Abstract
Objectives
Ocrelizumab demonstrated significant clinical benefit for the treatment of relapsing (RMS) and primary progressive (PPMS) multiple sclerosis (MS), an incurable disease characterized by disability progression. This study evaluated the clinical and economic impact of ocrelizumab relative to current clinical practice, including other disease-modifying therapies (DMT), available in Portugal.
Methods
Markov models for MS were adapted to estimate the impact of ocrelizumab across three patient populations: treatment-naïve RMS, previously treated RMS, and PPMS. Health states were defined according to the Expanded Disability Status Scale. For RMS, the model further captured the occurrence of relapses and progression to secondary progressive multiple sclerosis (SPMS). A lifetime time-horizon and Portuguese societal perspective were adopted.
Results
For RMS patients, ocrelizumab was estimated to maximize the expected time (years) without progression to SPMS (10.50) relative to natalizumab (10.10), dimethyl fumarate (8.64), teriflunomide (8.39), fingolimod (8.38), interferon β-1a (8.33) and glatiramer acetate (8.18). As the most effective option, with quality-adjusted life year (QALY) gains between 0.3 and 1.2, ocrelizumab was found to be cost-saving relative to natalizumab and fingolimod, and presented incremental cost-effectiveness ratios (ICER) below €16,720/QALY relative to the remaining DMT. For PPMS patients, the ICER of ocrelizumab versus best supportive care was estimated at €78,858/QALY.
Conclusions
Ocrelizumab provides important health benefits for RMS and PPMS patients, comparing favourably with other widely used therapies. In RMS, ocrelizumab was revealed to be either cost-saving or have costs-per-QALY likely below commonly accepted cost-effectiveness thresholds. In PPMS, ocrelizumab fills a clear clinical gap in the current clinical practice. Overall, ocrelizumab is expected to provide good value for money in addressing the need of MS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study evaluated the lifetime costs and consequences of ocrelizumab relative to current clinical practice, with special focus given to clinically meaningful outcomes. |
Model-based estimates demonstrate that ocrelizumab is expected to provide important lifetime health benefits in multiple sclerosis: maximizing expected time without progression to secondary progressive multiple sclerosis, enhancing time in mild disease stages and reducing the annual number of relapses. |
Ocrelizumab is expected to represent good value for money in addressing the needs of relapsing multiple sclerosis patients, while also filling a clear clinical gap in current clinical practice for primary progressive multiple sclerosis. |
1 Introduction
Multiple sclerosis (MS) is a chronic immune-mediated neurodegenerative disorder of the central nervous system clustered into different disease phenotypes [1]. The most common phenotype of MS is relapsing-remitting multiple sclerosis (RRMS), characterised by alternation of relapse and remission phases. About 85% of patients with MS are initially diagnosed with RRMS, with propensity to transform over time into secondary progressive multiple sclerosis (SPMS). Approximately 15% of the patients are diagnosed with primary progressive multiple sclerosis (PPMS), which progresses without relapses from the onset of the disease [2].
Despite the heterogeneity in clinical course, life expectancy may be shortened by about 7 years and mortality is almost threefold higher in individuals with MS relative to the general population [3]. In addition, in many individuals with MS, progression of the disease ultimately leads to severe disability with limited ability to walk and quality-of-life (QoL) impairment [4, 5].
Multiple sclerosis imposes a considerable socioeconomic burden on society. Implications for health systems, affected individuals, their families, and caregivers involve excessive health care resource utilisation, significant out-off pocket expenses and important productivity costs due to early retirement and high demand for informal care provided at home by family and friends [6, 7].
Disease-modifying therapies (DMTs) for MS coming to the market over the past 2 decades have led to improved outcomes including clinically significant decreases in the Expanded Disability Status Scale (EDSS) score or substantially lower rates of worsening and evolution to SPMS in the long-term in RRMS patients when compared to earlier natural history studies [8].
Since 1993, when the first interferon became available, several DMTs for MS were approved in EU by the European Commission in both injectable and oral formulations [9]. The expanding therapeutic armamentarium for MS ranges from lower efficacy peginterferon, glatiramer acetate, interferons and teriflunomide to more recent and higher efficacy alemtuzumab, natalizumab and ocrelizumab [10]. Broadened opportunities to manage and control disease activity allow individualised therapy, but patients and providers must balance considerations about efficacy, safety, persistence/adherence and treatment costs in a shared-decision process in the interest of individuals with MS to maintain a vibrant and meaningful life [11].
Ocrelizumab, a humanised anti-CD20 monoclonal antibody, is the only approved treatment in the EU and USA in primary-progressive as well as in relapsing forms of MS (RMS). Progressive multiple sclerosis is still the greatest therapeutic challenge facing the MS community today.
