Abstract
Background
Targeted temperature management (TTM) has been shown to improve neurological outcomes and survival in patients resuscitated from cardiac arrest; however, the cost effectiveness of multiple TTM methods is not well studied.
Objective
This study aimed to evaluate the cost effectiveness of intravascular temperature management (IVTM) using Thermogard XP compared with surface cooling methods after cardiac arrest in the England from the perspectives of the UK national health service and Personal Social Services.
Methods
We developed a multi-state Markov model that evaluated IVTM (Thermogard XP) compared with surface cooling using two different devices (Blanketrol III and Arctic Sun 5000) over a short-term and lifetime time horizon. Model input parameters were obtained from the literature and local databases. We assumed a hypothetical cohort of 1000 patients who required TTM after cardiac arrest per year in the England. The outcomes were costs (in £, year 2019 values) and quality-adjusted life-years (QALYs), discounted at 3.5% annually. Deterministic and probabilistic sensitivity analyses were undertaken to examine the effect of alternative assumptions and uncertainty in model parameters on the results.
Results
The cost-effectiveness analysis determined that Thermogard XP resulted in direct cost savings of £2339 and £2925 (per patient) compared with Blanketrol III and Arctic Sun 5000, respectively, and a gain of 0.98 QALYs over the patient lifetime. The probabilistic sensitivity analysis demonstrated that the probability of Thermogard XP being cost saving would be 69.2% and 65.3% versus the Arctic Sun 5000 and Blanketrol III, respectively.
Conclusion
Implementation of IVTM using Thermogard XP can lead to cost savings and improved patient quality of life versus surface cooling methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intravascular temperature management (IVTM) is associated with better neurological outcomes and survival rates than are surface cooling methods over hospital discharge duration. |
IVTM led to lower costs in the long term and was associated with better quality of life than surface cooling methods. |
Using IVTM in patients with cardiac arrest can be considered a cost-saving strategy. |
1 Introduction
Sudden cardiac arrest (SCA) is the third leading cause of death in Europe. Overall, less than 8% of patients experiencing out-of-hospital cardiac arrest (OHCA) survive to hospital discharge in England [1], an average that is also seen across Europe [2]. When cardiac arrest occurs in hospital (IHCA), higher survival rates of 15–34% across Europe are observed [2]. Independent of the setting of the event (OHCA or IHCA), SCA leads to loss of consciousness and death unless emergency resuscitation is given and the heart can be restarted [3]. Severe neurological injury has been considered to be the main consequence of SCA following successful resuscitation [4]. Irreversible brain injury is the most common cause of death in the post-cardiac arrest phase [5]. The limited available evidence also suggests that the health system costs associated with SCA in England and beyond are significant [6,7,8,9].
According to the literature, targeted temperature management (TTM) following cardiac arrest improves neurological and survival outcomes [10]. Leading medical societies recommend temperature management as part of the standard of care for patients with cardiac arrest [11]. In England, OHCA affects 30,000 people each year, corresponding to an incidence of 53 per 100,000 inhabitants [1]. Of these, approximately one-quarter can be hospitalised with a return of spontaneous circulation (ROSC) for post-resuscitation therapy [1]. Around 84% of these patients remain unconscious/comatose and are therefore indicated to receive TTM [12].
Multiple methods of TTM are in clinical use [13]. Different cooling methods have specific capabilities of extracting heat, which translate to varying rates of achieving the intended target temperature and varying abilities to accurately and precisely maintain a target temperature and control the rewarming phase after the TTM protocol [14]. TTM induced by surface cooling systems (SCS), intravascular temperature management (IVTM), and a combination of cooling methods has become standard therapy following cardiac arrest and is recommended by the UK National Institute for Health and Care Excellence (NICE) [3].
SCS works by circulating cold fluid or cold air through blankets or pads that are wrapped around the patient [14]. Conversely, IVTM (Thermogard XP®) controls a patient's body temperature through central venous heat exchange [13]. It can be used to induce and maintain TTM in critically ill patients after cardiac arrest [15]. IVTM uses central venous catheters placed in the subclavian, internal jugular, or femoral veins. Temperature control is achieved by circulating cool or warm saline in a closed loop through the catheter’s balloon [15].
The objective of this study was to estimate the costs and effectiveness of IVTM using Thermogard XP versus SCS as standard of care among a hypothetical cohort of 1000 patients who need TTM after cardiac arrest per year in the UK national health service (NHS).
2 Methods
2.1 Model Overview
We developed a de novo economic model based on the current pathway for TTM following cardiac arrest. The population was a hypothetical cohort of 1000 patients (aged ≥ 18 years) with ROSC after cardiac arrest who were admitted to critical care with cardiac arrest as a primary or secondary diagnosis, as documented with the code ‘I460 Cardiac arrest with successful resuscitation’ from the International Classification of Diseases and Related Health Problems, Tenth Revision [16].
