Abstract
Background
Proprotein convertase subtilisin/kexin type 9 inhibitors, such as evolocumab, are cholesterol-lowering drugs effective in lowering lipid levels in high-risk patients with primary hypercholesterolemia or mixed dyslipidemia.
Objective
This study assessed the cost effectiveness of evolocumab in combination with lipid-lowering therapies (LLTs) compared with LLTs alone, from a public healthcare perspective in the Kingdom of Saudi Arabia (KSA).
Methods
A Markov cohort state transition model was used, incorporating efficacy estimates from the FOURIER clinical trial and baseline cardiovascular event rates observed in clinical practice. Other model inputs were extracted from the literature and Saudi sources.
Results
In patients with clinically evident atherosclerotic cardiovascular disease and baseline low-density lipoprotein cholesterol ≥ 70 or ≥ 100 mg/dL, adding evolocumab to a maximally tolerated statin, with or without ezetimibe, was associated with incremental cost-effectiveness ratios (ICERs) of Saudi Arabian riyal (SAR) 109,274 ($US60,708) per quality-adjusted life-year (QALY) gained and SAR75,163 ($US41,757) per QALY gained, respectively. The ICER was SAR22,391 ($US12,440) per QALY gained in patients with heterozygous familial hypercholesterolemia. Sensitivity analysis results were robust to changes in model parameters and fell below the willingness-to-pay threshold of up to three times gross domestic product per capita in 2019 (SAR264,813 [$US147,118]).
Conclusion
Evolocumab can be considered a cost-effective treatment option for patients with atherosclerotic cardiovascular disease or heterozygous familial hypercholesterolemia in the KSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the Kingdom of Saudi Arabia (KSA), many patients with clinically evident atherosclerotic cardiovascular disease (ASCVD) or heterozygous familial hypercholesterolemia (HeFH) do not achieve adequate reductions in levels of low-density lipoprotein cholesterol (LDL-C) despite lipid-lowering therapies (LLTs) being widely available, leading to substantially increased cardiovascular event rates and associated costs. |
Evolocumab in combination with LLTs is a cost-effective treatment choice for patients with clinically evident ASCVD and HeFH whose LDL-C levels are not well controlled with LLTs alone. |
Decision makers may consider evolocumab as a useful option in improving the care of patients with ASCVD or HeFH in the KSA. |
1 Introduction
Cardiovascular disease (CVD) is one of the leading causes of mortality worldwide and was responsible for more than 17.7 million deaths in 2015 globally [1]. In Saudi Arabia, CVD accounts for 45.7% of all deaths, with an estimation of 41,000 deaths every year [2]. CVD incurs a high economic burden and high resource consumption. In Saudi Arabia, the direct medical costs for each patients with CVD was estimated to be $US10,710 per event [3].
Elevated low-density lipoprotein cholesterol (LDL-C) is one of the most important modifiable risk factors for atherosclerotic CVD (ASCVD) [4, 5]. Individuals with familial hypercholesterolemia (FH), a hereditary condition that leads to life-long raised LDL-C levels, are also particularly vulnerable to cardiovascular events [6,7,8]. Lipid-lowering therapies (LLTs), mainly statins, are prescribed to reduce LDL-C levels. However, many patients do not achieve an adequate reduction in LDL-C level despite widely available LLTs [9, 10] and so remain vulnerable to excess cardiovascular events.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, such as evolocumab, are a modern class of cholesterol-lowering drugs that have proven efficacy in lowering lipid levels in high-risk patients with primary hypercholesterolemia or mixed dyslipidemia with demonstrated safety over 5 years [11,12,13,14,15].
Evolocumab has been shown to be a consistent and efficacious treatment in reducing LDL-C levels, regardless of patient characteristics or background therapy [16].
However, the perceived added value of evolocumab could vary from country to country, as the reported incremental cost-effectiveness ratio (ICER) for evolocumab ranged from $US51,687 to 1,336,221 in ASCVD and from $US35,225 to 503,000 in heterozygous familial hypercholesterolemia (HeFH), indicating that PCSK9 inhibitors may be cost effective in some countries but not in others [17,18,19]. Differences in cost effectiveness are mainly because of different population characteristics, CVD risk factors, efficacy assumptions, and drug prices [17]. There is a need to support healthcare decision makers with economic evaluations to ensure scarce healthcare resources are invested efficiently.
The aim of this study was to assess the cost effectiveness of evolocumab as an add-on treatment for patients with clinically evident ASCVD and patients with HeFH whose LDL-C levels are not controlled with conventional LLTs, from a public healthcare perspective for the Kingdom of Saudi Arabia (KSA).
2 Materials and Methods
2.1 Patient Population
A base-case simulation used real-world data to model the Saudi patient population. Three patient populations were considered in the economic model: two subgroups of patients with clinically evident ASCVD (LDL-C ≥ 70 and ≥ 100 mg/dL) and patients with HeFH. Because of resource limitations and the absence of a complete dataset, data from multiple sources were used to inform patient population characteristics, as detailed in Table 1.
