Abstract
Background
In the randomised controlled trial ECHELON-2 (NCT01777152; January 2013), brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin and prednisone (CHP) demonstrated improved efficacy compared with CHOP (CHP and vincristine) in frontline CD30+ peripheral T-cell lymphoma (PTCL), an aggressive cancer with poor survival. In ECHELON-2, 70% of patients had systemic anaplastic large cell lymphoma (sALCL), a subtype of PTCL. Of sALCL patients who progressed from BV+CHP and CHOP, 36% (n = 17) and 56% (n = 36) received subsequent BV-containing therapy, respectively. As BV re-treatment was not funded in England at the time, our objective was to estimate adjusted efficacy and cost-effectiveness by excluding BV re-treatment from BV+CHP.
Methods
To remove the effects of BV re-treatment, the inverse probability of censoring weights (IPCW) and two-stage estimator (TSE) approaches, with and without re-censoring, were applied to overall survival (OS) in the BV+CHP arm of the ECHELON-2 sALCL population. Cost-effectiveness was determined in a three-state partitioned survival (PartSA) model from the perspective of the National Health Service (NHS) in England.
Results
The unadjusted hazard ratio (HR) for death in patients with sALCL with BV+CHP versus CHOP was 0.54 (95% CI 0.34, 0.87; p = 0.011). The model base case used TSE analysis without re-censoring, which provided an adjusted HR for death of 0.55 (95% CI 0.33, 0.86; p = 0.014). Incremental cost-effectiveness ratios (ICERs) including and excluding re-treatment with BV were £29,760/QALY and £27,761/QALY, respectively.
Conclusion
TSE without re-censoring provided the most clinically plausible estimate of survival whilst retaining sufficient information for OS extrapolation. After adjustment for BV re-treatment, BV+CHP remains an efficacious and cost-effective treatment in frontline sALCL compared with CHOP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the Phase III trial ECHELON-2, BV+CHP improved clinical outcomes compared with the current standard-of-care treatment for frontline sALCL, CHOP. |
During the trial, a number of patients in the BV+CHP arm subsequently received re-treatment with BV. Such re-treatment with BV was not funded in England at the time. |
This analysis attempts to remove the effects of re-treatment with BV from the BV+CHP arm using statistical methods designed for adjusting for bias due to crossover in clinical trials. |
Removing the effects of re-treating patients with BV following frontline BV+CHP maintains the efficacy and cost-effectiveness of BV+CHP compared with CHOP. |
1 Introduction
Systemic anaplastic large cell lymphoma (sALCL) is a subtype of peripheral T-cell lymphoma (PTCL); a rare and aggressive heterogeneous subset of non-Hodgkin lymphoma (NHL) characterised by a lack of T-cell related protein marker expression [1, 2], and poor survival [3,4,5,6]. sALCL accounts for approximately 15.8% of all PTCL cases in Europe, with up to 160 newly diagnosed cases of sALCL estimated in the UK annually [4, 5]. Patients are typically diagnosed with late-stage disease (Stage III–IV), and experience systemic symptomatology (B-symptoms, i.e., fever, night sweats, weight loss), requiring therapeutic intervention. Prognosis for patients is poor, with 5-year overall survival rates varying between 13 and 70% [4,5,6,7] depending on the presence or absence of mutant anaplastic lymphoma kinase (ALK), International Prognostic Index (IPI) score, and age [7,8,9].
Current UK treatment guidelines for frontline therapy recommend a maximum of six cycles of systemic combination chemotherapy, consisting of cyclophosphamide (C), doxorubicin (H), vincristine (O) and prednisone (P) (CHOP) [10,11,12,13,14,15]. Despite its position as the current standard-of-care, the evidence base for the use of CHOP in frontline treatment of sALCL lacks robust randomised, controlled, comparative studies, and is largely based on evidence stemming from the use of CHOP in the treatment of diffuse-large B-cell lymphoma, a subtype of NHL [16]. The overall treatment goal for individuals with newly diagnosed sALCL is to attain a deep, durable response with frontline therapy, inducing long-term remission and potentially curing underlying disease. The 5-year failure-free survival of patients with sALCL varies between 36 and 60% when considering key prognostic factors [4]. The risk of relapse after frontline treatment is highest in the first 2 years; patients who are disease free and have not relapsed within 2 years have a low likelihood of future relapse [17]. A retrospective analysis of 775 patients from the USA, Sweden and Canada concluded that the risk of relapse and death significantly decreased for patients with PTCL who remained disease free for 2 years after frontline treatment, and survival approached general population mortality; this trend was consistent for patients with sALCL [17].