The outcomes of interest in the analysis included improved neurological outcomes at hospital discharge and over a lifetime time horizon, long-term survival rates, reduced adverse events (AEs), total costs for each strategy, and incremental cost per quality-adjusted life-year (QALY) as a measure of the value of health outcomes gained. For each treatment arm, costs and outcomes were aggregated based on a series of decisions and events that were represented in the model structure. The structure of the model was the same for the two treatment strategies. The recommended discount rate in the UK of 3.5% per annum for both costs and benefits was applied [17]. As per NICE guidelines [18], the base-case model considered all costs and health effects from the perspective of the UK NHS and Personal Social Services. To fully capture the costs and benefits of Thermogard XP and the comparator(s), a lifetime time horizon was applied in the base-case analysis.
2.2 Intervention and Comparator
The intervention in this study was IVTM using Thermogard XP, applied in a hospital setting to control a patient's body temperature through central venous heat exchange. The current European Resuscitation Council and European Society of Intensive Care Medicine guidelines on post-resuscitation care recommend TTM for adults after either OHCA or IHCA with any initial rhythm who remain unresponsive after ROSC [11]. Body temperature is to be maintained at a constant value between 32 and 36 °C for at least 24 h. As comparators, to address heterogeneity in device costs, we considered Arctic Sun 5000 and Blanketrol III as two different devices that may be used as part of the surface cooling technique. Surface cooling is considered standard care in the NHS, based on the interventional procedure guidelines of TTM following cardiac arrest developed by NICE [3].
2.3 Model Structure
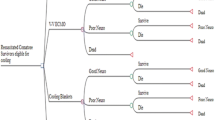
The economic model was developed in Microsoft Excel. A two-part economic model was developed, consisting of a short-term decision tree and a long-term Markov model. The decision tree has a short time horizon that estimates costs and effectiveness up until hospital discharge, and the Markov model is used to follow individual patients with good and poor neurological outcomes over the analysis’ lifetime time horizon, in annual cycles. The Markov model comprises three health states used for survivors: good neurological outcome, poor neurological outcome, and death. The neurological outcome is defined based on cerebral performance category (CPC), which is a validated scoring system for early stratification of neurological outcomes after cardiac arrest [19]. Based on this tool, CPC 1 and CPC 2 were considered good neurological outcomes, and CPC 3 and CPC 4 were considered poor neurological outcomes [19]. The probabilities of incurring different neurological outcomes in the decision tree component of the model were determined at hospital discharge following the cardiac arrest event and were presumed to remain constant thereafter. All patients experiencing different levels of neurological outcomes entered the long-term survival Markov model, where they remained until they died according to long-term mortality based on their assigned level of neurological outcome. The model structure is illustrated in Fig. 1.
Economic model structure. Percentage of good neurological outcome = (1 − probability of poor neurological outcome derived from meta-analysis) (see Table 1); percentage of poor neurological outcome = (1 − mortality rate in each arm derived from meta-analysis). Percentage of mortality = mortality rate in each arm derived from meta-analysis. The probability of a poor neurological outcome was derived from the meta-analysis (see Table 1)
2.4 Model Inputs
2.4.1 Clinical Efficacy
We conducted a subgroup meta-analysis based on data from studies included in two recent reviews and meta-analyses [13, 20] to estimate the pooled probability of unfavourable neurological outcomes and mortality over the hospital admittance duration (short-term decision tree). Since these two recent, larger meta-analyses did not categorise studies in terms of time or duration of admittance, with all included studies with different time horizons pooled together, their output was not suitable for direct inclusion in our own analysis. Therefore, to increase the reliability of our model and to ensure consistency with our short-term horizon (at hospital discharge), we used data from the included studies and conducted a separate re-pooled analysis to estimate neurological outcome and mortality probabilities over the initial hospital discharge period (see the electronic supplementary material [ESM] SI-I).
We estimated the pooled probability of a neurological outcome with IVTM versus SCS. As evidence was lacking, we assumed the same clinical efficacy for two different surface cooling devices (Blanketrol III and Arctic Sun 5000). Finally, we calculated the percentage of patients in each health state in our decision tree model over a short-term time horizon according to results of the pooled estimate of clinical efficacy.
To estimate long-term mortality based on the level of neurological outcome, we digitalised data from published Kaplan–Meier estimates for survival according to CPC score [21] and used them to estimate the parameters for parametric survival curves based on the methodology provided by Hoyle and Henley [22]. Parametric survival models were fitted to the overall survival data to extrapolate survival beyond the period of observation seen during the studies. Transition probabilities between health states for the Markov model were determined from these parametric survival functions. All estimated parametric survival functions are presented in the ESM (SI-II). All model input parameters are presented in Table 1.