The majority of population characteristics for the ASCVD populations were obtained from the Saudi Acute Myocardial Infarction Registry (STARS-1), a registry for patients with acute myocardial infarction (MI) that was conducted across 50 hospitals across the KSA, focusing on the public population sector [20].
Where data were not available from the STARS-1 dataset, alternative sources were used, such as Al Sifri et al. [21], a multicenter observational study investigating the prevalence of lipid abnormalities and cholesterol values in patients with stable dyslipidemia in the KSA. Baseline mean LDL-C levels were obtained from Altowaijri et al. [22].
For patients with HeFH, population characteristics were sourced from Al-Rasadi et al. [23]. This study assessed the prevalence, management, and outcomes of HeFH in patients with dyslipidemia in the Arabian Gulf. The model assumed that the discharge diagnoses observed in this patient population were representative of the underlying natural history for patients with HeFH in the KSA.
2.2 Model Structure
A previously published Markov state transition model was adapted for the Saudi public healthcare setting to estimate the cost effectiveness of evolocumab in lowering LDL-C for patients with ASCVD or HeFH [18, 24,25,26,27,28]. The model was populated with local inputs for baseline patient characteristics, background therapy, event rates, background mortality, and costs. The model used a lifetime time horizon and public healthcare perspective, and costs and outcomes were discounted at an annual rate of 3.0% [29]. Scenario analyses exploring alternative discount rates are provided in the electronic supplementary material (ESM). The model considered an annual cycle length consistent with other economic evaluation studies in CVD [30].
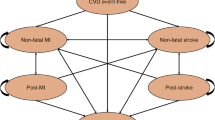
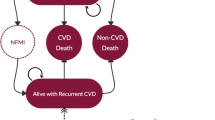
The model comprised eight main health states: no ASCVD, nonfatal MI, post-MI, nonfatal ischemic stroke (IS), post-IS, other ASCVD, cardiovascular death, and non-cardiovascular death (Fig. 1). The ‘other ASCVD’ health state captured less severe cardiovascular events, namely unstable angina, transient ischemic attack, revascularization without MI, and peripheral artery disease (i.e., peripheral revascularization and vascular amputations). The ‘nonfatal MI’ and ‘nonfatal IS’ health states covered the initial year period after the event, and post-event health states covered subsequent years, to account for differences in risk, costs, and utilities between initial and subsequent years.
Additionally, the model included combined health states that comprised either two or three post-event health states, created to track patients’ cardiovascular event history over time.
2.3 Model Inputs
2.3.1 Clinical Data
2.3.1.1 Atherosclerotic Cardiovascular Disease Population
The baseline nonfatal cardiovascular event rates for patients with clinically evident ASCVD were derived from the Truven MarketScan database [18, 31]. The rate of cardiovascular death was estimated separately by combining National Health and Nutrition Examination Survey mortality files (2004–2012) and the National Vital Statistics Mortality Report 2012 [18, 31].
Patients in the KSA have an elevated baseline cardiovascular event risk compared with a practice-based population from North America, so the baseline rate was increased by an adjustment factor based on geographic region, adjusted for REACH risk score (hazard ratio [HR] 1.54; 95% confidence interval [CI] 1.11–2.15) [32]. This yielded a cardiovascular event rate of 9.85, expressed as per 100 patient-years.
The baseline cardiovascular event rates were adjusted by age and LDL-C level using a published HR for age and a rate ratio for LDL-C levels [33, 34].
2.3.1.2 Heterozygous Familial Hypercholesterolemia Population
Published risk equations were applied to population characteristics from the RUTHERFORD-2 clinical trial to predict the aggregate 10-year risk of cardiovascular events in patients with and without a cardiovascular event history [35,36,37].
Patients with HeFH have a longer exposure to higher LDL-C levels, so they have a higher baseline cardiovascular risk than estimates based on the Framingham and REACH risk equations [38, 39]. Consequently, to approximate the risk for patients with HeFH, we adjusted the initial predicted aggregate cardiovascular event rate by using published event odds ratios of patients with HeFH compared with those without HeFH, while adjusting for several risk factors (13.2 [95% CI 10.0–17.4] in subjects with HeFH off therapy; 10.3 [95% CI 7.8–13.8] in subjects with HeFH on therapy). These reported odds ratios were used to calculate the rate ratios of cardiovascular events in patients with HeFH compared with other patients with hyperlipidemia [40]. To mimic the routine clinical setting, the risks of treated and untreated groups were pooled to account for the mix of patients with or without cardiovascular event history. A rate ratio of 7.1 (95% CI 5.7–8.7) was applied to the rates of events initially predicted to adjust for the increased risk in the modeled HeFH population [40].
