Abstract
Background
Coronary artery disease (CAD) is the most common type of heart and circulatory disease and is the leading cause of death worldwide. The current diagnostic pathway can lead to patient complications and is also extremely costly. A new medical device, the CADScor System (Acarix AB), was developed for the acoustic detection of CAD before patients undergo invasive diagnostic procedures.
Objectives
Our objective was to assess the cost utility of the CADScor System for the diagnosis of CAD at an early stage in the diagnostic testing pathway in England.
Methods
A two-part economic model, consisting of a decision tree and Markov model, was developed to consider the cost utility (cost per quality-adjusted life-year [QALY] gained) of introducing the CADScor System for the diagnosis of CAD. The decision tree component explored the short-term costs and diagnostic outcomes associated with introduction of the test compared with the existing testing pathway. A Markov model was developed to explore the longer-term health and economic implications of the condition and original diagnosis, with costs and health effects estimated over different time horizons. Parameter uncertainty was explored in deterministic and probabilistic sensitivity analyses.
Results
Base-case results indicated that the CADScor System would result in cost savings (− £131 per patient) and a marginal increase in QALYs (0.00001) over a 1-year time horizon. Probabilistic results indicated that the intervention had a > 99% probability of being cost effective at a willingness-to-pay threshold of £20,000 per QALY gained and 100% probability of being cost saving. Results from the deterministic sensitivity analyses indicated that variations in parameters related to the accuracy and cost of the CADScor System, and the prevalence of CAD, had the greatest impact on the overall results. The overall cost saving was estimated to be over £12.3 million per 100,000 eligible patient population.
Conclusions
The CADScor System is a potentially cost-saving test for the diagnosis of CAD. When initiated before the use of non-invasive cardiac imaging tests such as computed tomography coronary angiography, the test reduced costs to the healthcare service over various time horizons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The existing diagnostic pathway for coronary artery disease (CAD) is costly and can lead to patient complications. A new medical device, the CADScor system (Acarix AB), was developed for the acoustic detection of CAD before patients undergo invasive diagnostic procedures. |
An economic model was developed to consider the costs and clinical outcomes associated with the introduction of this new device to the diagnostic testing pathway for CAD in England. |
Results of the analysis indicated that the introduction of the CADScor system has the potential to lead to cost savings and improved patient outcomes. |
1 Introduction
Coronary artery disease (CAD) is the most common type of heart and circulatory disease and is the leading cause of death worldwide [1]. CAD is a condition that develops due to a narrowing or blockage of the coronary arteries, which decreases blood supply to the heart [2]. This blockage, and the resulting interruption of blood flow, is often as a result of the walls of the arteries becoming “clogged” because of a build-up of cholesterol and fatty deposits (or atheroma), in a process known as atherosclerosis [2]. This leads to a partial, or total, blockage of blood flow and oxygen to the heart, which causes the heart to contract less efficiently. Over time, as the situation worsens, this can result in the patient experiencing issues such as angina, nausea and light-headedness, pain or discomfort in the arms or shoulders, heart attack, and heart failure. Common risk factors for CAD include high blood pressure, high blood cholesterol levels, tobacco smoking, obesity, inactivity, excessive alcohol consumption and unhealthy eating habits. Risk of CAD also increases with age, with men having a higher risk of experiencing the condition from age 45 onwards, and women having an increased risk from age 55. Genetics and family history are also linked to the probability of developing the condition [3].
In the UK, CAD is the most common cause of premature death and is responsible for around 64,000 deaths each year. This equates to an average of 180 people per day. The disease kills one in seven men and one in 12 women, with more than twice as many women dying from CAD as from breast cancer [1]. In addition to the high mortality rate, morbidities associated with related symptoms have a detrimental impact on quality of life. Currently, approximately 2.3 million people live with the condition in the UK (1.5 million men and 830,000 women), highlighting the extent of the issue [1]. The economic consequences of the disease are also significant. The British Heart Foundation estimated that CAD cost the healthcare system £1.8 billion in 2009, with the costs of hospital care accounting for 56% of the total cost. In addition, productivity loss associated with mortality and morbidity was estimated to cost the UK over £3 billion, with 33% of this cost due to death, 14% due to illness amongst those of working age and the remaining £1.7 billion due to costs associated with providing informal care to patients [4].