2.4.2 Quality of Life
To estimate the total QALYs gained in the Markov model, survival time was adjusted by health-related quality of life. The utility values/quality-of-life inputs included utilities associated with health states and the disutility associated with treatment-related AEs.
Utility weights were sourced from multiple studies. In the base case, we used results from a UK-based study [23]; however, we also applied data from other relevant studies to estimate the range of utility weights. Detailed information regarding sources of utility value are presented in the ESM (SI-III).
Health-related quality of life associated with cardiac arrest survival based on patient's CPCs was derived from relevant published literature and previous economic evaluation studies in this area and are presented in Table 1 [24]. Following the literature, we assumed a constant utility following survival after cardiac arrest [9].
2.4.3 Adverse Events
The most common AEs associated with IVTM and SCS included shivering, temperature overcooling, local or skin injury, deep venous thrombosis (DVT), serious bleeding requiring transfusion, arrhythmias, pneumonia, and sepsis. We derived the risk difference in AEs in IVTM versus SCS groups based on the results of a recent random-effects meta-analysis study [13]. The risk difference in occurrence of AEs, along with the disutility of each event, are presented in Table 1. Utility decrements or disutilities associated with AEs are equivalent to the annual reduction in utility due to an AE that was derived from previous economic evaluations or studies [25,26,27,28].
2.4.4 Costs
The following costs were included in the model: annual capital cost and consumable cost of Thermogard XP and of each SCS, the cost associated with a stay in the intensive care unit (ICU) and hospital ward, and costs associated with the treatment of AEs. All cost input parameters are reported in Table 1. The cost of Thermogard XP was extracted from the Medtech innovation briefing developed by NICE and NHS supply chain [15], and the costs of the SCS devices were derived from different Medtech innovation briefings developed by NICE [29]. Healthcare resource utilisation costs associated with particular neurological outcomes, including length of stay, were derived from a UK single-centre cost study that estimated the hospital costs of patients with OHCA treated in ICUs, evaluated using the national tariff-based system [9]. The long-term cost of poor and good neurological outcomes were sourced from a US-based study [30]. We converted international cost-effectiveness data to UK prices [31] and used gross domestic product per capita in purchasing power standards to convert US-based costs to UK-specific costs.
AE costs were obtained from NHS reference costs [32] and were adjusted by the rate of each AE in the two arms. Costs were measured in UK pound sterling (£), year 2019 values.
2.5 Analysis
The cumulative estimates of costs and effectiveness were reported for each treatment arm. The incremental cost per life-year and incremental cost per QALY gained were reported. Deterministic sensitivity analyses (DSAs) were conducted to investigate the impact of key assumptions and parameter values used in the base-case analysis using the hypothetical increases or decreases of 25%. The results were reported using tornado diagrams and supporting tables. The model also allows for probabilistic sensitivity analysis (PSA) using Monte Carlo simulation to be performed. This form of analysis is used to estimate parameter uncertainty. To conduct the PSA, probabilistic distributions were assigned to each input in the model and used to randomly select new plausible values. Each new sampled value applied in the model and the new results of the model were recorded. This process was repeated for a large number of iterations (10,000) to produce a distribution of results from the model. The outcomes were reported using cost-effectiveness scatter plots and cost-effectiveness acceptability curves (CEACs).
3 Results
3.1 Clinical Efficacy
The results of the subgroup meta-analysis on neurological outcome (Fig. 2) indicate that seven studies [37, 42,43,44,45,46,47] compared the neurological outcomes associated with IVTM versus a SCS over a hospital discharge duration. The meta-analysis showed that the pooled estimates of the probability of poor neurological outcomes with IVTM and SCS were 0.60 (95% CI: 0.52–0.69) and 0.69 (95% CI: 0.63–0.75), respectively. IVTM had a lower probability of unfavourable neurological outcomes than did SCS (odds ratio [OR] 0.73; 95% confidence interval [CI] 0.61–0.88). Figure 3 shows that 12 studies compared the mortality associated with IVTM versus SCS over a hospital discharge duration [37,42,43,44,45,46,47,48,49505152]. The meta-analysis showed that the pooled estimates of probability of mortality during hospital discharge in IVTM and SCS group were 0.43 (95% CI: 0.40–0.47) and 0.48 (95% CI: 0.47–0.50), respectively. IVTM was associated with a lower probability of mortality than surface methods (OR 0.87; 95% CI 0.75–1.02).