Clinical trial data demonstrate ocrelizumab superiority as an effective and well-tolerated treatment for patients with MS, including those with suboptimal response to an adequate course of other DMTs [12, 13] and in early line patients with 91.2% of patients with no evidence of disease activity (NEDA) at 6 months and 84.8% at 1 year [14]. New long-term data further reinforce ocrelizumab as a highly effective treatment for people with MS [15, 16].
In this study, we report on the pharmacoeconomic value of ocrelizumab, relative to contemporary clinical practice at the time of presentation to the Portuguese Health Authority for pricing and reimbursement purposes for the treatment of RMS and PPMS. Besides the usual cost, quality adjusted life year (QALY) and incremental cost-effectiveness ratio (ICER) outcomes, special focus was given to the expected lifetime value of ocrelizumab in terms of clinically meaningful outcomes such as time without progression, time spent in mild disease stages, time spent in high impact and limiting disease stages and annualised relapse rates.
2 Methods
2.1 Modelling Multiple Sclerosis
There is no curative treatment available for MS, and the current therapies aim to reduce the risk of relapses and slow disability progression [9]. Relapses are the clinical expression of the acute, self-limited, focal inflammatory events occurring episodically within the central nervous system of MS patients. Progression is defined for the purposes of research as a steady deterioration in neurologic function observed during at least 6 or 12 months [17].
The pattern of early relapses and late progression in MS lead to the notion of two distinct phases: a relapsing remitting phase, and a progressive phase [17]. An international consensus has defined the clinical course of MS by three phenotypes: relapsing-remitting (RRMS), secondary progressive (SPMS) and primary progressive (PPMS) [2]. Unlike RRMS, in which patients experience attacks of symptoms followed by periods of improvement or remission, PPMS is typically characterised by a progressive decline from onset, with occasional temporary plateaus or minor improvement.
In the literature, the short-term and long-term cost-effectiveness implications of DMTs for MS have typically been estimated via Markov modelling using the EDSS score to define health states and model disease progression and the occurrence of relapses over time [18]. This approach can be traced back to the pioneering study of Parkin et al. [19] with subsequent modelling improvements including the use of one-point EDSS change as a clinically relevant variation [20, 21].
Previously validated discrete-time Markov models for MS [22,23,24] were adapted to estimate the lifetime costs and effectiveness of ocrelizumab, relative to contemporary clinical practice at the time of presentation to the Portuguese Health Authority for pricing and reimbursement purposes for the treatment of RMS and PPMS, in 2018. As part of the assessment scope of the Portuguese Health Authority, for RMS, interferon β-1a, dimethyl fumarate, glatiramer acetate, teriflunomide, fingolimod, and natalizumab were selected as relevant comparators. For PPMS, the relevant comparator was considered to be best supportive care (BSC) [25]. Inactive SPMS was not included in this study.
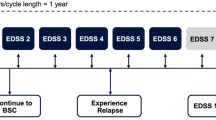
For both RMS and PPMS models, health states were defined according to the Expanded Disability Status Scale (EDSS 0 to 9) [26]. For RMS, the model further captures the occurrence of relapses and the progression to SPMS. The conceptual models are illustrated in Fig. A1 (available in the supplementary material). In each Markov cycle (1-year), patients in model (A) (RMS) can transit between EDSS scores within “RRMS-on treatment”; discontinue treatment and transition to “RRMS-off treatment”; progress to SPMS; or transition to death. In model (B) (PPMS) patients are assumed to start in one of the 10 EDSS scores under “PPMS-on treatment”, they then may discontinue treatment and transit to “PPMS-off treatment” or die.
This cost-effectiveness analysis assumes the Portuguese societal perspective. A lifetime time-horizon with annual cycles was adopted. The lifetime time-horizon was required to account for the long-term effect of non-curative treatments in chronic MS. Effectiveness was expressed in terms of time spent in each EDSS state, life years (LY) and QALY, and specifically for RRMS time spent without progression to SPMS and time spent in SPMS after progression according to the level of disability. Following the Portuguese guidelines for economic evaluation studies [27] a discount rate of 5% was applied to both costs (direct and indirect) and effectiveness. Final results were expressed in incremental costs per QALY. Probabilistic sensitivity analysis was performed using second order Monte Carlo simulations.
2.2 Base-Case Inputs: Effectiveness
In order to capture the natural history of the disease, models were parameterised with data retrieved from long-term registries [28,29,30,31] and Portuguese-specific data were included whenever available [32,33,34]. The relative treatment effects of interventions versus placebo (natural history) were retrieved from ocrelizumab clinical trials [12, 13, 15] and indirect treatment comparisons [35]. Specifically, for the RMS model, hazard ratios (HR) for the 12-week confirmed disability progression (CDP) and rate ratios for the annualised relapse rate (ARR) were used, as reported in McCool et al. (Table D16) [35]. For the PPMS model, 24-week CDP data from the ORATORIO open-label extension study was used (HR 0.60, 95% CI 0.40–0.89), considering time to sustained disability progression at 24 weeks, adjusting for crossover with the rank-preserving structural failure time model. Relative treatment effects related to disability progression were applied to the forward transitions in terms of EDSS health states, for both the RMS and PPMS models. Relative treatment effects were applied while patients are on treatment only and were assumed constant throughout the entire time-horizon.