2.3.2 Mortality
We estimated non-CVD mortality by subtracting the proportion of CVD-related deaths from the Saudi general population mortality, taken from life tables by age and sex for the KSA, published by the World Health Organization (WHO) [41]. The proportion of CVD-related deaths was assumed to be the same as observed in a Spanish data source to substitute unavailable Saudi CVD-only mortality estimates [42]. CVD mortality was modeled separately, dependent on the incidence of cardiovascular events predicted in the model.
2.3.3 Treatment Effect Data
The predicted effectiveness of evolocumab in reducing CVD event rates was derived from the relative LDL‐C reduction from baseline in the FOURIER study. The LDL-C reduction at week 48 was assumed to remain constant over the modeled time horizon [12]. This assumption is aligned with the observed sustained reductions in LDL-C for up to 5 years of evolocumab treatment in the open-label, randomized extension study OSLER-1 [15, 43]. This cost-effectiveness analysis used a rate ratio of 0.73 (95% CI 0.70–0.77) per 1 mmol/L (39 mg/dL) of LDL-C reduction for nonfatal MI events, 0.77 (95% CI 0.70–0.85) for nonfatal IS events, and 0.86 (95% CI 0.82–0.90) for cardiovascular death events, as reported by the Cholesterol Treatment Trialists' Collaboration (CTTC) [34].
The rate ratios per mmol/L of LDL-C reduction observed in the FOURIER trial (after accounting for study duration) were aligned with those from the CTTC meta-analysis. It has been well-documented that it takes time for the benefit of LLT to become evident [44,45,46,47]. To account for this delayed treatment effect, prespecified landmark analyses were performed in FOURIER, in which patients who were alive and included in follow-up at the end of the first year formed the group at risk to estimate the effect of evolocumab on outcomes beyond the first year. These analyses showed that the magnitude of the relative risk reduction of major cardiovascular events increased over time, from 16% during the first year to 25% beyond the first year. Compared with the statin-based CTTC meta-analysis, treatment with evolocumab had very similar effects on the risk of major cardiovascular events per 1 mmol/L of LDL-C reduction, for years 0 to 1 and years 1 to 2 [12]. Furthermore, the results from the FOURIER trial are consistent with the results of a Mendelian randomization study showing that variants in the genes encoding PCSK9 and 3-hydroxy-3-methylglutaryl-coenzyme A reductase (the target of statins) were associated with nearly identical effects on the risk of cardiovascular events per unit decrease of LDL-C [48]. In addition, a meta-analysis of 49 studies comparing the effects of statins and eight nonstatin LLTs (including PCSK9 inhibitors) demonstrated that lowering LDL-C levels was associated with a consistent proportional improvement in cardiovascular outcomes [49]. Importantly, the reduction in risk of major cardiovascular events observed in the FOURIER trial, when adjusted for duration of follow-up, is similar to that of statins based on the CTTC meta-analysis [50]. The treatment effect in our model was, therefore, based on the CTTC relationship between LDL-C reduction and reduced rates of cardiovascular events.
2.3.4 Treatment Persistence and Discontinuation
Treatment discontinuation was included in the economic model, using FOURIER Kaplan–Meier estimates for discontinuation for any reason other than death (Table 2) [51]. Given that the LDL-C reduction of 59% estimated in the FOURIER trial already incorporated the effect of treatment discontinuation in LDL-C lowering, no further adjustments were applied to the treatment effect after patients discontinued.
2.3.5 Resource Use and Costs
2.3.5.1 Medication Costs
Maximally tolerated statin (with or without ezetimibe) was chosen as the background LLT on top of which evolocumab may be administered as an adjunct therapy. Background LLT statin proportions applied in the model were sourced from Alburikan et al. [52], and proportions of concomitant ezetimibe were sourced from Al Sifri et al. [21]. For simplicity, we assumed that low-intensity statins reported were equal to moderate-intensity statins. The composition of background LLT is detailed, by population, in Table 1.
Medication costs were calculated using the price provided by the National Unified Procurement Company (NUPCO), which is the company responsible for the centralized procurement, warehousing, distribution, and re-exporting of pharmaceuticals, medical equipment, and supplies for the benefit of all public hospitals and healthcare facilities. NUPCO provides drugs with a fixed tender price for all governmental hospitals and health centers. The costs used in the model are presented in the ESM. No administration costs were considered for evolocumab since the prefilled pen is designed for self-administration by the patient.
2.3.5.2 Health State Costs
Table 2 summarizes the costs per modeled health state from the Saudi public healthcare perspective, as applied in this economic evaluation. Health state costs were calculated using real-world data from the local setting. Only direct medical costs were considered in this study, including the costs of outpatient visits, diagnostic tests, laboratory tests, medications, procedures, emergency room visits, and hospital stay [22]. Direct medical costs were collected from different public institutions in Riyadh, the KSA, and a weighted average was calculated. All cost data were collected in 2019 and adjusted for inflation to 2020 values. In addition, the estimated costs were presented in Saudi Arabian riyal (SAR) and $US ($US1 = SAR3.75).
