs in Ghanaian cedi (local currency) and converted it to 2021 purchasing power parity (PPP) in international US dollars (US$) using a standard web-based PPP converter [48]. The statistical analysis was performed in STATA version 14.0 (StataCorp. LLC, College Station, TX, USA), while graphs were extracted from Microsoft Excel (Microsoft Corporation, Armonk, NY, USA).
3 Results
3.1 Description of Participants
We recruited 426 patients in the AMR cohort from 5752 blood cultures performed between June and December 2021, which represents 7.4% (95% CI 6.8–8.1%) of eligible patients among those who had blood cultures performed, and 23.2% (95% CI 21.4–25.3) among those with BSI (n = 1836). Of those recruited, we lost 4.2% (18 patients) to follow-up and four refused consents, representing a 94.8% completion rate (AMR cohort, n = 404). We matched participants in the AMR cohort to 152 and 404 patients in the susceptible and uninfected cohorts, respectively, thus bringing the total number of participants studied to 960. The reason for the low number of patients in the susceptible cohort was the high prevalence of AMR; thus, it was not possible to find suitable matching susceptible controls for more than half the patients in the AMR cohort.
The percentage of female participants were approximately 52%. Furthermore, 46%, mainly adults, were admitted to a medical ward. Approximately 88% of the participants possessed active health insurance meant to offset part of their medical bills. The three cohorts had similar distributions of age, bacterial isolates, treatment ward, hospital, region of residence, and insurance status.
Mortality, measured as the proportion of patients who died during the study, was higher in the AMR cohort (15.4%, 95% CI11.9–18.9) compared with the susceptible cohort (5.3%, 95% CI 1.7–8.9), and the uninfected cohort (2.5%, 95% CI 2.3–4.0). Twelve patients in the AMR cohort died after discharge, while 50 patients died before discharge. We identified comorbidity in more than 90% of participants in the AMR and susceptible cohorts, with a McCabe score for severity of underlying illness of 25% and 22%, respectively. The composition of the AMR cohorts, in terms of bacterial types, was very similar (Table 1).
3.2 Hospital Resource Used
For all the measurable indicators of hospital resource use, patients in the AMR cohort used more resources than those in the susceptible cohort, who in turn used more resources than those in the uninfected cohort. For example, participants in the AMR cohort stayed approximately 5 days (95% CI 3.9–5.9) and 8 days (95% CI 7.2–8.6) longer in the hospital compared with their counterparts in the susceptible and uninfected cohorts, respectively (Table 2). The LOS distribution in the groups assumed a normal distribution (Fig. 2). Among the selected group of patients infected with AMR organisms of interest, the NBR analysis (electronic supplementary material [ESM] Tables S1 and S2) showed a positive association between AMR and LOS (p < 0.01). Likewise, the marginal effect estimates of the true LOS due to AMR after controlling for overdispersion in the datasets was 5.1 days (95% CI 4.1–6.0) compared with the susceptible cohort. The extra LOS was lower for admissions to the surgical ward (2.3 days; p = 0.05) and AMR caused by Klebsiella spp. (1.3 days; p = 0.04). Of interest to this study and rarely reported is the estimated difference in doctor’s time consumed by patients, measured in minutes, while on admission. We found staff time used by AMR patients to be significantly different compared with the remaining two cohorts (p < 0.01). For example, the AMR cohort consumed 317 (95% CI 201–433) and 529 (95% CI 457–601) more minutes of hospital staff time than the susceptible and uninfected cohorts, respectively.
3.3 Unit Costs
Table 3 presents the unit cost of frequently accessed laboratory tests, available antibiotics, accommodation, and feeding as relevant to the hospital settings. In the laboratory test category, a charge per service ranges from US$15.2 to US$52. The least- and highest-priced antibiotics in supply were doxycycline (100 mg; US$0.1–US$0.3) and Merrem/meropenem (1 g; US$70–US$211), respectively. The feeding cost per day was the same for all inpatients, but the accommodation fee varied by preference.