In Table 1 we present data sources for model parametrisation. The annual discontinuation probability of treatment with ocrelizumab was estimated based on data from the OPERA trials (12% discontinuation at 96 weeks, resulting in an annualised probability of 6.19%) while for each of the comparators the discontinuation rate was estimated combining this result with an odds ratio versus ocrelizumab from the NMA [35].
Mortality estimates are applied separately from disability progression as this allows the application of adjustable MS subtype specific mortally by EDSS state. A weighted average of the general population all-cause mortality rate [34] was calculated based upon the female to male ratio of MS patients [32]. Multiple sclerosis standard mortality ratios [23] are applied to the all-cause weighted average mortality rates to derive the risk of death for MS patients in different EDSS states.
EQ-5D-3L-based QoL utilities with Portuguese preferences for RMS and PPMS patients by EDSS level were retrieved from Sá et al. [33]. Quality-of-life weights (utilities) used to estimate QALYs are presented in Table 2. Utility weights for SPMS patients were assumed to be 0.045 [37] lower than the corresponding RMS EDSS level utility weights. Caregiver QoL impairment in the form of a disutility was included in both models, using a maximum disutility value of 0.14 (retrieved from NICE TA127 [36]), weighted for each EDSS level with the percentage of time that caregivers spend caring for MS patients at each EDSS stage, relative to the maximum time that is spent in care (Table 2). Time spent in care for each EDSS level was retrieved from the study of Tyas et al. [38].
Quality-of-life calculations for the RMS model assumed a loss of utility of 0.071 for relapse [37] with an average duration of 1.5 months [39]. The PPMS model included utility loss associated with fatigue (− 0.13, for all EDSS levels) and upper limb disfunction (− 0.06 for EDSS levels ≥ 5) [40], with the proportion of patients with fatigue or upper limb dysfunction in the absence of DMT based on medical opinion (as in the NICE appraisal [41], and validated by local clinical experts). Additionally, QoL decrements were also incorporated for adverse events, sourced from the National Institute for Health and Care Excellence (NICE, UK) technology appraisal guidance of daclizumab for treating RRMS [42].
2.3 Base-Case Inputs: Costs
Direct and indirect MS management costs were considered in the cost-effectiveness analysis. Treatment costs included DMT costs, administration costs, and monitoring costs (Table 2). Yearly monitoring costs were calculated according to each DMT´s summary of product characteristics (SPC) and unit costs from the comprehensive price lists of the Portuguese Ministry of Health [43]. Annual administration costs of €263 and €46 were calculated for natalizumab and ocrelizumab, based on recommended SPC posology and method of administration, including premedication for infusion-related reactions and a unit cost of €20.2 per infusion [43].
Annual direct and indirect costs for RMS and PPMS patients by EDSS score were estimated using the results reported in Sá et al. [33]. Direct costs included both medical (consultations, tests and medications) and non-medical costs (community services, investments and informal care). Disease-modifying therapy and hospitalisation costs were excluded from direct costs reported in Sá et al. [33] in order to avoid double counting as these are captured separately in the cost-effectiveness analysis. Indirect costs included short- and long-term production losses (sick leave, early retirement, invalidity). Expanded Disability Status Scale-related direct and indirect costs for SPMS patients were based on RMS costs increased by €473 and €1969, respectively, based on the results from Tyas et al. [38]. The reference year for costs is 2018, aligned with the original pricing and reimbursement submission to the Portuguese Health Authority. Costs related to EDSS health states and relapses, retrieved from Sá et al. [33], were uprated from 2015 to 2018, using the relevant consumer price indexes for health published by the Portuguese National Institute for Statistics. The results with an updated reference year for costs (2021) is included in the scenario analysis (reported in the supplementary material).
2.4 Additional Analysis
Scenario analysis and probabilistic sensitivity analysis (PSA) were performed to assess the influence of key assumptions and variables used in the model. In each of the scenarios assessed, a reduced number of variables and/or assumptions were altered, while maintaining the remaining variables and assumptions unchanged relative to the base case. The list of the scenarios assessed (and their respective results) are presented in Tables B1 and B2 in the supplementary material. For the PSA, the inputs for these variables were randomly drawn from pre-specified statistical distributions in order to calculate a corresponding ICER value and related uncertainty, presented in the form of acceptability curves and the probability of each DMT being cost effective. The parameters subject to PSA and the corresponding statistical distributions are presented in Table A1 in the supplementary material.