Because of a lack of data availability for the cost of cardiovascular death in the KSA, these costs were estimated based on estimations from expert cardiologist opinion. The cost of revascularization was calculated using the proportion of patients who underwent coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI) in our study (18 and 82%, respectively) multiplied by the direct medical costS of CABG and PCI [22, 53]. These costs were combined to calculate the expected annual cost of residing in each health state included in the model.
2.3.6 Utilities
In the absence of utility values for the Saudi setting, CVD health state utilities were derived from a general population sample in the UK that used time trade-off methods (Table 2) [54]. Patient health-related quality of life is anticipated to be lower for patients who have experienced multiple events than for those who have experienced a single event. There are three standard approaches combining health utility information as defined in Ara and Wailoo [55]. However, these methods have not been validated. The multiplicative method was used in this economic analysis to estimate the utilities for the combined health states, as these were considered the most clinically plausible by a leading clinician practicing in KSA. Scenario analyses exploring the additive and minimum methods as an alternative to the multiplicative method are provided in the ESM. The model used the same CVD health state utilities for patients with ASCVD and those with HeFH (primary and secondary prevention). However, the HeFH population without a previous cardiovascular event (i.e., in the ‘no ASCVD’ health state) were assigned sex-specific, age-dependent utility values estimated from a Belgian National Health Survey (as measured by the EQ-5D) [56]. Once primary prevention patients with HeFH had a cardiovascular event, the model used the CVD health state utilities described in Table 2.
2.4 Base-Case Analyses
Key outcomes included total and incremental life-years, quality-adjusted life-years (QALYs) and costs, and the ICER. Since there is no established cost-effectiveness threshold in the KSA, we assumed the threshold to be three times the Saudi gross domestic product (GDP) for the year 2019 (SAR264,813) in accordance with the WHO recommendations [57].
2.5 Sensitivity Analyses
One-way sensitivity analysis was performed, where efficacy parameters, rate adjustment factors, and health state utilities in the model were varied individually between their upper and lower bounds. We assumed a standard error of 10% of the mean values to calculate the 95% CIs for cost inputs. Probabilistic sensitivity analysis was also conducted to fully examine the combined effect of parameter uncertainty on the incremental cost per QALY gained. Values for parameters were sampled by Monte‐Carlo simulation with 1000 iterations in each loop, where stabilization had been achieved.
3 Results
3.1 Base Case
The incremental costs, QALYS, and ICER estimates associated with the addition of evolocumab to conventional background LLT treatment compared with background LLT alone are presented in Table 3.
In the clinically evident ASCVD population with baseline LDL-C ≥ 70 mg/dL (1.8 mmol/L), adding evolocumab to background LLT generated more costs (SAR101,985 [$US56,658]) than conventional background LLT alone but also generated more life-years (0.92) and QALYs (0.93). This resulted in an ICER of SAR109,274 ($US60,708) for each additional QALY gained.
In the clinically evident ASCVD population with baseline LDL-C ≥ 100 mg/dL (2.6 mmol/L), adding evolocumab to background LLT generated more costs (SAR91,134 [$US50,630]) than conventional background LLT alone but also generated more life-years (1.22) and QALYs (1.21). This resulted in an ICER of SAR75,163 ($US41,757) per QALY gained.
In the patients with HeFH, adding evolocumab to background LLT generated more costs (SAR69,511 [$US38,617]) than conventional background LLT alone but also generated more life-years (3.21) and QALYs (3.10). This resulted in an ICER of SAR22,391 ($US12,440) per QALY gained.
3.2 Sensitivity Analyses
3.2.1 Deterministic Sensitivity Analysis
One-way sensitivity analyses showed that, across all patient populations, ICER values were most sensitive to the cardiovascular event rate ratio (per 1 mmol/L LDL-C reduction) for cardiovascular death and, in ASCVD populations, to the baseline cardiovascular event rate increase for the Middle East (Fig. 2).
Tornado diagrams using upper and lower bound of parameters: a ASCVD and baseline low-density lipoprotein cholesterol ≥ 70 mg/dL, b ASCVD and baseline low-density lipoprotein cholesterol ≥ 100 mg/dL, c heterozygous familial hypercholesterolemia. ASCVD atherosclerotic cardiovascular disease; CV cardiovascular; HeFH heterozygous familial hypercholesterolemia; HSU health state utility; IS ischemic stroke; LDL-C low-density lipoprotein cholesterol; LLT lipid lowering therapy; MI myocardial infarction; oASCVD other atherosclerotic cardiovascular disease; RV revascularization; SAR Saudi Arabian Riyal; VB vascular beds; Y year.
3.2.2 Probabilistic Sensitivity Analysis
Probabilistic results by patient population are reported in the ESM. Figure 3 shows the cost-effectiveness acceptability curves for the three patient populations. The results showed that evolocumab reached a probability of being 100% cost effective at SAR169,480 ($US94,156) for the ASCVD, LDL-C ≥ 70 mg/dL group; SAR116,517 ($US64,732) for the ASCVD, LDL-C ≥ 100 mg/dL group; and at only SAR42,370 ($US23,539) for the HeFH group.