3.4 Direct Patient Cost of Antimicrobial Resistance (AMR)
Overall, AMR patients recorded a higher mean direct patient cost of US$3676 (95% CI US$3448–US$3904) compared with the susceptible cohort (US$2759, 95% CI US$2615–US$2903) and the uninfected cohort (US$2390, 95% CI US$2313–US$2467) (Table 4). The estimated difference in the mean direct patient costs of US$917 (95% CI US$536–US$1298) between the AMR and susceptible cohorts was significant (p < 0.01). Direct medical costs accounted for 84.8% of the cost per AMR patient, and was similar for the remaining two cohorts. Furthermore, AMR patients had 31.6% of the direct cost covered by health insurance compared with 34.9% and 35.9% for the susceptible and uninfected cohorts, respectively. We observed that the post-discharge review cost was high for the susceptible and uninfected cohorts because they were discharged early and had more time for outpatient review of illness after discharge before exiting the study (Table 4).
The stratified results show that the direct patient costs were much higher at KBTH in general than at KATH. Between cohorts, we observed a noticeable 7.5% increase in direct patient cost among AMR patients at KBTH relative to their counterparts at KATH (ESM Tables S2 and S3). Regarding bacterial types, there was a noticeable difference, but not significant. For instance, MRSA patients had a 9.8% and 3.1% higher direct patient cost compared with those identified with Klebsiella pneumoniae and E. coli infections resistant to 3GC, respectively (ESM Tables S4–S6).
3.5 Productivity Loss Due to AMR (Indirect Patient Cost)
Patients and carers who reported not working for income constituted 23%, 22.4%, and 27.2% of the AMR, susceptible, and uninfected cohorts, respectively. Of those who work for income, the estimated mean lost working days due to absenteeism from work was 19.7 days (95% CI 19.2–20.3) for the AMR cohort compared with 15.9 days (95% CI 15.3–16.6) for the susceptible cohort and 12.7 days (95% CI 12.2–13.2) for the uninfected cohort. Between groups, the lost working days due to absenteeism accounted for 93.5%, 89.0%, and 88.1% of the respective indirect patient costs. Thus, presenteeism cost contributed 7–12% of the productivity loss. There was no significant difference in amount between the cohorts, although the susceptible cohort tended to be highest. Furthermore, we note that the extra patient cost of AMR after combining the direct and indirect costs was approximately equivalent to the participants’ reported mean monthly household consumption expenditure (Table 5).
In the stratified model, we found no significant difference in productivity loss between AMR and the susceptible cohorts in the KATH sample, but there was a significant difference in the KBTH strata (ESM Table S8).
3.6 Adjusted Result for Mean Extra Cost Due to AMR
After adjusting for the severity of the underlying illness, we observed a negligible change in the mean extra cost due to AMR (Table 6). For instance, in the overall sample comparing the AMR cohort with the susceptible cohort, the mean extra direct patient cost decreased by US$7.00, however the difference was not statistically significant. In the stratified result, the decline in excess total patient cost was US$41 at KATH, but an increase of US$162 at KBTH. Furthermore, the stratified result for the endpoint LOS and patient costs for those who survived the 30 days of follow-up were much similar, except for the mean total extra cost due to AMR, estimated as the difference in costs between the AMR and susceptible cohorts, reduced by almost half (ESM Table S9).
3.7 Who Pays the Direct Patient Cost?
The question of who bears the direct medical cost of AMR is relevant to inform the public about how AMR affects society economically. Our analysis shows that families, defined as households excluding the patient, pay approximately 44% of the direct patient cost less health insurance cover. Donations received from extended families and friends other than the household, plus contributions from the Department of Social Welfare, non-governmental organizations, and individual philanthropic gestures amount to 22.9%, with the remaining 33% borne directly by the patient without help from household members. The trend was similar in all three cohorts (Fig. 3).
Main source of funding out-of-pocket hospital bills by patient cohort, excluding health insurance. δHousehold, excluding the patient. *The patient, excluding the household. **Donations received from extended relatives/friends. ***Includes Social Welfare Fund, NGO, Religious group, and individual philanthropic gestures.
3.8 Summary
In summary, from the patient’s point of view, the unadjusted mean extra cost of an AMR infection is US$1300 and US$1923 relative to the susceptible and uninfected cohorts, respectively. About one-third of the additional expenses resulted from productivity losses. With an estimated annual AMR risk of 7.5% (740/9864) at both hospitals, the estimated yearly cost of AMR amounts to US$962,000 compared with the susceptible cohort, and US$1,423,020 relative to the uninfected cohort (Table 7). The estimated extra costs due to AMR had a positive significant association with not only LOS but also female sex (ESM Table S10).