3 Results
3.1 RMS
Ocrelizumab is estimated to maximise mean time without progression to SPMS (10.50 years) relative to natalizumab (10.10 years), dimethyl fumarate (8.64 years), teriflunomide (8.39 years), fingolimod (8.38 years), interferon β-1a (8.33 years) and glatiramer acetate (8.18 years) (Table 3), corresponding to increments in time without progression to SPMS in favour of ocrelizumab of 3.9%, 21.5%, 25.0%, 25.2%, 26.0% and 28.3%, respectively.
Additionally, treatment with ocrelizumab is estimated to enhance time in mild disability MS stages (up to EDSS 2.5) while minimising time spent in high impact and limiting disease stages characterised by EDSS ≥ 6. For example, on average, in patients with RMS, ocrelizumab is expected to provide between 5.9 and 58.6% more time in EDSS 0 to 2.5 than natalizumab (5.96 years vs 5.63 years) and glatiramer acetate (5.96 years vs 3.76 years), respectively.
Both natalizumab and ocrelizumab are estimated to reduce the number of attacks (relapses) relative to the other compared DMTs. Estimated annualised relapse rate was: natalizumab (0.36); ocrelizumab (0.38); fingolimod (0.39); dimethyl fumarate (0.40); glatiramer acetate (0.42); teriflunomide (0.42); interferon β-1a (0.42).
Expected discounted lifetime LYs, QALYs, and total costs for each DMT are shown in Table 4, arranged in order of decreasing estimated QALYs. The incremental analyses presented reports the incremental cost-effectiveness ratio (€/QALY) of ocrelizumab utilisation relative to the other DMTs. Discounted life expectancy (LYs) is anticipated to be the highest in patients treated with ocrelizumab (15.24 LYs) and the lowest for glatiramer acetate (15.04 LYs). Corresponding QALY results were 3.22 QALY for ocrelizumab, 2.92 QALY for natalizumab and below 2.30 QALY for the remaining DMTs. Quality-adjusted life year gains with ocrelizumab may range between 0.3 QALY relative to natalizumab to 1.2 QALY in comparison to glatiramer acetate.
Discounted total costs including DMT-related costs, AE-related costs, MS-related healthcare costs and indirect costs over the projected lifetime, were approximately €320,811 for ocrelizumab and for other DMTs ranged from €353,206 for natalizumab to €302,255 for subcutaneous interferon β-1a.
For patients with RMS, glatiramer acetate was dominated by interferon β-1a, fingolimod was dominated by teriflunomide, and natalizumab was dominated by ocrelizumab. Teriflunomide and dimethyl fumarate were extendedly dominated by ocrelizumab. Ocrelizumab’s relevant comparison was against interferon β-1a, in which ocrelizumab was expected to provide gains of 1.11 QALY for an additional €18,556, resulting in an ICER of €16,727/QALY. Against the remaining comparators, ocrelizumab is dominant or the ICERs were lower (Fig. 1).
3.2 PPMS
On average, ocrelizumab is expected to increase (undiscounted) life expectancy of PPMS patients by 0.55 LY (25.15 vs 24.59 years) relative to best supportive care (BSC). This result follows from the disease progression delay provided by ocrelizumab, which is expected to increase the amount of time at lower EDSS levels (up to EDSS 6), while decreasing the amount of time spent at higher EDSS levels, remarkably reducing time at health states perceived as ‘worse than death’ (EDSS levels 8 and 9, presenting negative QoL utilities) by 1.38 years (Table 5). Adjusting for QoL, ocrelizumab is expected to provide on average 1.27 QALYs (discounted), which is an additional 0.80 QALYs relative to BSC, with substantial utility decrements due to fatigue (−0.97) and upper limb dysfunction and informal care (−0.38).
Treatment with ocrelizumab is expected to augment overall treatment costs by €63,085/patient (discounted), mainly related to drug costs, despite reduced indirect and other direct costs, corresponding to an incremental cost-effectiveness ratio (ICER) over BSC of €78,858/QALY (Table 4).
3.3 Probabilistic Sensitivity Analysis
Mean costs and QALYs estimated through PSA were, overall, consistent with corresponding deterministic estimates. In the RMS population, natalizumab and fingolimod were dominated by ocrelizumab and in the comparisons against subcutaneous interferon β-1a, teriflunomide, glatiramer acetate and dimethyl fumarate, treatment with ocrelizumab results in ICERs ranging from €17,469/QALY to €8339/QALY. In the PPMS population, ocrelizumab provided an average gain of 0.78 QALYs at the expense of an average increase in total costs of €63,074, resulting in an ICER of €81,052/QALY (Table 4).
Among the interventions considered for the RMS population, ocrelizumab proved to be the most cost-effective alternative for a willingness to pay (WTP) greater or equal to €18,000/QALY, reaching a probability of cost-effectiveness of 0.7 at a WTP of €43,000/QALY (Fig. 2a). For the PPMS population, ocrelizumab shows a higher probability of being cost-effective relative BSC for WTP values higher than €81,000/QALY, reaching a probability around 0.7 for a WTP of €100,000/QALY (Fig. 2b).