Cost-effectiveness acceptability curves for a ASCVD and baseline low-density lipoprotein cholesterol ≥ 70 mg/dL, b ASCVD and baseline low-density lipoprotein cholesterol ≥ 100 mg/dL, c heterozygous familial hypercholesterolemia. ASCVD atherosclerotic cardiovascular disease; CEAC cost-effectiveness acceptability curve; HeFH heterozygous familial hypercholesterolemia; LDL-C low-density lipoprotein cholesterol; LLT lipid lowering therapy; QALY quality-adjusted life year; SAR Saudi Arabian Riyal
4 Discussion
To the best of our knowledge, this is the first cost-effectiveness analysis to determine the value of evolocumab in the Middle East. In this study, we evaluated the economic value of adding evolocumab in patients with clinically evident ASCVD or HeFH whose LDL-C was not currently controlled by conventional LLT of maximally tolerated statin (with or without ezetimibe) from a Saudi healthcare perspective. Our analysis showed that adding evolocumab to LLT therapy (with or without ezetimibe) would be considered cost effective among all study populations when compared with conventional LLT alone, as the ICER estimates fell below the assumed willingness-to-pay threshold of three times the Saudi GDP (SAR264,813 [$US147,118]). This suggests that adding evolocumab to conventional background LLT therapy would provide value as secondary prevention in patients with ASCVD or HeFH in the KSA.
The study showed that the HeFH population had the highest QALY and life-year gain and the lowest incremental cost and ICER estimates, followed by patients with ASCVD with baseline LDL-C ≥ 100 mg/dL and then patients with ASCVD with LDL-C ≥ 70 mg/dL.
Our results are in line with previous cost-effectiveness analysis studies conducted in the USA, Spain, and Sweden. In the USA, Fonarow et al. [58] found that adding evolocumab to statin therapy with or without ezetimibe in patients with ASCVD was cost effective and was associated with 0.33 QALYs and an ICER estimate of $US91,610. Although this study used a social perspective, our study resulted in higher QALY gains and a lower ICER in this population. The difference in QALYs reported in Fonarow et al. [58] and our study are mainly because both population age (65.5 vs. 55.8) and CVD baseline risk (4.2 vs. 9.85) were estimated from a clinical trial, which underestimates the rates compared with rates in actual clinical practice.
Similar results were also reported by Gandra et al. [25] from the US payer perspective and Villa et al. [24] from the Spanish health system perspective. These studies, which included patients with ASCVD with baseline LDL-C ≥ 100 mg/dL and patients with HeFH, found that evolocumab was a cost-effective option in both populations for secondary prevention [24, 25]. However, in our study, QALY gains and ICER values were better for both ASCVD groups. Another study, conducted in Sweden by Lindgren et al. [59] and including all three populations, found that adding evolocumab would be cost effective, with the exception of patients with ASCVD with baseline LDL-C ≥ 70 mg/dL. However, this was not the case in our study, where the addition of evolocumab was considered cost effective in this group, which was in line with findings from a recent Swedish study, that evolocumab was shown to be cost effective in very high-risk populations [28].
Several studies conducted in the USA, Canada, and Australia yielded different results. In these studies, the ICER estimates were above the willingness-to-pay thresholds [60,61,62]. However, the evolocumab price used in our analysis was lower than that in most of these countries. In addition, some of these studies were conducted before the price of evolocumab was lowered by almost 60% in the USA in 2018. Variation in model results can also be explained by the diversity of healthcare systems, study perspectives, and willingness-to-pay thresholds.
Overall, one-way sensitivity analyses suggested these findings were robust to changes in the model input parameters. All estimated ICERs fell below the threshold of SAR264,813 ($US147,118) in the KSA. Despite higher medication costs, the incremental reduction in cardiovascular events, corresponding reductions in hospitalizations, and revascularizations resulting from the addition of evolocumab offset the medication cost. Tornado plots (Fig. 2) show that ICER values were most sensitive to the cardiovascular event rate ratio (per 1 mmol/L LDL-C reduction) for cardiovascular death and, in ASCVD populations, to the baseline cardiovascular event rate increase for the Middle East and for IS rates after 1 year. This is similar to what was reported by Fonarow et al. [18], where IS event rate and cardiovascular deaths after 1 year were the most sensitive parameters to changes in both the FOURIER trial patients and the US clinical practice groups.
The results of the probabilistic sensitivity analyses suggested that the model outcomes were generally robust to simultaneous variation of all parameters according to their estimated uncertainty margins. For the three groups, evolocumab can be considered ~100% cost effective at the assumed willingness-to-pay threshold. The results of the CEACs showed that the HeFH group reached the probability of being 100% cost effective at only 16% of the assumed willingness-to-pay threshold. For the ASCVD groups, the 100% probability of being cost effective was reached at 44 and 64% of the assumed willingness-to-pay threshold for LDL-C ≥ 100 and ≥ 70 mg/dL, respectively. Our results are in line with the probabilistic sensitivity analysis reported by Gandra et al. [63], who reported that, when evolocumab was added to standard of care it reached 100% cost effectiveness for the HeFH population, and other ASCVD groups reached 87% probability of being cost effective compared with 100% for both ASCVD groups in our study.