Patients with sALCL uniformly present with CD30 expression (CD30+), a type I transmembrane receptor, which, upon stimulation, may activate pro-proliferative and pro-tumourigenic signalling pathways [18, 19]. Brentuximab vedotin (BV) is an antibody-drug conjugate, composed of an anti-CD30 monoclonal antibody conjugated by a protease-cleavable linker to the microtubule-disrupting drug monomethyl auristatin E (MMAE) [20,21,22]. In 2014, BV monotherapy received marketing authorisation from the European Medicines Agency (EMA) for relapsed/refractory (R/R) sALCL. It has become the standard-of-care for R/R sALCL in the UK following a positive National Institute for Health and Care Excellence (NICE) recommendation in 2017 [10]. BV in combination with cyclophosphamide, doxorubicin and prednisone (BV+CHP) received marketing authorisation in frontline sALCL [23] in 2020 on the basis of the pivotal Phase III, double-blind, double-dummy, randomised, controlled trial ECHELON-2, in which BV+CHP demonstrated superiority over CHOP for all primary and key alpha-controlled secondary endpoints. In ECHELON-2, patients with sALCL were a pre-specified study population, and represented 70% (n = 316) of the enrolled population. Randomisation was stratified by histological subtype according to local pathology assessment (ALK-positive sALCL versus all other histologies), and baseline IPI score (0–1 vs. 2–3 vs. 4–5). The sALCL population of ECHELON-2 included 15 patients with sALCL from the UK across four centres.
In the ECHELON-2 intention-to-treat (ITT) population, the risk of a progression-free survival (PFS) event and death was reduced by 29% (stratified hazard ratio (HR): 0.71 [95% confidence interval (CI): 0.54, 0.93]; p = 0.011) and 34% (HR: 0.66 [95% CI 0.46, 0.95]; p = 0.0244), respectively, in patients receiving BV+CHP compared with CHOP. Patients diagnosed with sALCL who received BV+CHP had a 41% reduction in risk of a PFS event (HR: 0.59 [95% CI 0.42, 0.84]; p = 0.0031), and a 46% reduced risk of death (HR: 0.54 [95% CI 0.37, 0.867]; p = 0.0096) compared with those receiving CHOP [24]. Furthermore, 71% of patients with sALCL treated with BV+CHP achieved complete response compared with 53% treated with CHOP [24]. ECHELON-2 demonstrated a favourable efficacy and comparable safety profile for BV+CHP versus CHOP in both the ITT and sALCL populations. Common treatment-emergent adverse events (TEAEs) in the ITT population were similar in the BV+CHP and CHOP arms of the trial, including peripheral neuropathy (BV+CHP: 45%; CHOP: 41%). The majority of TEAEs were less than Grade 3, with the exception of treatment-emergent neutropenia. In ECHELON-2, patients who experienced disease progression following study treatment were eligible to receive BV monotherapy post-progression. Due to study blinding, clinicians were not influenced in treatment choice following relapse, resulting in a proportion of patients being re-treated with BV. In the sALCL subgroup, 36% (n = 17/47) and 57% (n = 36/63) of patients who had non-fatal PFS events received subsequent BV-containing therapy in the BV+CHP and CHOP arms, respectively.
In August 2020, NICE recommended BV+CHP for the frontline treatment of sALCL (TA641) [25]. At the time of the NICE appraisal of BV+CHP, re-treatment with BV was not reimbursed within England. Therefore, while the CHOP arm of ECHELON-2 (i.e., CHOP followed by BV monotherapy in R/R sALCL) is largely representative of clinical practice, the re-treatment with BV observed in the BV+CHP arm did not reflect the locally funded pathway. Therefore, using the unadjusted overall survival (OS) data from the ECHELON-2 trial may bias the estimates of clinical and cost-effectiveness, and adjustment of subsequent therapy was required to accurately reflect clinical practice in England.
Our objective was to estimate adjusted OS by removing the effect of re-treatment with BV in the BV+CHP arm, to reflect the locally funded pathway in England. This enabled us to assess the clinical and cost-effectiveness of BV+CHP in frontline sALCL from the perspective of the National Health Service (NHS) in England.