The first line of treatment for CAD usually involves the patient making healthy lifestyle changes, including quitting smoking, eating healthier foods and exercising regularly. Where alterations to lifestyle are unsuccessful, patients may be administered medication, including cholesterol-modifying medications, β-blockers, calcium channel blockers and aspirin (acetylsalicylic acid [ASA]), amongst others. Where prior treatments have failed to sufficiently improve and restore blood flow, some patients may go on to have more aggressive treatment options, including angioplasty and/or coronary artery bypass surgery [5]. However, it should be noted that the treatment pathway is heavily dependent on the stage of CAD with which the patient presents. For instance, patients may be administered pharmacological therapy for early-stage CAD or more advanced treatment for advanced CAD immediately whilst also being encouraged to make necessary lifestyle changes.
Prior to treatment, an accurate diagnosis of the condition needs to be made. The current management pathway, based on UK National Institute for Care and Excellence (NICE) clinical guidelines, begins with patients presenting with symptoms of stable CAD undergoing a physical examination and clinical history assessment [6]. Where CAD cannot be ruled out based on clinical assessment alone, it is recommended that patients are tested using computed tomography coronary angiography (CTCA) as a first-line diagnostic, which involves taking images of the coronary arteries. An electrocardiogram (ECG) test may also be done at this point to confirm diagnosis. Where CTCA has shown CAD or failed to rule out the condition, patients will be offered non-invasive functional imaging (NIFI) for myocardial ischaemia. Finally, where results of NIFI are inconclusive, invasive coronary angiography (ICA) will be used as a third-line test. These invasive diagnostic tests are both costly and present a risk of complications, highlighting the benefits of appropriately and accurately ruling out patients who do not have CAD at an earlier point in the diagnostic testing pathway [7].
The CADScor System (Acarix AB) is an innovative medical device that was developed for the acoustic detection of CAD before patients undergo CTCA [8]. Therefore, it would act as a first-line ‘rule-out’ test before patients are stratified to subsequent invasive testing. The device works by recording heart sounds, murmurs and vibrations and converting them into a CAD-score (range 0–99), using data from both the recording and patient characteristics (i.e., age, sex and blood pressure). Based on this CAD-score, patients are then either stratified to further testing or, alternatively, the score will suggest a low probability of CAD and no further investigation is recommended. While past studies have highlighted its strong diagnostic performance [7] and clinical viability [9], there is a lack of evidence on the potential economic impact associated with introducing the CADScor System as an early-stage rule-out test in the CAD diagnostic pathway. This study aims to develop an economic model to explore relevant costs and patient outcomes for England.
2 Methods
An economic decision-analytic model was developed in Microsoft Excel to reflect the clinical pathways typically followed by patients presenting with symptoms of stable CAD (mean age 57 years [10]). We calculated the mean age using data from the Hospital Episode Statistics database for patients who were referred for CAD diagnostic testing. The model was designed to reflect the diagnostic testing pathways that patients would typically follow both under current practice and after introduction of the CADScor System. Longer-term clinical pathways, depending on whether the patient was diagnosed/undiagnosed and based on the clinical events that patients may experience, were also modelled. Therefore, progression of patients through the model was dependent on whether or not they had CAD, the accuracy of the individual and combined diagnostic tests and the effectiveness of patient treatment/management once diagnosed/undiagnosed.
The recommended discount rate in the UK (3.5% per annum) was applied for both costs and benefits [11], with discounting applied after the first year of the model. All costs included in the model were considered from the English national health service (NHS) and personal social services perspective.