There is a high burden of noncommunicable diseases in the KSA, and these account for 73% of deaths (of which a major cause is CVD) [64]. PCSK9 inhibitors such as evolocumab, in addition to improved diet and exercise in line with European Society of Cardiology (ESC) guidelines, could play a major role in reducing mortality and improving health outcomes in the Saudi population. This evaluation provides insights as to the economic implications of evolocumab therapy if applied to eligible patients with ASCVD in Saudi clinical practice.
The analysis is associated with some limitations, namely the lack of utility values specific to the Saudi population. However, this information was derived from alternative international populations with assumptions to ensure the analysis was representative of the Saudi general population. Model inputs, including utility values were validated by a group of leading cardiologists practicing in the KSA to ensure they were representative of the KSA population. Second, the public healthcare system in the KSA is fragmented, which may lead to variations in the estimated healthcare costs across different healthcare sectors; results should therefore be interpreted with caution. Furthermore, real-world evidence about the efficacy and safety of evolocumab is lacking in the KSA. Even though FOURIER did not have a subgroup analysis specific to race, the results are still considered generalizable to the population in the KSA setting. We recognize the challenge in using clinical trial data in economic evaluation; however, it is currently the best available source for patient outcomes data to be used in economic evaluations given the lack of country-specific patient outcome data. Another limitation was that the model assumed that the LDL-C reduction at week 48 was assumed to remain constant over the modeled time horizon [12]. However, this assumption is aligned with the observed sustained reductions in LDL-C for up to 5 years of evolocumab treatment in the open-label, randomized extension study OSLER-1 [15, 43]. Finally, the latest guidelines from the ESC suggested a further possible subgroup of interest: patients with LCL-C baseline levels ≥ 55 mg/dL. As data currently available for this population in the KSA are limited, results would be associated with high uncertainty given the assumptions that would be required.
5 Conclusion
This economic evaluation suggests that evolocumab is a cost-effective treatment choice for patients with clinically evident ASCVD and HeFH whose LDL-C levels are not controlled with conventional LLTs. Decision makers may consider evolocumab as a useful option in improving the care of patients with ASCVD and HeFH in the KSA. Future economic research should consider new LDL-C guideline changes and take into account any utility values that will be established for a Saudi population.
References
Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25.
WHO. Noncommunicable Diseases (NCD): Country statistics and global health estimates by WHO and UN partners 2019. http://who.int/gho/mortality_burden_disease/en/.
Osman AM, Alsultan MS, Al-Mutairi MA. The burden of ischemic heart disease at a major cardiac center in Central Saudi Arabia. Saudi Med J. 2011;32(12):1279–84.
Bhatnagar D, Soran H, Durrington PN. Hypercholesterolaemia and its management. BMJ (Clin Res Ed). 2008;337:993.
Roth G, Fihn S, Mokdad A, Aekplakorn W, Hasegawa T, Lim S. WHO|High total serum cholesterol, medication coverage and therapeutic control: an analysis of national health examination survey data from eight countries. Bull World Health Organ. 2011;89:92–101.
Punekar RS, Fox KM, Richhariya A, Fisher MD, Cziraky M, Gandra SR, et al. Burden of first and recurrent cardiovascular events among patients with hyperlipidemia. Clin Cardiol. 2015;38:483–91.
Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;39(15):2999–3058.
Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2013;2014:129.
Pijlman AH, Huijgen R, Verhagen SN, Imholz BPM, Liem AH, Kastelein JJP, et al. Evaluation of cholesterol lowering treatment of patients with familial hypercholesterolemia: a large cross-sectional study in The Netherlands. Atherosclerosis. 2010;209:189–94.
Elis A, Zhou R, Stein EA. Effect of lipid-lowering treatment on natural history of heterozygous familial hypercholesterolemia in past three decades. Am J Cardiol. 2011;108:223–6.
Toth PP, Worthy G, Gandra SR, Sattar N, Bray S, Cheng LI, et al. Systematic review and network meta-analysis on the efficacy of evolocumab and other therapies for the management of lipid levels in hyperlipidemia. J Am Heart Assoc. 2017;6(10):e005367.
Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–22.
Giugliano RP, Pedersen TR, Park J-G, De Ferrari GM, Gaciong ZA, Ceska R, et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet. 2017;390(10106):1962–71.
Nicholls SJ, Puri R, Anderson T, Al E. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316(22):2373–84.
Koren M, Sabatine M, Giugliano R. Long-term low-density lipoprotein cholesterol–lowering efficacy, persistence, and safety of evolocumab in treatment of hypercholesterolemia: Results up to 4 years from the open-label osler-1 extension study. JAMA Cardiol. 2017;2:598–607.