3.4 Scenario Analysis
The results of the scenario analysis are presented in Tables B1 and B2 of the supplementary material. In PPMS the only scenarios with a relative increase (with respect to the base-case) in the ICER over 10% are the “Initial EDSS: 4” (increase of 14%) and the “Exclude upper limb disutilities” (increase of 11%). In RRMS, the scenarios with the highest relative increase in the ICER are the “Initial EDSS: 6” (increase of 21%) and the “No EDSS stopping rule” (increase of 12%). All other scenarios resulted in either a lower ICERs or in a higher ICERs with a relative increase inferior to 11%.
4 Discussion
Health technology assessment at the point of market entry is a common practice in European countries to assess the therapeutic and economic value of new pharmaceuticals and guide decisions about scarce health resource allocation. Ocrelizumab is a disease-modifying therapy recently approved by the Portuguese Ministry of Health (PMH) for the public financing of MS. The positive recommendation from the PMH included both the RMS and PPMS populations and ocrelizumab became the first DMT financed for PPMS in Portugal. In this study we report the cost-effectiveness analysis of the utilisation of ocrelizumab according to the Portuguese societal perspective, using a broad set of current clinical practice comparators.
Analyses of the natural history of MS have shown that conversion of RRMS to SPMS is a critical event, both because it implies the inexorable progression of disability and because available treatments have no efficacy in terms of modifying the course of the disease at this stage [45].
The results of our study confirm that currently, ocrelizumab is among the most effective treatment options for RMS. Our study suggests that ocrelizumab maximises time spent in lower EDSS levels in RMS while being more efficient than other DMTs in refraining the inevitable progression to SPMS. In patients with PPMS, ocrelizumab is estimated to provide a clinically relevant benefit over BSC with more than one year spent in EDSS levels lower than 6, which marks the border line for substantial disability and profound limitations to patients’ independence, including dependency from wheelchair. These findings are consistent with the post hoc analyses from the ORATORIO trial confirming ocrelizumab significantly delayed time to wheelchair requirement [46]. These results are potentially relevant because the EDSS score is the most widely used disability outcome measure in clinical trials in MS [47]. Additionally, delaying or even preventing disease progression in MS may reduce the societal economic burden of MS [48].
The value of ocrelizumab to society can be additionally characterised by the cost per quality adjusted life years (QALYs) preferred health technology assessment value approach. In this respect the results from our study suggest that ocrelizumab is the most effective of the compared RMS DMTs with QALY gains in the range of 0.3 to 1.2 QALY, while being cost saving relative to natalizumab and fingolimod and presenting ICERs extending from €7585/QALY to just under €16,720/QALY for the remaining DMTs under assessment. The value for money of ocrelizumab in PPMS is estimated at €78,858/QALY.
The literature with regard to the cost-effectiveness of DMTs for MS is extensive [49]. Far less common is the number of studies reporting the cost-effectiveness value of ocrelizumab [23, 24, 50, 51]. Likewise, in these studies ocrelizumab was found to be the DMT having the highest (Yang [23]; Zimmermann [51]) or among the highest effectiveness, and being a cost-effective treatment option for RMS. In the paper by Auguste et al. [24], the authors report the appraisal undertaken by NICE on ocrelizumab for treating PPMS in the UK. The analyses yielded an ICER of approximately GBP78,300 per QALY, which is in the same range of our result of €78,858 per QALY. NICE recommended ocrelizumab in the treatment of early PPMS in adults with imaging features characteristic of inflammatory activity. The public financing decision in Portugal was identical to that in the UK, with ocrelizumab being considered a cost-effective treatment for PPMS.
Our cost-effectiveness estimates should be interpreted in light of some limitations.
The results reported in this study reflect the pharmacoeconomic value of ocrelizumab as assessed and validated by the Portuguese Health Authority during the pricing and reimbursement process that took place between January 2018 and January 2020. The assessment scope, as defined by the Portuguese Health Authority, although contemporary at the time of submission, did not reflect certain other DMT for MS such as rituximab, alemtuzumab and cladribine, since at the time of assessment, they were either used off-label or were not yet approved for public financing in Portugal. A further limitation, reporting the pharmacoeconomic value of ocrelizumab as presented during the lengthy pricing and reimbursement process, relates to the fact that EDSS health state and relapse costs were uprated to 2018 values only. Notwithstanding, the impact of this limitation on the analysis results is expected to be small, as the consideration of a reference year for costs of 2021 (included as a scenario in the scenario analysis reported in the supplementary material) lead to similar cost-effectiveness results. This is mostly due to the small changes from 2018 until the end of 2021 in the reference unit costs, namely of the DMTs involved in the analysis.
When performing cost-effectiveness comparisons of multiple treatment options, investigators frequently face challenges related to the absence of head-to-head clinical trials. In this respect, investigators need to rely on indirect treatment comparisons in order to use relative comparative efficacy and safety evidence. The way these indirect treatment comparisons are planned and executed may influence the relative effectiveness of the indirectly compared treatments.