2 Methodology
2.1 Model Structure
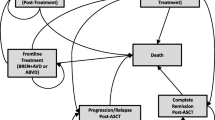
The primary outcome of the model was the incremental cost-effectiveness ratio (ICER), expressed as the cost per quality-adjusted life-year (QALY) gained, in line with NICE guidance [25]. Clinical efficacy, safety and health-related quality of life (HRQoL) data were derived from the pre-specified sALCL population of ECHELON-2. The model was programmed in Microsoft® Excel, and comprised a three-state partitioned survival model (PartSA) to evaluate the expected costs and outcomes of BV+CHP compared with CHOP as frontline therapy in sALCL from the perspective of NHS England. The PartSA comprises three mutually exclusive health states: (1) progression-free disease, (2) progressed disease (PD), and (3) death (Fig. 1). The proportion of patients in each of the health states were derived from the PFS and OS data from the sALCL population of ECHELON-2; the proportion of patients in the progression-free disease health state was estimated based on area under the PFS curve, the proportion of patients in the death health state was estimated based on 1 minus the area under the OS curve, and the proportion of patients within the PD state was estimated as the difference between the OS and PFS curves. The utilisation of the PartSA model structure is common in oncology, and has been implemented in all cost-effectiveness analyses for BV to date. It also aligns with key clinical endpoints of ECHELON-2, PFS and OS, and accurately reflects the nature of the condition. Unadjusted OS outcomes from ECHELON-2 informed the CHOP arm, as the subsequent therapies used were considered reflective of UK clinical practice (as confirmed with clinical experts and local guidelines) [13, 16, 26]. The treatment-switching analysis to remove the impact of re-treatment with BV described below was only applied to the BV+CHP arm.
Outcomes were extrapolated beyond ECHELON-2 and a lifetime time horizon (45 years) was utilised to capture the differences in expected costs and outcomes between model arms. A 21-day cycle length was used to reflect the typical duration of CHOP and BV+CHP treatment cycles. Half-cycle correction was implemented. Costs and outcomes were discounted at 3.5% in line with NICE guidance [27].
2.2 Efficacy Inputs
2.2.1 Adjustment for Re-Treatment with Brentuximab Vedotin (BV)
A detailed description of the methods used are described in the Online Supplementary Material (OSM), section 1, including an overview of the methodology, rationale for methods used, and outputs from these methods. To remove the effects of re-treatment with BV on OS, multiple methods were considered based on treatment-switching approaches described within the NICE Decision Support Unit (DSU) Technical Support Document (TSD) 16 [28], including: the naïve approach, rank-preserving structural failure time models (RPSFTM), the inverse probability of censoring weights (IPCW), and the two-stage estimator (TSE). The TSE and IPCW approaches were the a priori preferred approaches, as the risk of bias associated with naïve approaches and the lack of plausibility for the common treatment effect assumption required for the RPSFT approach made these less suitable. In practice, only the TSE provided logical estimates with plausible underlying assumptions. Therefore, this approach was used in the base case. Alternative methods considered are described in OSM section 1.
The simplified TSE was initially described by Latimer et al. [29]. Based on the assumption that all patients are at a similar stage of disease at the point of disease progression, the effect of re-treatment with BV-containing regimens on survival from the point of disease progression to death could be estimated. The point of disease progression was considered a ‘secondary baseline’, and survival post-progression then estimated from this secondary baseline. An accelerated failure time (AFT) model was estimated and included covariates and an indicator for whether subsequent BV-containing regimens were used. The AFT model was fitted to estimate the treatment effect for those re-treated with BV compared with those who were not in the BV+CHP arm.
Counterfactual survival times were then predicted for each patient using:
where \({T}_{{A}_{i}}\) is the time before disease progression for the \(i\)th individual, \({T}_{{B}_{i}}\) represents the time post-progression, and \({\theta }_{v}\) represents the treatment effect (time ratio) for BV re-treatment use in post-progression survival. This method requires the assumption of ‘no unmeasured confounders’ at the secondary baseline timepoint, and does not require modelling of the process by which patients are re-treated with BV following progression.
Decisions required for the application of the TSE include: (1) which AFT model to use, (2) which covariates to include in the model, and (3) whether or not to include re-censoring [30]. Weibull models were used to estimate post-progression survival, including \({\theta }_{v}\). This was because the generalised gamma model was unable to achieve convergence in several scenarios, presumably because of the relatively low number of patients in the BV+CHP arm who received re-treatment with BV. Note that the use of the Weibull model in this context is distinct from the survival models used in the long-term extrapolation of outcomes (Sect. 2.2.2).
Prognostic covariates tested for inclusion included response to frontline therapy, remission duration, receipt of consolidative autologous stem cell transplant (ASCT) therapy, and other baseline characteristics (IPI score, ALK status, age and region). Only statistically significant predictors were retained in the base-case analysis; these included IPI score, age and time-to-progression.
The process of adjusting survival times introduces an informative censoring bias [28]. For the TSE method, informative censoring is introduced because the counterfactual survival model involves adjusting survival times for those who received re-treatment with BV, but not for those who did not. For some re-treated patients, time of death may not have yet been observed in the unadjusted data, and the TSE would adjust their censoring times. This would result in informative censoring if there were an association between re-treatment with BV and prognosis. In the context of TSE, the process of re-censoring is summarised by Latimer et al. [31].