2.1 Model Overview
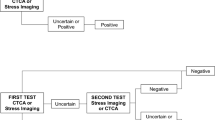
A two-part economic model consisting of a short-term decision tree and a long-term Markov model was developed. The decision tree component of the model was developed to capture the sequence of diagnostic tests in the intervention and comparator arms, whereas the Markov model captured the longer-term outcomes for patients diagnosed (or not) with CAD. In the Markov model, progression over the model time horizon was represented by various health states and clinical probabilities of progressing from one health state to the next. Model structures are shown in Figs. 1 and 2.
The decision tree model began at the point where all patients have previously undergone prior clinical assessment, having presented with symptoms of stable CAD. Based on UK NICE guidelines (CG95), 64-slice CTCA would be conducted in patients following this clinical assessment as would a 12-lead ECG test [6] if CAD could not be ruled out at this point (i.e., results were positive or equivocal). Therefore, where clinical assessment is unable to rule out CAD, or where ECG shows ST-T changes or Q waves, patients would receive CTCA. This is the point at which the decision tree model began, as it is also the point at which the CADScor System would be used, i.e., prior to CTCA but following initial clinical assessment. All subsequent model pathways were based on these same national guidelines [6]. Therefore, where CTCA could not rule out CAD (i.e., test results were positive or equivocal), patients in the intervention arm progressed to undergo NIFI testing using myocardial perfusion scan (MPS). Once again, where this second-line test could not rule out CAD, patients progressed to undergo a third-line ICA test. As highlighted, these pathways were based on official NICE guidelines [6] despite some evidence indicating that direct referral to ICA is not uncommon [12]. Therefore, it should be noted that, although the model pathways adhered strictly to the NICE guidelines [6], in practice, patients may skip lines of testing based on presentation and disease severity. Similarly, although methods such as fractional flow reserve may also be used to assess coronary blood flow in clinical practice as an aid to existing diagnostic methods, these were not accounted for in the model structure. Although the decision tree is simplistic, it nevertheless allowed for an estimation of the cost and health outcome implications of introducing the CADScor System to the existing pathway.
The pathways in the intervention (CADScor System) arm of the decision tree model were identical, except that a CADScor test was provided as a first-line diagnostic test instead of CTCA, with CTCA, MPS and ICA provided as second-, third- and fourth-line tests where CAD could not be ruled out or where the results of testing were equivocal. Patients undergoing testing with the CADScor System progressed to further testing if the test score exceeded 20, whereas a score ≤ 20 indicated a low probability of CAD and no requirement for further testing [8]. The CADScor System can classify patients as low risk (≤20), intermediate risk (21–29) and high risk (≥ 30). However, as no evidence exists as to how intermediate- and high-risk patients would be stratified in England, we assumed that patients in the intervention and comparator arms who tested positive or equivocal after the initial CADScor test followed the same lines of testing, i.e., no distinguishing between intermediate- and high-risk patients regarding subsequent testing after the CADScor test. Costs associated with testing patients were captured in the decision tree, as were the proportions of patients who could be categorised as positive and negative for CAD (including the accuracy of this diagnosis, i.e., true positive [TP], false positive [FP], true negative [TN], false negative [FN]). Therefore, potential per-patient cost savings, as well as change in accuracy of diagnosis, could be estimated. The structure of the decision tree model is shown in Fig. 1. The number of TP, FP, FN and TN cases were estimated using the following formulae:
A Markov-cohort model was developed to capture the 1-year costs and outcomes for patients in the post-diagnosis period. This model was designed to capture outcomes for patients diagnosed with CAD (both correctly and incorrectly) and amongst patients diagnosed as not having CAD (both correctly and incorrectly) based on progression across health states relevant to this patient population. The model structure was defined based on a model by Cowie et al. [13], which looked at the cost effectiveness of rivaroxaban in combination with ASA amongst a patient population with CAD or peripheral artery disease (PAD) in the UK. The model structure considered the long-term clinical complications most pertinent for this patient population and was considered to be the most appropriate structure from previous modelling studies identified.