3.2 Cost-Effectiveness Analysis
The results of the base-case cost analysis over a lifetime time horizon demonstrated that the average device cost per patient (annual cost of equipment along with consumable cost) of Thermogard XP was £932.91 per patient compared with £503.40 for Blanketrol III and £1075.30 for Arctic Sun 5000. Additionally, the cost of an ICU stay associated with treatment with Thermogard XP was £17,108 per patient compared with £17,204 with SCS. Considering the costs of hospital ward stay, AE treatments, and long-term costs, the total average discounted cost for the intervention was £82,846. In comparison, the average discounted cost for Blanketrol III and Arctic Sun 5000 was £85,185 and £85,771, respectively. Thermogard XP resulted in direct cost savings of £2339 and £2925 (per patient) compared with Blanketrol III and Arctic Sun 5000. Treatment with Thermogard XP led to an increase of 0.98 discounted QALYs relative to Blanketrol III and Arctic Sun 5000 over a lifetime time horizon (Table 2). The estimated incremental cost-effectiveness ratio (ICER) was dominant for Thermogard XP versus Arctic Sun 5000 and Blanketrol III over a lifetime horizon, i.e., the intervention was less costly and more effective than the comparator(s).
The results of the PSA are presented as a cost-effectiveness plane and a CEAC plot, respectively, for IVTM compared with SCS (Figs. 4, 5). The results of the cost-effectiveness plane demonstrated that, in the majority of cases, the ICER was located in the south-east quadrant, i.e., the intervention was less costly and more effective. The probability of the intervention being cost saving was 69.2% and 65.3% versus the Arctic Sun 5000 and Blanketrol III, respectively, based on the CEAC plot (Figs. 4, 5). In the DSA, key cost and outcome parameters were subject to hypothetical increases or decreases of 25% to determine the key drivers of the model results. Results from the one-way sensitivity analyses generally supported the base-case findings, and findings from the different scenario analyses are shown in Fig. 6a and b in terms of change in incremental cost versus Blanketrol III and Arctic Sun 5000, respectively. Regarding the results of DSA, the probability of neurological outcome (i.e., the neurological outcome health state that patients entered in the short term) had the most significant effect on the base-case results. In addition, in the base-case analysis, we assumed a cool line was used for intravascular catheters; however, considering different catheters for IVTM, such as Icy and Quattro, did not change the cost-effectiveness analysis results. The results of the base-case analysis over the hospital discharge time horizon are presented in the ESM (SI-IV).
4 Discussion
We performed a cost-effectiveness analysis to explore the impact of treating patients with IVTM using Thermogard XP compared with the SCS by Blanketrol III or Arctic Sun 5000, following a cardiac arrest. Results of the cost-effectiveness analysis over a lifetime time horizon indicated that this intervention was dominant compared with the SCS. To the best of our knowledge, this is the first cost-effectiveness analysis to evaluate IVTM using a Markov model.
According to the literature, TTM following cardiac arrest improves neurological and survival outcomes. Although different temperature management procedures to control cardiac arrest survival are cost effective [24, 53], identifying the most cost-effective cooling procedure is an area that needs to be explored.
A previous study determined that TTM with a cooling blanket among cardiac arrest survivors improves clinical outcomes and is a cost-effective intervention versus conventional care in the USA [24]. In this study, patients receiving TTM gained an average of 0.66 QALYs compared with conventional care, at an incremental cost of $US31,254. Another study evaluated different methods of temperature management, including blanket cooling, peritoneal lavage, and venovenous extracorporeal membrane oxygenation VV ECMO [53]. The results of this study showed that a cooling blanket was the most cost-effective intervention, with an ICER of $US58,329/QALY versus peritoneal lavage and VV ECMO.
Since recent reviews [13] determined that IVTM was associated with improved neurological outcomes compared with SCS among survivors resuscitated following cardiac arrest, economic evaluation assessing the costs and outcomes of IVTM methods is important to help healthcare policymakers identify the optimal procedure. Besides improving neurological outcomes and survival rates, IVTM is associated with a reduced burden of significant AEs, including temperature overshoot, arrhythmia, deep venous thrombosis, and shivering [13]. These clinical challenges will complicate the treatment process and delay hospital discharge, which will impose more costs on the healthcare system. For instance, shivering is a common side effect in TTM and can lead to cerebral and metabolic stress [40, 54] that, in turn, can lead to additional usage of sedation treatments, prolonged length of stay, and increased healthcare utilisation. Recent findings show that IVTM is associated with a reduced rate of shivering (as low as 2.36%) compared with SCS [37,38,39,40], which can be considered a positive clinical and economic impact of IVTM.
Furthermore, multiple studies [40, 45, 55, 56] have reported more rapid cooling and a more stable temperature profile (during both cooling and rewarming phases) with IVTM. This results in a faster time to reach target temperature and greater precision in maintaining the patient’s temperature, two parameters that are repeatedly described as favourably impacting clinical outcomes following cardiac arrest [57,58,59,60,61,62]. The time- and cost-saving potential provided by this practical dimension was indirectly considered through the clinical outcome probabilities entered in the model presented.