4 Discussion
We conducted a prospective parallel cohort study to evaluate the LOS and the patient cost of AMR using prospective data collected from 960 participants in 8 months at two tertiary hospitals in Ghana. We found that the mean LOS for the AMR cohort increased by 38.6% and 81.4% compared with the susceptible and uninfected cohorts, respectively. The extra LOS due to AMR translated into more hospital resources used by AMR patients, including staff time and laboratory tests, with the latter corresponding to a significant increase in the direct patient costs paid by the patients. Additionally, the cost of reduced productivity due to reduced effectiveness while present at work and absent from work was approximately 35% and 75% more for AMR patients compared with the susceptible and uninfected cohorts, respectively. The mean total excess cost per patient due to AMR relative to the susceptible and uninfected cohorts was US$1300 and US$1923, corresponding to an annual estimate of US$962,000 and approximately US$1.4 million at both hospitals, respectively. We have shown that the patient cost of AMR has societal implications because the government, friends, and relatives of the patient support the payment of hospital bills directly or indirectly. Furthermore, had we included the productivity loss of caregivers/relatives of adult patients, this burden would have been higher.
The stratified result by bacteraemia type and study site shows similar increases in the cost for AMR patients compared with the remaining two cohorts. Nonetheless, we observed a negligible difference in expenses for AMR patients at the KBTH compared with the KATH and the same for patients with MRSA relative to patients with Klebsiella pneumoniae and E. coli infections resistant to 3GC.
Compared with other studies, we found similarities and differences in study outcomes. The excess LOS due to AMR is comparable with the findings by Mauldin and colleagues based on a similar study conducted in a University Hospital in South Carolina, USA, where the estimated excess LOS due to AMR was approximately 5 days compared with a susceptible BSI cohort [22]. Two other studies conducted in Hanoi, and Boston, USA, reported lower and higher LOS impacts of 2.1 days and 6.5 days, respectively [10, 49]. Some studies link the extra LOS due to AMR to the causative bacterial type, the severity of the illness, and delayed access to empirically prescribed antibiotics occasioned by the patient’s inability to timely mobilize funds to purchase more expensive antibiotics needed to speed their recovery post-AMR susceptibility test [35, 46]. In our study, the proportion of the participants with BSI and severe underlying illness due to comorbidity was similar for both the AMR and susceptible cohorts. Therefore, we think the extra LOS was due to factors including delayed therapy because the more expensive antibiotics such as vancomycin and meropenem used to treat severe BSI are not covered by Ghana’s national health insurance scheme (NHIS) [50]. This means that relatively poor patients may need a day or two to mobilize funds to purchase more expensive antibiotics/drugs not covered by the NHIS, which further prolongs their duration of hospital stay. In the absence of AMR, there may be a substantial potential saving of approximately 2000 (2020) hospital bed-days compared with the susceptible cohort. Again, assuming there was no AMR and all 960 participants studied during the data collection had only BSI, the hospital bed savings will be more than we have reported using the total number of observations in the susceptible cohort.
Using 2021 as a base year for comparing our cost estimates with other studies, we found our estimated extra patient cost of AMR was less compared with another study conducted in a hospital in Connecticut, USA, which shows that the excess expense per patient due to AMR was equivalent to US$1871 [23]. Furthermore, an increasing number of studies, mainly conducted in Europe and the US, report a higher patient cost of AMR with variation in excess cost depending on the bacteraemia type, sample size, study perspective, and location [21, 46, 51,52,53]. Nonetheless, we are cautious in comparing our estimated costs with available studies conducted in Europe and the US due to complete differences in socioeconomic circumstances. From the crude result, the absence of AMR could reduce patient cost by approximately 25%, equivalent to US$962,000 in annual total cost savings when compared with the susceptible cohort at both hospitals combined. Approximately 30% of the patient cost savings may result from reduced opportunity costs due to improved productivity in the absence of AMR. This is in line with a study conducted in Europe by the European Centre for Disease Control, which found that almost 40% of the total patient cost resulting from productivity losses due to AMR could have been averted in the absence of AMR [18].
In this study, we showed that AMR infections do increase LOS and patient cost of admission independently of severity. We believe the difference in the patient cost of AMR between study sites may be due to regional differences in the prices of goods and services such as transportation, and differences in absenteeism costs due to higher income among KBTH patients, which affect the patient cost. Again, the small variations in the unit cost of antibiotics put some patients at a disadvantage depending on the availability and brand.