Our effectiveness results for the RMS population are influenced by the systematic review and network meta-analysis comparing ocrelizumab with other treatments for RMS, performed by McCool et al. [35]. These authors report that ocrelizumab has an efficacy superior to or comparable with all other currently approved DMTs, suggesting ocrelizumab was more effective in reducing the risk of 12-week confirmed disability progression. These results are corroborated by other recently published network meta-analyses [52,53,54]. Nonetheless, caution is needed when interpreting differential effectiveness between treatment options without solid head-to-head randomised controlled clinical trials (RCTs). Moreover, risk of bias in trials and heterogeneity between trials should also be considered, as trial conditions may differ from real-world clinical practice, where a patient’s history may influence disease progression or therapeutic choices [23].
An important consideration in estimating cost-effectiveness relates to events occurring outside the timescale of published trials. The RCT period, from which treatment effects have been used is shorter than the lifetime time horizon used in the cost-effectiveness modelling. This is recognised as an additional source of uncertainty. Disease progression was calculated based on EDSS scores. Despite EDSS scores being widely used in clinical trials [47], their utilisation and interpretation in clinical practice decision making may hold different utility.
Quality-of-life weights (utilities) used to calculate QALYs were not obtained from ocrelizumab RCT. Rather, we used EQ-5D-3L based QoL utilities with Portuguese preferences for RMS and PPMS patients by EDSS level from the study of Sá et al. [33]. This option was based on the fact that in the OPERA I and II clinical trials only the change from baseline in the SF-36 quality-of-life physical-component was assessed [12]. It represents a restrictive view of QoL because it lacks important information about the impact on mental domains. With respect to the PPMS cost-effectiveness analysis reference case, it should be noted that additional utility decrements associated with fatigue and upper limb dysfunction were included. This option is questionable since it can result, at least to some extent, in a double counting of the impact of these symptoms on patients’ QoL, and cost-effectiveness results were sensitive to these assumptions.
Natural history data, used to model transition between EDSS scores among untreated patients, were retrieved from long-term registries [28,29,30,31]. Due to the retrospective nature of these registries and older timeframe (1980–2009) they may inaccurately reflect current MS cohorts. However, a different alternative is unrealistic because of the increasing number of treatment options available since the late 1990s.
Despite the aforementioned limitations, there is valuable information about the utilisation of ocrelizumab in real-world conditions that may complement and support the daily decision-making process about the value of different DMT for MS. An accumulating body of evidence supports the effectiveness outcomes and the impact of ocrelizumab in suppressing disease activity with a favourable and consistent safety profile [55], superior persistence and adherence to treatment [56], lower work and activity impairment versus patients treated with other DMTs [57] and predictability and lower treatment costs relative to other infused disease-modifying therapies [58].
5 Conclusions
We demonstrated ocrelizumab to provide important health benefits as a therapy for both RMS and PPMS. For PPMS, ocrelizumab fills a clear clinical gap in current clinical practice. In the case of RMS, we established ocrelizumab’s economic value to be either cost-saving or with cost per QALY likely below commonly accepted cost-effectiveness thresholds. Overall, ocrelizumab is expected to provide good value for money as a treatment option to address the needs of MS patients.
References
Dobson R, Giovannoni G. Multiple sclerosis—a review. Eur J Neurol. 2019;26(1):27–40.
Lublin FD, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–86.
Lunde HMB, et al. Survival and cause of death in multiple sclerosis: a 60-year longitudinal population study. J Neurol Neurosurg Psychiatry. 2017;88(8):621–5.
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907–11.
Gil-González I, et al. Quality of life in adults with multiple sclerosis: a systematic review. BMJ Open. 2020;10(11): e041249.
Jennum P, et al. The socioeconomic consequences of multiple sclerosis: a controlled national study. Eur Neuropsychopharmacol. 2012;22(1):36–43.
Heinzlef O, et al. Economic burden of the out-of-pocket expenses for people with multiple sclerosis in France. PharmacoEconomics-Open. 2020;4(4):593–603.
University of California, S.F.M.E.T, et al. Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol. 2016;80(4):499–510.
Montalban X, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler J. 2018;24(2):96–120.
Lucchetta RC, et al. Disease-modifying therapies for relapsing–remitting multiple sclerosis: a network meta-analysis. CNS Drugs. 2018;32(9):813–26.
Rae-Grant A, et al. Comprehensive systematic review summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):789–800.
Hauser SL, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–34.
Montalban X, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209–20.
Vollmer T et al. Recently Diagnosed Early-Stage RRMS: NEDA, ARR, Disability Progression, Serum Neurofilament and Safety: 1-Year Interim Data From the Ocrelizumab Phase IIIb ENSEMBLE Study (2261). 2021, AAN Enterprises.