In this study, we found that IVTM is likely to be the most cost-effective strategy among current temperature management procedures for delivering TTM after cardiac arrest. The probability of poor neurological outcomes was the main driver in this analysis, based on results from the DSA. There is a significant difference between the ICU and hospital ward length of stay associated with poor and good neurological outcomes in the UK healthcare system. As a result, the probability of neurological outcome parameters has a significant effect on costs, clinical outcomes, and economic evaluation results. One strength of our study is that we used multiple sources of high-level evidence to inform the probability of clinical outcomes in IVTM and SCS. Additionally, our incorporation of uncertainty in model inputs and assumptions in the results should provide reassurance that the overall findings are not materially affected by uncertain evidence.
The limitations of our study should also be acknowledged. Because UK-based studies on the cost estimation of long-term costs associated with cardiac arrest in terms of severity of neurological outcomes were lacking, we extracted data from a US-based source and converted international cost-effectiveness data to UK values. We also assumed that the neurological outcome status of survivors of cardiac arrest was constant over a lifetime time horizon. We also combined CPC 1 and CPC 2 as good and CPC 3 and CPC 4 as poor neurological outcome status, which did not allow for variation in long-term care costs associated with each individual neurological state. To model this assumption, we derived the rate of each neurological status including CPC1, CPC2, CPC3, and CPC4 [21], and we then estimated the weighted average hazard ratio by adjusting the rate of CPC1 and CPC 2 for good and CPC3 and CPC 4 for poor neurological outcome. We also assigned the incremental cost of poor neurological outcomes among patients in this condition until death. Moreover, the main source of Kaplan–Meier estimates for survival according to CPC category score and rate of each status was a US-based study [21], and we used the most recent UK national life table data to estimate age- and sex-adjusted hazard ratios for the final survival parameters in the model [63].
5 Conclusion
Our findings show that using IVTM is associated with better neurological outcomes and survival rates over both short-term and long-term time horizons. In addition, IVTM improves life-year gains and reduces the total costs per patient over a lifetime time horizon versus surface cooling methods for managing patients’ temperature after cardiac arrest.
References
Hawkes C, Booth S, Ji C, et al. Epidemiology and outcomes from out-of-hospital cardiac arrests in England. Resuscitation. 2017;110:133–40. https://doi.org/10.1016/j.resuscitation.2016.10.030.
Gräsner JT, Herlitz J, Tjelmeland IBM, et al. European Resuscitation Council Guidelines 2021: epidemiology of cardiac arrest in Europe. Resuscitation. 2021;161:61–79. https://doi.org/10.1016/j.resuscitation.2021.02.007.
Overview | Therapeutic hypothermia following cardiac arrest | Guidance | NICE. https://www.nice.org.uk/guidance/ipg386. Accessed 7 Aug 2020
Vaity C, Al-Subaie N, Cecconi M. Cooling techniques for targeted temperature management post-cardiac arrest. Crit Care. 2015. https://doi.org/10.1186/s13054-015-0804-1.
Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30(11):2126–8. https://doi.org/10.1007/s00134-004-2425-z.
Gage H, Kenward G, Hodgetts TJ, Castle N, Ineson N, Shaikh L. Health system costs of in-hospital cardiac arrest. Resuscitation. 2002;54(2):139–46. https://doi.org/10.1016/s0300-9572(02)00099-0.
Geri G, Scales DC, Koh M, et al. Healthcare costs and resource utilization associated with treatment of out-of-hospital cardiac arrest. Resuscitation. 2020;153:234–42. https://doi.org/10.1016/j.resuscitation.2020.04.032.
Efendijev I, Folger D, Raj R, et al. Outcomes and healthcare-associated costs one year after intensive care-treated cardiac arrest. Resuscitation. 2018;131:128–34. https://doi.org/10.1016/j.resuscitation.2018.06.028.
Petrie J, Easton S, Naik V, Lockie C, Brett SJ, Stümpfle R. Hospital costs of out-of-hospital cardiac arrest patients treated in intensive care; a single centre evaluation using the national tariff-based system. BMJ Open. 2015. https://doi.org/10.1136/bmjopen-2014-005797.
Barbarawi M, Alabdouh A, Barbarawi O, et al. Targeted temperature management in cardiac arrest patients with an initial non-shockable rhythm: a systematic review and meta-analysis. Shock. 2020;54(5):623–30. https://doi.org/10.1097/SHK.0000000000001550.
Nolan JP, Sandroni C, Böttiger BW, et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: post-resuscitation care. Resuscitation. 2021;161:220–69. https://doi.org/10.1016/j.resuscitation.2021.02.012.