An important consequence of re-censoring is that longer-term information is discarded, which can be problematic for cost-effectiveness analyses requiring extrapolation of long-term survival [30]. Studies investigating treatment switching have concluded that analyses should be conducted with and without re-censoring [31]. In the present scenario, historical data and clinical experts suggested that the hazard of death in patients with sALCL reduces significantly for patients who have remained disease free for 2 years after frontline treatment, and survival approached general population mortality. Therefore, in discarding long-term data, re-censoring may omit the observed change in the shape of the hazard.
On the basis that the objective was to fit parametric survival models to trial data to extrapolate into the future, where long-term trends are highly important, the base-case analysis excludes re-censoring. Sensitivity analysis was performed including re-censoring.
2.2.2 Extrapolation of Overall Survival (OS) and Progression-Free Survival (PFS)
Parametric survival models were used to extrapolate OS and PFS data beyond the observed period from ECHELON-2 (data cut-off: 15 August 2018). Visual inspection of the log-cumulative hazard plots and hypothesis testing by means of the Schoenfeld test of residuals with respect to time suggested that the proportional hazards assumption was not violated (see OSM section 6). Therefore, a joint modelling approach, in which the effect of treatment is represented by a coefficient within a statistical model estimated in both BV+CHP and CHOP arms, was adopted in the base case. Parametric curves were fitted to the PFS and OS data from ECHELON-2; models using both unadjusted and counterfactual ‘adjusted’ outcomes (OS only) were estimated separately to estimate long-term outcomes (generalised gamma, Weibull, exponential, log-normal, log-logistic and Gompertz) in line with NICE Technical Support Document 14 [32]. Clinical experts were asked to select the most representative predictions from the candidate curves. Feedback indicated that the risk of relapse would be low after 2 years for patients who remain progression-free and this would align with a risk of death similar to the general population. The generalised gamma distribution aligned with the expected HR over time and was selected for both PFS and OS in the base case. Generalised gamma curves were applied in the model base case (OS and PFS) and yielded the most conservative cost-effectiveness results of the distributions considered.
In the long term, the risk of mortality was estimated based on the age- and gender-matched general population and adjusted using a standardised mortality ratio of 1.21. This mortality ratio was based on UK clinical input and reflects the small reduction in life-expectancy associated with increased rates of cardiac toxicity, and a small increased risk of secondary primary malignancies.
2.2.3 Other Clinical Parameters
In ECHELON-2, BV+CHP was administered across a 21-day cycle for a maximum of six to eight cycles. The base case was informed by the average duration from the trial (6.2 and 5.8 treatment cycles of BV+CHP and CHOP, respectively). This aligned with expected clinical practice in the UK; unanimous clinical feedback indicated that a maximum of six cycles is used in Europe in frontline sALCL and PTCL. The sALCL population from ECHELON-2 informed the following inputs: adverse events (Grade 3–4 TEAEs occurring in ≥ 5% of patients, apart from Grade 1–2 diarrhoea, which was identified by clinical experts as being of clinical interest), subsequent therapy and consolidative stem cell transplantation (SCT)/radiotherapy. All inputs are reported in OSM section 2.
2.3 Health-Related Quality of Life (HRQoL)
During ECHELON-2, EQ-5D-3L data were collected until patient death or study closure, whichever came first. The EQ-5D-3L tariff from Dolan [33] was applied to individual responses to generate EQ-5D-3L index scores. At baseline, 310 valid EQ-5D-3L questionnaires were available for analysis. Mean EQ-5D-3L was 0.60 (standard deviation (SD): 0.37), with an imbalance between the treatment arms (BV: 0.58, CHOP: 0.63; p = 0.2413). In the base case, covariates representing how close an observation was to the time of patients’ death were included to allow prediction of HRQoL as patients approached death, in addition to baseline EQ-5D, age, presence of adverse events and SCT.
As may be expected, HRQoL declined significantly as patients approached death (Table 1). Additionally, given the severity of episodes of Grade 3–4 peripheral neuropathy, a decrement of – 0.33 (taken from Swinburn et al. [34]) was applied to the number of events per patient across the time horizon, for the duration of 4.34 days in the BV+CHP arm and 3.65 days in the CHOP arm, estimated from ECHELON-2 [25, 34]. This effect was considered based on clinical feedback.
2.4 Costs
The cost year implemented in the analysis was 2018/2019; list prices were used for all treatments and do not reflect any confidential discounts. Drug acquisition costs used were taken from UK-specific public sources [35]. For BV, the method of moments was used to calculate the distribution of cost, based on observed weight at baseline in ECHELON-2. Costs for adverse events and administration were taken from the NHS Reference Costs [36]. It was assumed that all patients would be concomitantly administered granulocyte colony stimulating factor as primary prophylaxis, as this is expected in UK clinical practice [37].