Stroes E, Robinson JG, Raal FJ, Dufour R, Sullivan D, Kassahun H, et al. Consistent LDL-C response with evolocumab among patient subgroups in PROFICIO: a pooled analysis of 3146 patients from phase 3 studies. Clin Cardiol. 2018;41(10):1328–35.
Azari S, Rezapour A, Omidi N, Alipour V, Behzadifar M, Safari H, et al. Cost-effectiveness analysis of PCSK9 inhibitors in cardiovascular diseases: a systematic review. Heart Fail Rev. 2019.
Fonarow G, Keech A, Pedersen T. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:1069–78.
Korman MJ, Retterstøl K, Kristiansen IS, Wisløff T. Are PCSK9 inhibitors cost effective? Pharmacoeconomics. 2018;36(9):1031–41.
Alhabib KF, Kinsara AJ, Alghamdi S, Al-Murayeh M, Hussein GA, AlSaif S, et al. The first survey of the Saudi acute myocardial infarction registry program: main results and long-term outcomes (STARS-1 Program). PLoS ONE. 2019;14(5):e0216551.
Al Sifri S, Al Shammeri O, Al Jaser S, Alkhenizan A, Bin Shafi Shafiurrehman A, Morcos B, et al. Prevalence of lipid abnormalities and cholesterol target value attainment in patients with stable coronary heart disease or an acute coronary syndrome in Saudi Arabia. Saudi Med J. 2018;39(7):697–704.
Altowaijri AA, Balkhi N, Alghamdi B. PCV50 economic burden of major cardiovascular diseases and ischemic stroke in Saudi Arabia: a cost of illness study. Value Health. 2020;23:S495–6.
Al-Rasadi K, Al-Zakwani I, Alsheikh-Ali AA, Almahmeed W, Rashed W, Ridha M, et al. Prevalence, management, and outcomes of familial hypercholesterolemia in patients with acute coronary syndromes in the Arabian Gulf. J Clin Lipidol. 2018;12(3):685-92.e2.
Villa G, Lothgren M, Kutikova L, Lindgren P, Gandra SR, Fonarow GC, et al. Cost-effectiveness of evolocumab in patients with high cardiovascular risk in Spain. Clin Ther. 2017;39:771-86.e3.
Gandra S, Villa G, Fonarow G, Lothgren M, Lindgren P, Somaratne R, et al. Cost-effectiveness of LDL-C lowering with evolocumab in patients with high cardiovascular risk in the United States. Clin Cardiol. 2016;39:313–20.
Toth PP, Danese M, Villa G, Qian Y, Beaubrun A, Lira A, et al. Estimated burden of cardiovascular disease and value-based price range for evolocumab in a high-risk, secondary-prevention population in the US payer context. J Med Econ. 2017;20:555–64.
Borissov B, Urbich M, Georgieva B, Tsenov S, Villa G. Cost-effectiveness of evolocumab in treatment of heterozygous familial hypercholesterolaemia in Bulgaria: measuring health benefit by effectively treated patient-years. J Market Access Health Policy. 2017;5:1412753.
Landmesser U, Lindgren P, Hagström E, van Hout B, Villa G, Pemberton-Ross P, et al. Cost-effectiveness of proprotein convertase subtilisin/kexin type 9 inhibition with evolocumab in patients with a history of myocardial infarction in Sweden. Eur Heart J Qual Care Clin Outcomes. 2020.
Sanders G, Neumann P, Basu A, Brock D, Feeny D, Krahn M. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–103.
Inc. A. A systematic review of cardiovascular outcomes-based cost effectiveness analyses of lipid lowering therapies. Amgen data on file. 2016.
Jena A, Blumenthal D, Stevens W, Chou J. Value of improved lipid control in patients at high risk for adverse cardiac events. Am J Manag Care. 2016;22:199–207.
Abtan J, Bhatt DL, Elbez Y, Sorbets E, Eagle K, Ikeda Y, et al. Residual ischemic risk and its determinants in patients with previous myocardial infarction and without prior stroke or TIA: insights from the REACH registry. Clin Cardiol. 2016;39:670–7.
Wilson PWF, D’Agostino R, Bhatt DL, Eagle K, Pencina MJ, Smith SC, et al. An international model to predict recurrent cardiovascular disease. Am J Med. 2012;125:695–703.
CTTC. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376:1670–81.
D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53.
Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:331–40.
Amgen Inc. RUTHERFORD-2 (Study 20110117) Clinical study report. Amgen data on file. 2014.
Goldberg AC, Hopkins PN, Toth PP, Ballantyne CM, Rader DJ, Robinson JG, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S1-8.
Ohman EM, Bhatt DL, Steg PG, Goto S, Hirsch AT, Liau CS, et al. The REduction of Atherothrombosis for Continued Health (REACH) Registry: an international, prospective, observational investigation in subjects at risk for atherothrombotic events-study design. Am Heart J. 2006;151(4):786.e1-10.