Thomassen A, Wernberg M. Prevalence and prognostic significance of coma after cardiac arrest outside intensive care and coronary units. Acta Anaesthesiol Scand. 1979;23(2):143–8. https://doi.org/10.1111/j.1399-6576.1979.tb01434.x.
Bartlett ES, Valenzuela T, Idris A, et al. Systematic review and meta-analysis of intravascular temperature management vs. surface cooling in comatose patients resuscitated from cardiac arrest. Resuscitation. 2020;146:82–95. https://doi.org/10.1016/j.resuscitation.2019.10.035.
(23) (PDF) Efficacy of different cooling technologies for therapeutic temperature management: a prospective intervention study. ResearchGate. https://www.researchgate.net/publication/322073031_Efficacy_of_different_cooling_technologies_for_therapeutic_temperature_management_A_prospective_intervention_study. Accessed 11Aug 2020.
Summary | Thermogard XP for therapeutic hypothermia after cardiac arrest | Advice | NICE. https://www.nice.org.uk/advice/mib37/chapter/summary. Accessed 7 Aug 2020.
National Clinical Coding Standards ICD-10 5th Edition (2021). 250.
Methods for the Economic Evaluation of Health Care Programmes—Research Database, The University of York. https://pure.york.ac.uk/portal/en/publications/methods-for-the-economic-evaluation-of-health-care-programmes(8f69bcee-cdac-44fa-871c-f821470df60a).html. Accessed 7 Aug 2020.
guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781.pdf. https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. Accessed 7 Aug 2020.
Rittenberger JC, Raina K, Holm MB, Kim YJ, Callaway CW. Association between cerebral performance category, modified Rankin scale, and discharge disposition after cardiac arrest. Resuscitation. 2011;82(8):1036–40. https://doi.org/10.1016/j.resuscitation.2011.03.034.
Calabró L, Bougouin W, Cariou A, et al. Effect of different methods of cooling for targeted temperature management on outcome after cardiac arrest: a systematic review and meta-analysis. Crit Care Lond Engl. 2019;23(1):285. https://doi.org/10.1186/s13054-019-2567-6.
Phelps R, Dumas F, Maynard C, Silver J, Rea T. Cerebral Performance Category and long-term prognosis following out-of-hospital cardiac arrest. Crit Care Med. 2013;41(5):1252–7. https://doi.org/10.1097/CCM.0b013e31827ca975.
Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol. 2011;11:139. https://doi.org/10.1186/1471-2288-11-139.
Hurdus B, Munyombwe T, Dondo TB, et al. Association of cardiac rehabilitation and health-related quality of life following acute myocardial infarction. Heart. 2020;106(22):1726–31. https://doi.org/10.1136/heartjnl-2020-316920.
Merchant RM, Becker LB, Abella BS, Asch DA, Groeneveld PW. Cost-effectiveness of therapeutic hypothermia after cardiac arrest. Circ Cardiovasc Qual Outcomes. 2009;2(5):421–8. https://doi.org/10.1161/CIRCOUTCOMES.108.839605.
Preblick R, Kwong WJ, White RH, Goldhaber SZ. Cost-effectiveness of edoxaban for the treatment of venous thromboembolism based on the Hokusai-VTE study. Hosp Pract. 2015;43(5):249–57. https://doi.org/10.1080/21548331.2015.1099412.
Wehler E, Storm M, Kowal S, Campbell C, Boscoe A. A health state utility model estimating the impact of ivosidenib on quality of life in patients with relapsed/refractory acute myeloid leukemia. 1.
Stein EM, Yang M, Guerin A, et al. Assessing utility values for treatment-related health states of acute myeloid leukemia in the United States. Health Qual Life Outcomes. 2018. https://doi.org/10.1186/s12955-018-1013-9.
van Hoek AJ, Underwood A, Jit M, Miller E, Edmunds WJ. The impact of pandemic influenza H1N1 on health-related quality of life: a prospective population-based study. PLoS One. 2011;6(3): e17030. https://doi.org/10.1371/journal.pone.0017030.
The technology | Arctic Sun 5000 for therapeutic hypothermia after cardiac arrest | Advice | NICE. https://www.nice.org.uk/advice/mib112/chapter/The-technology. Accessed 11 Aug 2020.
Chan PS, McNally B, Nallamothu BK, et al. Long-term outcomes among elderly survivors of out-of-hospital cardiac arrest. J Am Heart Assoc. 2021;5(3):e002924. https://doi.org/10.1161/JAHA.115.002924.
Gosden TB, Torgerson DJ. Converting international cost effectiveness data to UK prices. BMJ. 2002;325(7358):275–6.
NHS reference costs 2015 to 2016. GOV.UK. https://www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016. Accessed 28 Aug 2019.