The costs of use of salvage chemotherapy, SCT and radiotherapy were informed by ECHELON-2. Treatment with subsequent BV monotherapy in the CHOP arm was assumed to be administered for six cycles. The costs of SCT were taken from related technology appraisals and inflated to the 2018/2019 cost year using inflation indices from the Personal Social Services Research Unit (PSSRU) [38]. Medical resource use costs and frequencies were informed by the London Cancer Alliance documentation on follow-up care with CHOP chemotherapy [37] and resource use estimates presented in the NICE economic evaluation of BV in R/R sALCL [25]. Different assumptions were made in pre- and post-progression health states to reflect the varying intensities of follow-up care, based on clinical input. The frequency and nature of monitoring modelled in the cost-effectiveness analysis were validated by clinical experts. Full details of the costs included in the analysis are reported in OSM sections 3 (primary therapy and concomitant medication) and 4 (subsequent/salvage therapies).
2.5 Sensitivity Analysis
Individual parameter uncertainty was tested using univariate sensitivity analysis, in which all model parameters were systematically and independently varied over a plausible range, determined by either the 95% CI or ± 15%, where no estimates of precision were available. The ICER was recorded at the upper and lower values to produce a tornado diagram. Scenario analyses were performed to explore structural changes and assumptions.
Joint parameter uncertainty was explored through probabilistic sensitivity analysis (PSA), in which all parameters were assigned distributions and varied jointly. Correlation between parameters in the time-to-event and EQ-5D models was preserved and sampled using multivariate normal distributions. Costs and medical resource use estimates were assumed to follow log-normal distributions, and proportions (such as the proportion of patients receiving ASCT) were sampled from beta distributions. The distribution of subsequent treatments was sampled from a Dirichlet distribution. 5000 Monte Carlo simulations were recorded. From this, results were plotted on the cost-effectiveness acceptability plane and the probability of being cost-effective was calculated at different willingness-to-pay (WTP) thresholds. Results of the scenario analysis are presented in OSM section 5.
3 Results
3.1 Adjustment for Re-Treatment with Brentuximab Vedotin (BV)
In the unadjusted analysis, patients in the sALCL population of ECHELON-2 who received BV+CHP had a 46% reduced risk of death compared with those receiving CHOP (HR: 0.54 (95% CI 0.37, 0.87); p = 0.0096). The statistical model predicting re-treatment in the IPCW approach had poor predictive power. Estimates from IPCW were consequently counterintuitive and closely aligned with naïve approaches that censor on receipt of re-treatment (HR: 0.46 (95% CI 0.27, 0.77); full details in OSM section 1). The TSE with and without re-censoring provided hazard ratios for death of 0.41 (95% CI 0.15, 0.87; p = 0.022) and 0.55 (95% CI 0.33, 0.86; p = 0.014), respectively. The model of post-progression survival used within the TSE analysis is presented in Table 2.
The adjusted OS data including and excluding re-censoring utilising the TSE method are presented in Fig. 2. The effect of adjustment on the Kaplan-Meier estimator was minor, reflecting the relatively low number of patients who received re-treatment. However, the loss of long-term follow-up in the BV+CHP arm in the re-censored analysis was pronounced, and as a consequence of discarding much of the observed data, the resulting point estimate was (counterintuitively) improved versus the unadjusted analysis. The BV+CHP arm of ECHELON-2 was associated with a reduction in the long-term hazard over time, ultimately leading to a sustained event-free period towards the end of study follow-up (Fig. 2). Thus, re-censoring was deemed to discard important evidence in the BV+CHP arm of the changing hazards over time and was not included in the base-case analysis. The long-term predicted OS and PFS including (adjusted) general population mortality are presented in Fig. 3.
Base-case extrapolations including general population mortality. a OS with adjustment for BV re-treatment, b PFS. BV+CHP brentuximab vedotin cyclophosphamide doxorubicin prednisone, CHOP cyclophosphamide doxorubicin vincristine prednisone, OS overall survival, PFS progression-free survival, sALCL systemic anaplastic large cell lymphoma
3.2 Cost-Effectiveness
In the base-case analysis, excluding re-treatment with BV, BV+CHP is associated with incremental costs of £40,826 and 1.47 incremental QALYs, resulting in an ICER of £27,761 per QALY gained versus CHOP (Table 3). The effect of removing re-treatment with BV reduced the ICER from £29,760 per QALY gained to £27,761 per QALY gained. This was due to reduced efficacy following the removal of re-treatment with BV in the BV+CHP arm, offset by lower costs (Table 3).