We were able to analyse patient costs for direct medical expenses on cost accounting for every unit of healthcare service consumed and confirmed by receipt of payments for the duration of the study. In our view, comparing the mean extra patient cost of AMR with participants’ mean monthly household expenditure makes it reasonable and a good motivation for behavioural change for appropriate antibiotic use by the population. This requires that the population is aware of the connection between antibiotic use and AMR, as indicated in our submitted study to the British Medical Journal (unpublished), which suggests the need for point-of-care information to patients about the health and economic implications of antibiotic use and AMR.
4.1 Strengths and Limitations
Strengths Our study included a large sample size with data collected prospectively for 8 months, with two separate comparator groups to the AMR cohorts. This allowed us to estimate the study endpoint precisely, evidenced by the narrow 95% CIs accompanying the endpoint mean LOS and costs. This study employed robust matching criteria that considered the timing of events in estimating the true LOS and costs due to AMR. The use of precision costing methodology, combined with the study design, makes our paper unique compared with many others, which only compare the AMR cohort with either a susceptible or uninfected cohort. The lack of published studies on the cost of AMR in LMIC settings and the non-existence of the same in the Ghanaian context means we have contributed to the health economics literature on the subject. Our findings provide baseline data for further studies in which we will evaluate AMR cost from the healthcare provider perspective and conduct a microsimulation analysis focusing on the long-term AMR cost for the entirety of Ghana. Again, we anticipate the findings can be used to motivate the population to use antibiotics appropriately to reduce out-of-pocket payments on healthcare due to AMR. Additionally, we believe our finding is timely and may support behavioural change in line with the objective of Ghana’s policy on antimicrobial use and resistance [54].
Limitations During the 8 months of data collection, there were three different labour union strikes in the health sector in Ghana, beginning with laboratory scientists who were assisting with the AMR surveillance and consequently affected participant recruitment for weeks. Without government intervention to call off the strikes, the incidents could have affected the sample size. Furthermore, the cost of goods and services increased three times in Ghana following hikes in fuel prices during the data collection, demanding that we review and appropriately adjust the unit costs to avoid underestimation. Dwelling on mean estimates of LOS and cost may not reflect individual circumstances and most likely contribute to the underestimation of LOS and the associated patient cost due to AMR. We limited the measurement of staff time consumed to doctors’ time spent on patients, which was available in the patient folder. This means we excluded the time nurses spent on patients because it was not recorded in patient folders to make it official for us to collect such data, which may lead to underestimation. There is still the possibility of misclassification, i.e., the likelihood that patients in the uninfected group may have had an infection, but the laboratory was unable to isolate the organism. This may be due to the fastidious nature of the organism or the requirement of special conditions for growth (e.g., anaerobic conditions), which were not available in the microbiology laboratory.
5 Conclusion
We have shown how AMR affects the LOS and the associated patient cost in Ghana using prospective data collected from two tertiary hospitals in Ghana. We anticipate our findings will encourage investment in AMR interventions by the government and others interested in reversing the prevalence and spread of AMR. Such interventions may include point-of-care AMR information to patients to motivate behaviour change for appropriate antibiotic use, as we have suggested in another study. Our case for behavioural change is that patients could save an amount equivalent to their mean monthly household expenditure without AMR.
References
World Health Organization. Antimicrobial resistance, Key facts. 2020. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Accessed 14 Jan 2021.
World Health Organization. Meeting report: WHO expert consultation meeting on antimicrobial resistance (AMR) health burden estimation 18-19 January 2018: Geneva, Switzerland: WHO. 2018. https://www.who.int/glass/events/2018/health-burden-estimation-meeting-report.pdf. Accessed 11 Jan 2022.
World Health Organization. Antimicrobial resistance: multi-country public awareness survey. 2015. https://apps.who.int/iris/bitstream/handle/10665/194460/9789241509817_eng.pdf;sequence=1. Accessed 11 Jan 2022.
O'Neill J. Review on Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. London: Review on Antimicrobial Resistance. 2014. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf. Accessed 19 Mar 2022.
de Kraker ME, Stewardson AJ, Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016. https://doi.org/10.1371/journal.pmed.1002184.
Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019. https://doi.org/10.1016/S1473-3099(18)30605-4.
Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer CY. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003. https://doi.org/10.1086/345476.