Ian S, Graham N, George W, et al. Health-related quality of life is better for cardiac arrest survivors who received citizen cardiopulmonary resuscitation. Circulation. 2003;108(16):1939–44. https://doi.org/10.1161/01.CIR.0000095028.95929.B0.
Fryback DG, Dunham NC, Palta M, et al. US norms for six generic health-related quality-of- life indexes from the national health measurement study. Med Care. 2007;45(12):1162–70. https://doi.org/10.1097/MLR.0b013e31814848f1.
Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. 1996;156(16):1829–36.
KetkiD R, Clifton C, JonC R, MargoB H. Neurological and functional status following cardiac arrest: method and tool utility. Resuscitation. 2008;79(2):249–56. https://doi.org/10.1016/j.resuscitation.2008.06.005.
Gillies MA, Pratt R, Whiteley C, Borg J, Beale RJ, Tibby SM. Therapeutic hypothermia after cardiac arrest: a retrospective comparison of surface and endovascular cooling techniques. Resuscitation. 2010;81(9):1117–22. https://doi.org/10.1016/j.resuscitation.2010.05.001.
Tømte Ø, Drægni T, Mangschau A, Jacobsen D, Auestad B, Sunde K. A comparison of intravascular and surface cooling techniques in comatose cardiac arrest survivors. Crit Care Med. 2011;39(3):443–9. https://doi.org/10.1097/CCM.0b013e318206b80f.
Deye N, Cariou A, Girardie P, et al. Endovascular versus external targeted temperature management for patients with out-of-hospital cardiac arrest: a randomized, controlled study. Circulation. 2015;132(3):182–93. https://doi.org/10.1161/CIRCULATIONAHA.114.012805.
Glover GW, Thomas RM, Vamvakas G, et al. Intravascular versus surface cooling for targeted temperature management after out-of-hospital cardiac arrest—an analysis of the TTM trial data. Crit Care. 2016;20(1):381. https://doi.org/10.1186/s13054-016-1552-6.
Home. NHS Supply Chain. https://www.supplychain.nhs.uk/. Accessed 8 July 2021.
Look X, Li H, Ng M, et al. Randomized controlled trial of internal and external targeted temperature management methods in post-cardiac arrest patients. Am J Emerg Med. 2018;36(1):66–72. https://doi.org/10.1016/j.ajem.2017.07.017.
Oh SH, Oh JS, Kim YM, et al. An observational study of surface versus endovascular cooling techniques in cardiac arrest patients: a propensity-matched analysis. Crit Care. 2015. https://doi.org/10.1186/s13054-015-0819-7.
Pittl U, Schratter A, Desch S, et al. Invasive versus non-invasive cooling after in- and out-of-hospital cardiac arrest: a randomized trial. Clin Res Cardiol. 2013;102(8):607–14. https://doi.org/10.1007/s00392-013-0572-3.
Sonder P, Janssens GN, Beishuizen A, et al. Efficacy of different cooling technologies for therapeutic temperature management: a prospective intervention study. Resuscitation. 2018;124:14–20. https://doi.org/10.1016/j.resuscitation.2017.12.026.
Ferreira IA, Schutte M, Oosterloo E, et al. Therapeutic mild hypothermia improves outcome after out-of-hospital cardiac arrest. Neth Heart J. 2009;17(10):378–84. https://doi.org/10.1007/BF03086288.
Kim KH, Shin SD, Song KJ, et al. Cooling methods of targeted temperature management and neurological recovery after out-of-hospital cardiac arrest: a nationwide multicenter multi-level analysis. Resuscitation. 2018;125:56–65. https://doi.org/10.1016/j.resuscitation.2018.01.043.
de Waard MC, Banwarie RP, Jewbali LSD, Struijs A, Girbes ARJ, Groeneveld ABJ. Intravascular versus surface cooling speed and stability after cardiopulmonary resuscitation. Emerg Med J. 2015;32(10):775–80. https://doi.org/10.1136/emermed-2014-203811.
Flint AC, Hemphill JC, Bonovich DC. Therapeutic hypothermia after cardiac arrest: performance characteristics and safety of surface cooling with or without endovascular cooling. Neurocrit Care. 2007;7(2):109–18. https://doi.org/10.1007/s12028-007-0068-y.
Flemming K, Simonis G, Ziegs E, Diewok C, Gildemeister R, Wunderlich C, Strasser RH. Comparison of external and intravascular cooling to induce hypothermia in patients after CPR. GMS German Medical Science. 2006;4
Forkmann M, Kolschmann S, Holzhauser L, et al. Target temperature management of 33 °C exerts beneficial haemodynamic effects after out-of-hospital cardiac arrest. Acta Cardiol. 2015;70(4):451–9. https://doi.org/10.1080/AC.70.4.3096893.