Univariate sensitivity analysis suggested the most influential parameter was the estimated treatment effect for BV+CHP versus CHOP in OS from the TSE model (Fig. 4a). The model was moderately sensitive to other model parameters, including those in the statistical model predicting EQ-5D. The scenario that included re-censoring resulted in an ICER of £25,424. Most scenarios that considered alternative distributions for OS and PFS resulted in lower estimates of the ICER (with the exception of the Gompertz distribution, which resulted in a 2% increase in the ICER). Scenarios that reduced the time horizon led to increased ICERs as the costs of BV+CHP are incurred upfront, but benefits are accrued over the lifetime of patients (see OSM section 5). Omitting any confidential discounts on acquisition costs, the probability BV+CHP is cost-effective versus CHOP at a WTP threshold of £30,000 was estimated at 54% (Fig. 4).
4 Discussion and Conclusion
This cost-effectiveness analysis has demonstrated that for the treatment of frontline sALCL, BV+CHP is associated with incremental costs of £40,826, an incremental life-year (LY) gain of 1.96 years, and an incremental gain of 1.47 QALYs, compared with CHOP. The resulting ICER is £27,761 per QALY gained, and therefore BV+CHP is a cost-effective treatment based on NICE’s standard WTP threshold of £30,000. The probabilistic ICER was £28,383, which is congruent with the deterministic ICER of £27,761. The proportion of simulations considered cost-effective at ICER thresholds of £20,000 and £30,000 (omitting any confidential discounts) were of 10% and 54%, respectively.
The quality of the clinical evidence from the ECHELON-2 clinical trial contributes to the strength of the treatment switching and economic analyses. ECHELON-2 is a high-quality double-blind, double-dummy, randomised, controlled trial that compares BV+CHP with the current standard-of-care in UK clinical practice, CHOP. Five UK centres participated in ECHELON-2, and clinical experts confirmed that the patient population is reflective of UK clinical practice. Furthermore, the trial provides data for 316 patients with sALCL and has a median follow-up of 36.2 months, representing a large dataset with relatively long follow-up.
The results from the economic model were considered robust when testing for structural and parameter uncertainty. The one-way sensitivity analyses identified that the results of the economic model are largely driven by the improved survival in the BV+CHP arm relative to the CHOP arm. In the base case, to reflect UK clinical practice, the impact of re-treatment with BV was removed from the BV+CHP arm. However, results remain cost-effective when using both the adjusted and unadjusted data. Notably, the conclusion of cost-effectiveness is maintained under all parametric curve choices.
The uncertainty associated with the treatment-switching analyses has been explored through different treatment-switching methodologies, including IPCW and TSE. The failure of the IPCW to produce plausible estimates of the treatment effect was likely caused by a low number of observations of those receiving re-treatment with BV, and the lack of detailed information on time-varying confounders from which the possibility of re-treatment could be predicted. The assumptions underpinning the TSE method appeared plausible, and results were in line with clinical expectations. Note that the point estimate HR for BV+CHP compared with CHOP improved in the statistical adjustments of OS and remains statistically significant, despite the additional uncertainty introduced through treatment-switching adjustments.
Where available, published recommendations have been adhered to and these have been referenced where appropriate. Finally, inputs and extrapolated endpoints informing the economic model were validated by 12 clinical experts from the UK across two advisory boards and multiple interactions. This allowed the model to be developed to accurately reflect the outcomes for standard-of-care in clinical practice.
To adjust estimates of survival to better reflect local reimbursement realities, our analyses utilised a novel application of existing methods. These methods were applied in the base-case scenario of NICE submission TA641 (BV+CHP in the treatment for untreated sALCL) [25], which received a positive recommendation in 2020. A limitation of this cost-effectiveness analysis is that the sALCL population is a subgroup within the ECHELON-2 trial and not the full ITT population. However, this was a pre-specified subgroup that formed the majority of the population (n = 316/452; 70%) and retained the rigour of a large data set with substantial follow-up. The model also assumes a starting age equal to that of the ECHELON-2 sALCL cohort (52 years). This is slightly younger than suggested by other sources for a UK population. However, sensitivity analysis has demonstrated that this makes only a modest difference to the cost-effectiveness of BV+CHP.
In conclusion, we consider BV+CHP to be a cost-effective frontline treatment option for adults with previously untreated sALCL irrespective of whether re-treatment with BV is available.
References
Tang T, et al. Peripheral T-cell lymphoma: review and updates of current management strategies. Adv Hematol. 2010;2010: 624040. https://doi.org/10.1155/2010/624040.
Herbst H, et al. Immunoglobulin and T-cell receptor gene rearrangements in Hodgkin’s disease and Ki-1-positive anaplastic large cell lymphoma: dissociation between phenotype and genotype. Leuk Res. 1989;13(2):103–16. https://doi.org/10.1016/0145-2126(89)90134-3.
Foss FM, Zinzani PL, Vose JM, Gascoyne RD, Rosen ST, Tobinai K. Peripheral T-cell lymphoma. Blood. 2011;117(25):6756–67. https://doi.org/10.1182/blood-2010-05-231548.