WHO Regional Office for Europe/European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2022–2020 data. Copenhagen: WHO Regional Office for Europe. 2022. https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2022-2020-data. Accessed 9 Jun 2022.
Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022. https://doi.org/10.1016/S0140-6736(21)02724-0.
Peters L, Olson L, Khu DTK, Linnros S, Le NK, Hanberger H, et al. Multiple antibiotic resistance as a risk factor for mortality and prolonged hospital stay: a cohort study among neonatal intensive care patients with hospital-acquired infections caused by gram-negative bacteria in Vietnam. PLoS ONE. 2019. https://doi.org/10.1371/journal.pone.0215666.
Laxminarayan R. The overlooked pandemic of antimicrobial resistance. Lancet. 2022. https://doi.org/10.1016/S0140-6736(22)00087-3.
Aiken AM, Mturi N, Njuguna P, Mohammed S, Berkley JA, Bacteraemia Surveillance Group. Risk and causes of paediatric hospital-acquired bacteraemia in Kilifi District Hospital, Kenya: a prospective cohort study. Lancet. 2011. https://doi.org/10.1016/S0140-6736(11)61622-X.
The Brooklyn Antibiotic Resistance Taskforce. The cost of antibiotic resistance: effect of resistance among Staphylococcus aureus, Klebsiella pneumonia, Acinetobacter bauman Pseudomonas seudmonas aeruginosa on length of hospital stay. Infect Control Hosp Epidemiol. 2002. https://doi.org/10.1086/502018.
Wong JG, Chen MI, Win MK, Ng PY, Chow A. Length of stay an important mediator of hospital-acquired methicillin-resistant Staphylococcus aureus. Epidemiol Infect. 2016. https://doi.org/10.1017/S0950268815002733.
Kraker MEA, Davey PG, Grundmann H, on behalf of the BURDEN study group. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteraemia: Estimating the burden of antibiotic resistance in Europe. PLoS Med. 2011. https://doi.org/10.1371/journal.pmed.1001104.
Roope LSJ, Smith RD, Pouwels KB, Buchanan J, Abel L, et al. The challenge of antimicrobial resistance: what economics can contribute. Science. 2019. https://doi.org/10.1126/science.aau4679.
Sipahi OR. Economics of antibiotic resistance. Expert Rev Anti Infect Ther. 2008. https://doi.org/10.1586/14787210.6.4.523.
ECDC/EMEA. The bacterial challenge: time to react. 2009. https://www.ema.europa.eu/en/documents/press-release/bacterial-challenge-time-react-call-narrow-gap-between-multidrug-resistant-bacteria-eu-development_en.pdf. Accessed 26 Feb 2022.
Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathogens Glob Health. 2015;109(7):309–18. https://doi.org/10.1179/2047773215Y.0000000030.
Centres for Disease Control and Prevention, US Department of Health and Human Services. Antibiotic resistance threats in the United States. Atlanta: CDC; 2013. http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed 5 Mar 2022.
Maragakis LL, Perencevich EN, Cosgrove SE. Clinical and economic burden of antimicrobial resistance. Expert Rev Anti Infect Ther. 2008. https://doi.org/10.1586/14787210.6.5.751.
Mauldin PD, Salgado CD, Hansen IS, Durup DT, Bosso JA. Attributable hospital cost and length of stay associated with healthcare-associated infections caused by antibiotic-resistant gram-negative bacteria. Antimicrob Agents Chemother. 2010;54(1):109–15. https://doi.org/10.1128/AAC.01041-09.
Capitano B, Leshem OA, Nightingale CH, Nicolau DP. Cost effect of managing methicillin-resistant Staphylococcus aureus in a long-term care facility. J Am Geriatr Soc. 2003. https://doi.org/10.1034/j.1601-5215.2002.51003.x.
Barasa E, Solange H, Fenny AP, Omaswa F, Moosa S, et al. The state of universal health coverage in Africa: Report of the Africa Health Agenda International Conference Commission. Nairobi. Kenya. 2021. https://repository.amref.ac.ke/handle/123456789/211. Accessed on 15 June 2022
University of York. Antibiotics found in some of the world’s rivers exceed ‘safe’ levels. 2019. https://www.york.ac.uk/news-and-events/news/2019/research/antibiotics-found-in-some-of-worlds-rivers/. Accessed 11 Jan 2020.