Rosman J, Hentzien M, Dramé M, et al. A comparison between intravascular and traditional cooling for inducing and maintaining temperature control in patients following cardiac arrest. Anaesth Crit Care Pain Med. 2018;37(2):129–34. https://doi.org/10.1016/j.accpm.2016.08.009.
Gajarski RJ, Smitko K, Despres R, Meden J, Hutton DW. Cost-effectiveness analysis of alternative cooling strategies following cardiac arrest. Springerplus. 2015;4:427. https://doi.org/10.1186/s40064-015-1199-9.
Kirk A, McDaniel C, Szarlej D, Rincon F. Assessment of antishivering medication requirements during therapeutic normothermia: effect of cooling methods. Ther Hypothermia Temp Manag. 2016;6(3):135–9. https://doi.org/10.1089/ther.2016.0001.
Precision and Safety of an Intravascular Temperature Management System for Postcardiac Arrest Syndrome Patients: A Multicenter Clinical Trial (COOL-ARREST JP)-PubMed. https://pubmed.ncbi.nlm.nih.gov/32348714/. Accessed 14 Oct 2021.
Lopez-de-Sa E, Juarez M, Armada E, et al. A multicentre randomized pilot trial on the effectiveness of different levels of cooling in comatose survivors of out-of-hospital cardiac arrest: the FROST-I trial. Intensive Care Med. 2018;44:1807–15. https://doi.org/10.1007/s00134-018-5256-z.
Liao X, Zhou Z, Zhou M, et al. Effects of endovascular and surface cooling on resuscitation in patients with cardiac arrest and a comparison of effectiveness, stability, and safety: a systematic review and meta-analysis. Crit Care Lond Engl. 2020;24(1):27. https://doi.org/10.1186/s13054-020-2731-z.
Arrich J, Herkner H, Müllner D, Behringer W. Targeted temperature management after cardiac arrest. A systematic review and meta-analysis of animal studies. Resuscitation. 2021;162:47–55. https://doi.org/10.1016/j.resuscitation.2021.02.002.
Sendelbach S, Hearst MO, Johnson PJ, Unger BT, Mooney MR. Effects of variation in temperature management on cerebral performance category scores in patients who received therapeutic hypothermia post cardiac arrest. Resuscitation. 2012;83(7):829–34. https://doi.org/10.1016/j.resuscitation.2011.12.026.
Stanger D, Kawano T, Malhi N, et al. Door-to-targeted temperature management initiation time and outcomes in out-of-hospital cardiac arrest: insights from the continuous chest compressions trial. J Am Heart Assoc. 2019;8(9): e012001. https://doi.org/10.1161/JAHA.119.012001.
May TL, Lary CW, Riker RR, et al. Variability in functional outcome and treatment practices by treatment center after out-of-hospital cardiac arrest: analysis of International Cardiac Arrest Registry. Intensive Care Med. 2019;45(5):637–46. https://doi.org/10.1007/s00134-019-05580-7.
Matsuzaki M, Matsumoto N, Nagao K, et al. Impact of induced therapeutic hypothermia by intravenous infusion of ice-cold fluids after hospital arrival in comatose survivors of out-of-hospital cardiac arrest with initial shockable rhythm. Circ J Off J Jpn Circ Soc. 2021;85(10):1842–8. https://doi.org/10.1253/circj.CJ-20-0793.
National life tables: UK—Office for National Statistics. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables. Accessed 15 Mar 2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study is independent research funded by ZOLL Medical. The sponsor is aware of the content of the submitted material but did not play any role nor exert any influence in the design of the study, the conduct of the research, or the reporting of results.
Conflict of interest
MJ and AM are employees of Optimax Access Ltd and MBH is an employee of Device Access Ltd. Both Optimax Access and Device Access Ltd received funds from ZOLL to conduct this study. TRK has received research funding from ZOLL. MY is an assistant professor at Mercer University, was employed by Optimax Access at the time of conducting the work, and received consulting fees for its contribution to this study. MRH is a freelance health economist and has no conflict of interest.
Author contributions
MJ conceived the study question. MY, MJ, and MRH developed the analysis plan, conducted the economic model, and drafted the manuscript. All authors contributed to the study design, provided input on the model, and read and approved the final draft of the manuscript.
Availability of data and material
The model used in this study was provided to the journal’s peer reviewers for their reference when reviewing the manuscript.
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
All authors approve the manuscript and gave their consent for submission and publication.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Javanbakht, M., Mashayekhi, A., Hemami, M.R. et al. Cost-Effectiveness Analysis of Intravascular Targeted Temperature Management after Cardiac Arrest in England. PharmacoEconomics Open 6, 549–562 (2022). https://doi.org/10.1007/s41669-022-00333-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-022-00333-7