Vose J, Armitage J, Weisenburger D, T. C. L. P. International. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–30. https://doi.org/10.1200/JCO.2008.16.4558.
Haematological Malignancy Research Network (HMRN). T-cell Lymphoma. https://www.hmrn.org/statistics/disorders/34. Accessed 10 Mar 2021.
Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin’s lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol. 1998;9(7):717–20. https://doi.org/10.1023/a:1008265532487.
Weisenburger DD, et al. Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood. 2011;117(12):3402–8. https://doi.org/10.1182/blood-2010-09-310342.
Gleeson M, et al. Outcomes following front-line chemotherapy in peripheral T-cell lymphoma: 10-year experience at The Royal Marsden and The Christie Hospital. Leuk Lymphoma. 2018;59(7):1586–95. https://doi.org/10.1080/10428194.2017.1393671.
Sibon D, et al. Long-term outcome of adults with systemic anaplastic large-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte trials. J Clin Oncol. 2012;30(32):3939–46. https://doi.org/10.1200/JCO.2012.42.2345.
National Institute for Health and Care Excellence (NICE). TA478: Brentuximab vedotin for treating relapsed or refractory systemic anaplastic large cell lymphoma. 2017. https://www.nice.org.uk/guidance/ta478. Accessed 14 Aug 2020.
Abouyabis AN, Shenoy PJ, Sinha R, Flowers CR, Lechowicz MJ. A Systematic Review and Meta-Analysis of Front-line Anthracycline-Based Chemotherapy Regimens for Peripheral T-Cell Lymphoma. ISRN Hematol. 2011;2011: 623924. https://doi.org/10.5402/2011/623924.
d’Amore F, et al. Peripheral T-cell lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v108–15. https://doi.org/10.1093/annonc/mdv201.
Dearden CE, et al. Guidelines for the management of mature T-cell and NK-cell neoplasms (excluding cutaneous T-cell lymphoma). Br J Haematol. 2011;153(4):451–85. https://doi.org/10.1111/j.1365-2141.2011.08651.x.
National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: T-Cell Lymphomas. Version 2.2019. http://med.stanford.edu/content/dam/sm/cutaneouslymphoma/documents/2018_Dec_t-cell.pdf. Accessed 26 Jan 2021.
Robinson M, Podjonjak T, Turner B, Field F. A survey of clinical practice for the front-line treatment of peripheral Tcell lymphoma (PTCL) in the United Kingdom. In: PCN515. Presented at: 2019-11, ISPOR Europe 2019, Copenhagen, Denmark. 2019.
Dearden CJ, Pettengell RR, Devereux S, Cwynarski K, Whittaker S, McMillan A. Guidelines for the management of mature T-cell and NK-cell neoplasms (excluding cutaneous T-cell lymphoma). Br J Haematol. 2013. https://b-s-h.org.uk/media/2895/t-nhl-guideline-3-8-13-updated-with-changes-accepted-v1-rg.pdf. Accessed 10 Mar 2021.
Maurer MJ, et al. International Assessment of Event-Free Survival at 24 Months and Subsequent Survival in Peripheral T-Cell Lymphoma. J Clin Oncol. 2017;35(36):4019–26. https://doi.org/10.1200/JCO.2017.73.8195.
Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119(3):651–65. https://doi.org/10.1182/blood-2011-04-325225.
Mir SS, Richter BW, Duckett CS. Differential effects of CD30 activation in anaplastic large cell lymphoma and Hodgkin disease cells. Blood. 2000; 96(13): 4307–12. [Online]. https://www.ncbi.nlm.nih.gov/pubmed/11110706.
Horwitz S, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229–40. https://doi.org/10.1016/S0140-6736(18)32984-2.
Donato EM, Fernandez-Zarzoso M, Hueso JA, de la Rubia J. Brentuximab vedotin in Hodgkin lymphoma and anaplastic large-cell lymphoma: an evidence-based review. Onco Targets Ther. 2018;11:4583–90. https://doi.org/10.2147/OTT.S141053.
Schonberger S, et al. Brentuximab vedotin exerts profound antiproliferative and pro-apoptotic efficacy in CD30-positive as well as cocultured CD30-negative germ cell tumour cell lines. J Cell Mol Med. 2018;22(1):568–75. https://doi.org/10.1111/jcmm.13344.
European Medicines Agency (EMA). Committee for Medicinal Products for Human use (CHMP) agenda for the meeting on 14-17 October 2019. https://www.ema.europa.eu/en/documents/agenda/agenda-chmp-agenda-14-17-october-2019-meeting_en.pdf. Accessed 26 Jan 2021.