Wolinsky JS, et al. Long-term follow-up from the ORATORIO trial of ocrelizumab for primary progressive multiple sclerosis: a post-hoc analysis from the ongoing open-label extension of the randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2020;19(12):998–1009.
Hauser SL, et al. Five years of ocrelizumab in relapsing multiple sclerosis: OPERA studies open-label extension. Neurology. 2020;95(13):e1854–67.
Confavreux C, Vukusic S. The clinical course of multiple sclerosis. Handb Clin Neurol. 2014;122:343–69.
Guo S, et al. Cost-effectiveness analyses in multiple sclerosis: a review of modelling approaches. Pharmacoeconomics. 2014;32(6):559–72.
Parkin D, et al. A cost-utility analysis of interferon beta for multiple sclerosis. Health Technol Assess (Winchester, Engl). 1998;2(4):iii–54.
Kobelt G, et al. Cost-utility analysis of interferon beta-1b in secondary progressive multiple sclerosis. Int J Technol Assess Health Care. 2000;16(03):768–80.
van Munster CE, Uitdehaag BM. Outcome measures in clinical trials for multiple sclerosis. CNS Drugs. 2017;31(3):217–36.
Cortesi PA, et al. The value and sustainability of ocrelizumab in relapsing multiple sclerosis: a cost-effectiveness and budget impact analysis. Farmeconomia. Health Econ Therap Pathways. 2019. https://doi.org/10.7175/fe.v20i1.1435.
Yang H, et al. Cost-effectiveness analysis of ocrelizumab versus subcutaneous interferon beta-1a for the treatment of relapsing multiple sclerosis. J Med Econ. 2017;20(10):1056–65.
Auguste P, et al. Ocrelizumab for treating patients with primary progressive multiple sclerosis: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2020;38(6):527–36.
INFARMED I.P. Relatório de Avaliação Prévia do Medicamento para Uso Humano em Meio Hospitalar; DCI - Ocrelizumab. 2020. https://www.infarmed.pt/documents/15786/1424140/Relat%C3%B3rio+p%C3%BAblico+de+avalia%C3%A7%C3%A3o+de+Medicamento+Ocrevus+%28DCI+ocrelizumab%29+2020/5f7e7157-561c-6e22-5582-199404eedb1f. Accessed 18 June 2020.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–1444.
da Silva E et al. Orientações metodológicas para estudos de avaliação económica de medicamentos, in Autoridade Nacional do Medicamento e Produtos de Saúde IP. 1998.
Palace J, et al. UK multiple sclerosis risk-sharing scheme: a new natural history dataset and an improved Markov model. BMJ Open. 2014;4(1): e004073.
Scalfari A, et al. The natural history of multiple sclerosis, a geographically based study 10: relapses and long-term disability. Brain. 2010;133(7):1914–29.
Patzold U, Pocklington PR. Course of multiple sclerosis: first results of a prospective study carried out of 102 MS patients from 1976–1980. Acta Neurol Scand. 1982;65(4):248–66.
Pokorski RJ. Long-term survival experience of patients with multiple sclerosis. J Insurance Med N Y. 1997;29:101–6.
De Sa J, et al. The prevalence of multiple sclerosis in the District of Santarem, Portugal. J Neurol. 2006;253(7):914–8.
Sá MJ, et al. New insights into the burden and costs of multiple sclerosis in Europe: results for Portugal. Mult Scler J. 2017;23(2_suppl):143–54.
Statistics Portugal. Complete Life Tables Portugal 2015–2017. www.ine.pt. Accessed 29 Aug 2018.
McCool R, et al. Systematic review and network meta-analysis comparing ocrelizumab with other treatments for relapsing multiple sclerosis. Mult Scler Relat Disord. 2019;29:55–61.
NICE The National Institute for Health and Care Excellence, Natalizumab for the treatment of adults with highly active relapsing–remitting multiple sclerosis [TA127].
Orme M, et al. The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value Health. 2007;10(1):54–60.
Tyas D, et al. The distribution of the cost of multiple sclerosis in the UK: how do costs vary by illness severity? Value Health. 2007;10(5):386–9.
Gani R, et al. Cost-effectiveness analyses of natalizumab (Tysabri®) compared with other disease-modifying therapies for people with highly active relapsing-remitting multiple sclerosis in the UK. Pharmacoeconomics. 2008;26(7):617–27.
Daigl M, et al. Impact of disease activity measures on health utilities in PPMS. Value Health. 2017;20(9):A727–8.
NICE The National Institute for Health and Care Excellence, Single technology appraisal: Ocrelizumab for treating primary progressive multiple sclerosis [TA585]—company submission. 2018.
NICE The National Institute for Health and Care Excellence, Daclizumab for treating relapsing–remitting multiple sclerosis. Technology appraisal guidance [TA441].
PMH—Portuguese Ministry of Health, Diário da República, 1.ª série—N.º 132—Portaria n.º 207/2017 de 11 de Julho de 2017. 2018.