Labi A-K, Obeng-Nkrumah N, Bjerrum OES, Bediako-Bowan A, Sunkwa-Mills G, et al. Multi-centre point-prevalence survey of hospital-acquired infections in Ghana. J Hosp Infect. 2018. https://doi.org/10.1016/j.jhin.2018.04.019.
Newman MJ, Frimpong E, Donkor ES, Opintan JA, Asamoah-Adu A. Resistance to antimicrobial drugs in Ghana. Infect Drug Resist. 2011. https://doi.org/10.2147/IDR.S21769.
World Health Organization. AWaRe Classification. WHO access, watch, reserve, classification of antibiotics for evaluation and monitoring of use. 2021 Guideline. https://www.who.int/publications/i/item/2021-aware-classification. Accessed on 15 Nov 2022
Adebisi YA, Jimoh ND, Ogunkola IO, Uwizeyimana T, Olayemi AH, Ukor NA, Lucero-Prisno DE 3rd. The use of antibiotics in COVID-19 management: a rapid review of national treatment guidelines in 10 African countries. Trop Med Health. 2021;49(1):51. https://doi.org/10.1186/s41182-021-00344-w.
Klein EY, Milkowska-Shibata M, Tseng KK, Sharland M, Gandra S, Pulcini C, Laxminarayan R. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–15: an analysis of pharmaceutical sales data. Lancet Infect Dis. 2021;21(1):107–15. https://doi.org/10.1016/S1473-3099(20)30332-7.
Husereau D, Drummond M, Augustovski F, Standards CHEER, et al. (CHEERS 2022) explanation and elaboration: a report of the ISPOR CHEERS II Good Practices Task Force. Value Health. 2022;2022:25. https://doi.org/10.1016/j.jval.2021.10.008.
Cochrane Tool to assess risk of bias in cohort studies. (n.d). https://www.bing.com/search?q=Tool+to+Assess+Risk+of+Bias+in+Cohort+Studies&cvid=ae2e2ee2446048969f5a9980d8bb9db5&aqs=edge..69i57j69i60.1186j0j1&pglt=299&FORM=ANNTA1&PC=U531. Accessed 23 Apr 2022.
Komfo Anokye Teaching Hospital. About us: Historical background. 2021. https://kath.gov.gh/our-history/. Accessed 10 Mar 2022.
Korle-Bu Teaching Hospital. About us: A brief history. 2021. https://kbth.gov.gh/brief-history/. Accessed 10 Mar 2022.
Smith R, Coast J. The true cost of antimicrobial resistance. BMJ. 2013. https://doi.org/10.1136/bmj.f1493.
Nelson RE, Nelson SD, Khader K, Perencevich EL, Schweizer ML, et al. The magnitude of time-dependent bias in the estimation of excess length of stay attributable to healthcare-associated infections. Infect Control Hosp Epidemiol. 2015. https://doi.org/10.1017/ice.2015.129.
Manoukian S, Stewart S, Dancer S, Graves N, Mason H, McFarland A, Robertson C, Reilly J. Estimating excess length of stay due to healthcare-associated infections: a systematic review and meta-analysis of statistical methodology. J Hosp Infect. 2018. https://doi.org/10.1016/j.jhin.2018.06.003.
Fenny AP, Otieku E, Labi KA, Asante FA, Enemark U. Costs and extra length of stay because of neonatal bloodstream infection at a teaching hospital in Ghana. Pharmacoeconomics Open. 2021. https://doi.org/10.1007/s41669-020-00230-x.
Drummond MF, Sculpher MJ, Claxton K, Stddart GL, et al. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2015.
Simoens S. Health economic assessment: a methodological primer. Int J Environ Res Public Health. 2009. https://doi.org/10.3390/ijerph6122950.
Sevransky JE, Rothman RE, Hager DN, Bernard GR, Brown SM, et al. Effect of Vitamin C, Thiamine, and Hydrocortisone on ventilator- and vasopressor-free days in patients with sepsis: the VICTAS Randomized Clinical Trial. JAMA. 2021. https://doi.org/10.1001/jama.2020.24505.
von Dach E, Albrich WC, Brunel AS, Prendki V, et al. Effect of C-reactive protein-guided antibiotic treatment duration, 7-day treatment, or 14-day treatment on 30-day clinical failure rate in patients with uncomplicated gram-negative bacteraemia: a randomized clinical trial. JAMA. 2020. https://doi.org/10.1001/jama.2020.6348.