European Medicines Agency (EMA). Adcetris, INN-brentixumab vedotin. Summary of Product Characteristics. 2022. https://www.ema.europa.eu/en/documents/product-information/adcetris-epar-product-information_en.pdf. Accessed 26 Jan 2021.
National Institute for Health and Care Excellence (NICE). Brentuximab vedotin in combination for untreated systemic anaplastic large cell lymphoma, in Technology appraisal guidance. 2020. https://www.nice.org.uk/guidance/ta641/documents/final-appraisal-determination-document. Accessed 26 Jan 2021.
National Institute for Health and Care Excellence. NICE NG52. Non-Hodgkin's lymphoma: diagnosis and management. 2016. [Online]. https://www.nice.org.uk/guidance/ng52/evidence/full-guideline-2551524594. Accessed 11 May 2022.
National Institute for Health and Care Excellence (NICE). Guide to the methods of technology appraisal 2013. https://www.nice.org.uk/guidance/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. Accessed 26 Jan 2021.
Latimer NR, Abrams KR. NICE DSU technical support document 16: adjusting survival time estimates in the presence of treatment switching. London: NICE Decision Support Unit Technical Support Documents; 2014.
Latimer NR, et al. Adjusting for treatment switching in randomised controlled trials—a simulation study and a simplified two-stage method. Stat Methods Med Res. 2017;26(2):724–51. https://doi.org/10.1177/0962280214557578.
Latimer NR, Abrams KR, Siebert U. Two-stage estimation to adjust for treatment switching in randomised trials: a simulation study investigating the use of inverse probability weighting instead of re-censoring. BMC Med Res Methodol. 2019;19(1):69. https://doi.org/10.1186/s12874-019-0709-9.
Latimer NR, White IR, Abrams KR, Siebert U. Causal inference for long-term survival in randomised trials with treatment switching: Should re-censoring be applied when estimating counterfactual survival times? Stat Methods Med Res. 2019;28(8):2475–93. https://doi.org/10.1177/0962280218780856.
National Institute for Health and Care Excellence (NICE). NICE DSU Technical Support Document 14: Survival analysis for economic evaluations alongside clinical trials-extrapolation with patient-level data. 2013. http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf. Accessed 10 Mar 2021.
Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–108. https://doi.org/10.1097/00005650-199711000-00002.
Swinburn P, Shingler S, Acaster S, Lloyd A, Bonthapally V. Health utilities in relation to treatment response and adverse events in relapsed/refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Leuk Lymphoma. 2015;56(6):1839–45. https://doi.org/10.3109/10428194.2014.970542.
National Institute for Health and Care Excellence (NICE), British National Formulary (BNF). https://bnf.nice.org.uk/. Accessed 12 June 2019.
National Health Service (NHS), "Reference costs/NHS improvement. https://improvement.nhs.uk/resources/reference-costs/#rc1718. Accessed 12 Aug 2019.
National Health Service (NHS), "London Cancer Alliance. CHOP 21 +/- Rituximab for Non-Hodgkin's Lymphoma (NHL) protocol. 2011. http://www.londoncanceralliance.nhs.uk/media/36668/NHL_CHOP-R_Protocol_V1_1.pdf. Accessed 4 Jun 2019.
Personal Social Services Research Unit (PSSRU), "Unit Costs of Health and Social Care. 2018. https://www.pssru.ac.uk/project-pages/unit-costs/. Accessed 4 Jun 2019.
Acknowledgements
The authors would like to acknowledge Fiona Field and Meredith Little for providing clinical input, Eugene Benson for contributing to the development of the analysis, and Dr Jennifer Ferris of Source Health Economics for providing medical writing and editorial support in the development of the manuscript, which was funded by Takeda UK Limited.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was funded by Takeda UK Limited.
Conflicts of interest/Competing interests
HC is an employee of Takeda Pharmaceuticals International Co. TP is an employee of Takeda UK Limited. DT, EE, and FW are employees of Source Health Economics who received funding for this research.
Ethics approval
Not required.
Consent to participate
Not required.
Consent for publication
Not required.
Availability of data and material
No additional data or materials are available.
Code availability
The economic model used in this study is proprietary intellectual property.
Author contributions
HC and TP were responsible for study conception and design. DT performed the statistical analysis and prepared the first draft of this manuscript. EE and FW developed the economic model. All authors contributed to the interpretation of the results, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Cranmer, H., Trueman, D., Evers, E. et al. Brentuximab Vedotin Plus CHP in Frontline sALCL: Adjusted Estimates of Efficacy and Cost-Effectiveness Removing the Effects of Re-Treatment with Brentuximab Vedotin. PharmacoEconomics Open 6, 881–892 (2022). https://doi.org/10.1007/s41669-022-00349-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-022-00349-z