SPMS—Shared Services of the Portuguese Ministry of Health, Public Purchasing Catalogue.
Coret F, et al. Onset of secondary progressive multiple sclerosis is not influenced by current relapsing multiple sclerosis therapies. Mult Scler J Exp Transl Clin. 2018;4(2):2055217318783347.
Butzkueven H, et al. Risk of requiring a wheelchair in primary progressive multiple sclerosis: data from the ORATORIO trial and the MSBase registry. Eur J Neurol. 2022;29(4):1082–90.
Uitdehaag BM. Disability outcome measures in phase III clinical trials in multiple sclerosis. CNS Drugs. 2018;32(6):543–58.
Ness N-H, et al. Differentiating societal costs of disability worsening in multiple sclerosis. J Neurol. 2020;267(4):1035–42.
Iannazzo S, Iliza A-C, Perrault L. Disease-modifying therapies for multiple sclerosis: a systematic literature review of cost-effectiveness studies. Pharmacoeconomics. 2018;36(2):189–204.
Chirikov V, et al. Cost-effectiveness of alemtuzumab in the treatment of relapsing forms of multiple sclerosis in the United States. Value Health. 2019;22(2):168–76.
Zimmermann M, et al. Disease-modifying therapies for relapsing–remitting and primary progressive multiple sclerosis: a cost-utility analysis. CNS Drugs. 2018;32(12):1145–57.
Giovannoni G, et al. A systematic review and mixed treatment comparison of pharmaceutical interventions for multiple sclerosis. Neurol Therapy. 2020;9(2):359–74.
Liu Z, et al. Disease modifying therapies in relapsing-remitting multiple sclerosis: a systematic review and network meta-analysis. Autoimmun Rev. 2021;20(6): 102826.
Samjoo IA, et al. Efficacy classification of modern therapies in multiple sclerosis. J Comp Eff Res. 2021;10(6):495–507.
Fernandez-Diaz E, et al. Real-world experience of ocrelizumab in multiple sclerosis in a Spanish population. Ann Clin Transl Neurol. 2021;8(2):385–94.
Engmann NJ, et al. Persistence and adherence to ocrelizumab compared with other disease-modifying therapies for multiple sclerosis in US commercial claims data. J Manag Care Spec Pharm. 2021;27(5):639–49.
Neuberger EE, et al. Work productivity outcomes associated with ocrelizumab compared with other disease-modifying therapies for multiple sclerosis. Neurol Therapy. 2021;10(1):183–96.
Nicholas J, et al. Real-world cost of treatment for multiple sclerosis patients initiating and receiving infused disease-modifying therapies per recommended label in the United States. J Med Econ. 2020;23(8):885–93.
Acknowledgements
G. Marcelli contributed to the manuscript revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by Roche Farmacêutica e Química, Lda., Portugal.
Conflicts of interest/competing interest
PM, BV and JF are employed by Exigo Consultores. The study sponsor contracted with Exigo Consultores for the development of the research project. Exigo Consultores provided support in the form of salaries for authors but did not have any additional role in the study design, data collection and analysis, or preparation of the manuscript. CC has received compensation for advisory board/consulting services to Biogen, Janssen, Merck, Novartis, Roche, and Sanofi, and has been on the speakers’ bureau for Almirall, Biogen, BMS, Janssen, Merck, Novartis, Roche, and Sanofi. JC has received compensation for activity with Almirall, Biogen, Bristol-Myers-Squibb, Janssen, Merck, Novartis, Roche, Sanofi, and Zambon. AS has received compensation for activity with Astra Zeneca, Biogen, Merck, Novartis, Roche, and Sanofi. DF and IM are employees of Roche Farmacêutica e Química, Lda., Portugal.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication (from patients/participants)
Not applicable.
Availability of data and material
All data and material relevant to the analysis are presented in the outlined publication or supplementary material. The model used in this study was provided to the journal’s peer reviewers for their reference when reviewing the manuscript.
Code availability (software application or custom code)
The authors will answer any enquiries regarding the details of the analysis should these have not been answered by the information provided in the methods section.
Author contributions
PM: study design, data collection, analytical calculations, interpretation of results, drafting and critical revision of the manuscript; BV: study design, interpretation of results and critical revision of the manuscript; JF: interpretation of results, drafting and critical revision of the manuscript; CMC: interpretation of results and critical revision of the manuscript; JJC: interpretation of results and critical revision of the manuscript; AVS: interpretation of results and critical revision of the manuscript; DGF: study conception, study design, interpretation of results, critical revision of the manuscript; IM: study conception, study design, interpretation of results, critical revision of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Martins, P., Vandewalle, B., Félix, J. et al. Cost-effectiveness Analysis of Ocrelizumab for the Treatment of Relapsing and Primary Progressive Multiple Sclerosis in Portugal. PharmacoEconomics Open 7, 229–241 (2023). https://doi.org/10.1007/s41669-022-00381-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-022-00381-z