Villa G, Wong B, Kutikova L, Ray K, Mata P, Bruckert E. Prediction of cardiovascular risk in patients with familial hypercholesterolaemia. Eur Heart J Qual Care Clin Outcomes. 2017;3:274–80.
WHO. The World Health Organization: Global Health Observatory data repository. Life tables by country, Saudi Arabia, 2016.
Instituto Nacional de Estadística. Tablas de mortalidad. Madrid2015.
Koren M, Sabatine M, Giugliano R. Long-term evolocumab for the treatment of hypercholesterolemia. Presented at American Heart Association’s Scientific Sessions; Chicago, USA; November 10–November 14 2018. 2018.
Buchwald H, Varco RL, Matts JP, Long JM, Fitch LL, Campbell GS, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia. N Engl J Med. 1990;323:946–55.
Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. The IMPROVE-IT study. N Engl J Med. 2015;372:2387–97.
Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–61.
Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, et al. Helsinki heart study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. N Engl J Med. 1987;317:1237–45.
Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375:2144–53.
Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–97.
Ference BA, Ginsberg HN, Graham I, Kausik K, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–72.
Inc. A. FOURIER time to IP discontinuation. Amgen data on file. 2017.
Alburikan KA, Asiri RM, Alhammad AM, Abuelizz AA, Bawazeer GA, Aljawadi MH. Utilization and adherence to guideline-recommended lipid-lowering therapy at an academic medical center. Ann Saudi Med. 2017;37(4):276–81.
Confidential. Report of economic burden of major cardiovascular diseases and ischemic stroke in Saudi Arabia: a cost of illness study. 2019.
Matza LS, Stewart KD, Gandra SR, Delio PR, Fenster BE, Davies EW, et al. Acute and chronic impact of cardiovascular events on health state utilities. BMC Health Serv Res. 2015;15:173.
Ara R, Wailoo A. Using health state utility values in models exploring the cost-effectiveness of health technologies. Value Health J Int Soc Pharmacoecon Outcomes Res. 2012;15(6):971–4.
Belgian Health Interview Survey. Health related quality of life. 2013.
WHO. Cost effectiveness and strategic planning (WHO-CHOICE). 2015.
Fonarow GC, van Hout B, Villa G, Arellano J, Lindgren P. Updated cost-effectiveness analysis of evolocumab in patients with very high-risk atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4(7):691–5.
Lindgren P, Hagström E, Hout Bv, Villa G, Pemberton-Ross P, Arellano J, et al. Cost-effectiveness of evolocumab in atherosclerotic cardiovascular disease patients with varying risk profiles in Sweden. 2019.
Kazi D, Moran A, Coxson P, Penko J, Ollendorf D, Pearson S. Cost-effectiveness of pcsk9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743–53.
Kumar R, Tonkin A, Liew D, Zomer E. The cost-effectiveness of PCSK9 inhibitors—the Australian healthcare perspective. Int J Cardiol. 2018;267:183–7.
Lee TC, Kaouache M, Grover SA. Evaluation of the cost-effectiveness of evolocumab in the FOURIER study: a Canadian analysis. CMAJ Open. 2018;6(2):E162–7.
Gandra SR, Villa G, Fonarow GC, Lothgren M, Lindgren P, Somaratne R, et al. Cost-effectiveness of LDL-C lowering with evolocumab in patients with high cardiovascular risk in the United States. Clin Cardiol. 2016;39(6):313–20.
The burden of disease in Saudi Arabia 1990–2017: results from the Global Burden of Disease Study 2017. Lancet Planet Health. 2020;4(5):e195-e208.
Acknowledgements
The authors thank Dr Khalid Alhabib for his contribution to the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was funded by Amgen Inc.
Conflict of interest
BresMed was contracted by Amgen for the adaptation of the global model to Saudi settings. Ahmed Alghamdi, Bander Balkhi, Abdulaziz Altowaijri, and Nasser Al-shehri received consultation fees from BresMed. Rima Aziziyeh, Fawaz Aljanad, and Michael Urbich are employees of Amgen who are the manufacturers of evolocumab. Emily-Ruth Marriott and Lewis Ralph are employees of BresMed.
Availability of data and material
Not applicable
Ethics approval
Not applicable
Consent to participate
Not applicable
Code availability
Not applicable
Author contributions
All authors were involved in the study conception and the analysis and interpretation of data as well as the drafting of the manuscript. All authors have read and approved this version of the manuscript. All authors agree to be accountable for all aspects of the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Alghamdi, A., Balkhi, B., Altowaijri, A. et al. Cost-Effectiveness Analysis of Evolocumab for the Treatment of Dyslipidemia in the Kingdom of Saudi Arabia. PharmacoEconomics Open 6, 277–291 (2022). https://doi.org/10.1007/s41669-021-00300-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-021-00300-8