Ying P, Chen J, Ye Y, Ye J, Cai W. Adipose tissue is a predictor of 30-days mortality in patients with bloodstream infection caused by carbapenem-resistant Klebsiella pneumonia. BMC Infect Dis. 2022. https://doi.org/10.1186/s12879-022-07108-9.
Cameron AC, Trivedi PK. Microeconometrics using Stata. College Station: Stata Press; 2010.
Gujarati DN. Basic econometrics. Tata McGraw-Hill Education; 2009.
Gandra S, Barter DM, Laxminarayan R. Economic burden of antibiotic resistance: how much do we really know? Clin Microbiol Infect. 2014. https://doi.org/10.1111/1469-0691.12798.
Thom KA, Shardell MD, Osih RB, et al. Controlling for severity of illness in outcome studies involving infectious diseases: impact of measurement at different time points. Infect Control Hosp Epidemiol. 2008. https://doi.org/10.1086/591453.
Ian S, James T, Marcello M. A web-based tool for adjusting costs to a specific target currency and price year. Evid Policy. 2010;6:51–9.
Carmeli Y, Eliopoulos G, Mozaffari E, Samore M. Health and economic outcomes of vancomycin-resistant enterococci. Arch Intern Med. 2022. https://doi.org/10.1001/archinte.162.19.2223.
National Health Insurance Authority Medicines list. 2021. https://nhis.gov.gh/files/MedicinesList(2021).pdf. Accessed 11 Jan 2022.
van Rijt AM, Dik JH, Lokate M, Postma MJ, Friedrich AW. Cost analysis of outbreaks with Methicillin-resistant Staphylococcus aureus (MRSA) in Dutch long-term care facilities (LTCF). PLoS ONE. 2018. https://doi.org/10.1371/journal.pone.0208092.
Wozniak TM, Barnsbee L, Lee XJ, Pacella RE. Using the best available data to estimate the cost of antimicrobial resistance: a systematic review. Antimicrob Resist Infect Control. 2019. https://doi.org/10.1186/s13756-019-0472-z.
Thaden JT, Li Y, Ruffin F, Maskarinec SA, Hill-Rorie JM, et al. Increased costs associated with bloodstream infections caused by multidrug resistant Gram-negative bacteria are due primarily to patients with hospital-acquired infections. Antimicrob Agents Chemother. 2017. https://doi.org/10.1128/AAC.01709-16.
Republic of Ghana. Policy on antimicrobial use and resistance 1st Edition. 2017. https://www.moh.gov.gh/wp-content/uploads/2018/04/AMR-POLICY-A5_09.03.2018-Signed.pdf. Accessed 22 Feb 2022.
Acknowledgements
The authors acknowledge the support of the data collection team and the in-charge nurses and matrons at the various inpatient wards who facilitated access to the patients during participant recruitment. We also acknowledge Prof. Alexander Aiken at the Department of Infectious Disease Epidemiology, London School of Hygiene and Tropical Medicine, for his expert advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
Conceptualization: EO, APF, JALK, UE. Data curation and investigation: EO, AKL, AOO. Methodology: EO, APF, JALK, UE. Formal analysis and writing – original draft: EO. Project administration: EO, APF, JALK, UE. Supervision: APF, JALK, UE. Validation and writing—reviews: AKL, AOO, APF, JALK, UE. Visualization: EO.
Funding
A research training supplement was awarded to the corresponding author by the Graduate School of Health at Aarhus University to cover the cost of subsistence as a PhD student. The grant/award number is not applicable.
Competing interests
None.
Data availability statement
The anonymized data used for this study is available upon reasonable request to the corresponding author due to ethics review guidelines.
Ethics approval
Ethics approval for this study was granted by the Institutional Review Boards of KATH and KBTH, with reference numbers KATH-IRB/AP/030/21 and KBTH/MD/93/21, respectively.
Consent to participate
All the participants gave written informed consent and kept a copy for their personal reference.
Consent for publication (from patients and participants)
Not applicable.
Code availability
The file containing STATA codes used for the analysis is available upon request to the corresponding author.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Otieku, E., Fenny, A.P., Labi, AK. et al. Attributable Patient Cost of Antimicrobial Resistance: A Prospective Parallel Cohort Study in Two Public Teaching Hospitals in Ghana. PharmacoEconomics Open 7, 257–271 (2023). https://doi.org/10.1007/s41669-022-00385-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-022-00385-9