All patients in the model began at a point where they were defined as being ‘event free’. Patients accurately diagnosed as having CAD received treatment (ASA); patients accurately or inaccurately diagnosed as not having CAD did not receive treatment. We assumed that FP cases would be checked with ICA, which has 100% sensitivity, and therefore would be reclassified as TN. Although, in practice, some patients with stable angina may undergo revascularisation after being administered anti-anginal/anticoagulation medications, to simplify the model, we modelled only the first line of treatment. Additionally, patients who did not receive treatment when they should have been (i.e., FN cases) were assumed to have a higher risk of experiencing subsequent clinical events than TP patients receiving appropriate treatment (see Sect. 2.2.1). Patients could either remain in this health state, die from causes unrelated to CAD or progress to ‘first acute event’ having experienced a CAD-related event. Events modelled included myocardial infarction (MI) and stroke (divided into ischaemic stroke [IS] and intracranial haemorrhage [ICH]). Patients either died from this acute event or progressed to a ‘post first acute event’ health state to account for the differing costs, utilities and clinical probabilities associated with the aftermath of experiencing a major adverse cardiovascular event compared with the initial acute event health state. Alternatively, patients progressed directly from this first acute event health state directly to a second acute event health state (i.e., MI2 [e.g., if the first event was MI], IS or ICH). Where patients entered a post-first-event health state, they either remained in this post-event health state, died or progressed to experience a second cardiovascular event (‘second acute event’). Once again, patients either died from this second event or progressed to a second post-event health state (‘post second acute event’). Once in the second post-event health state, patients either remained in this health state or died. However, the possibility of patients experiencing additional complications, including venous thromboembolism and major extracranial non-fatal bleeding events within each health state, were also modelled.
The Markov model had a cycle length of 3 months; therefore, the probability of patients experiencing more than one adverse cardiovascular event per time cycle was considered negligible. Figure 2 is a simple representation of the model structure. Costs associated with the resources used in each health state were assigned to the model. Additionally, the quality of life of patients in each health state was represented by utility values. Both costs and utilities, as well as relevant clinical parameters, are described in Sect. 2.2.
2.2 Model Inputs
All model inputs are presented in the following section. Point estimate values for the clinical effectiveness, utility and cost parameters are presented in Table 1. Additionally, uncertainty around the point estimate, as well as a distribution of uncertainty, are presented where appropriate and available to allow for a probabilistic analysis to be undertaken.
2.2.1 Clinical Effectiveness Parameters
Clinical data were identified from relevant published literature to populate both the decision tree and Markov models. The prevalence of CAD amongst patients presenting with symptoms of stable CAD was derived from a previous cost-effectiveness analysis of stand-alone or combined non-invasive imaging tests for the diagnosis of stable CAD [14]. This study involved a health economic analysis based on data from the EVINCI (Evaluation of Integrated Cardiac Imaging for the Detection and Characterization of Ischemic Heart Disease) study, which looked at the performance of CTCA compared with alternative non-invasive stress-imaging modalities for the diagnosis of obstructive CAD in patients with stable chest pain symptoms. Given that this was a multi-national European trial, and given the similarity of patients with the patient population included in this analysis (patients presenting with symptoms of stable CAD), we considered the prevalence data appropriate for use in our economic analysis.
Data on the test accuracy of the CADScor System were sourced from a paper discussing risk classification of CAD using this new method [9]. The population included in the study were patients suspected of stable CAD, with the CADScor System being used to classify patients as having intermediate to low likelihood of having the condition. Patient data from 2245 patients were included in this retrospective analysis of existing cohorts, which is the largest amount of data available to inform a diagnostic accuracy assessment of the technology. In this study, heart sound recordings and patient data from three clinical studies were combined in a single dataset. In each of these studies, patients with a CTCA indicating CAD were referred for ICA. A CAD-score was then constructed by processing the audio recordings of heart sounds. The CADScor System was utilised to assess heart sound signals from patients. The Diamond–Forrester score was then used to calculate pre-test probability, with patients classified according to available guidelines for CAD as low (< 15%), intermediate (15–85%) and high (> 85%) risk. Intermediate-risk patients could then be reclassified according to their CAD-Score, with those with scores ≤ 20 reclassified as having a low risk of CAD. The diagnostic accuracy of the system could then be estimated.
Data on the accuracy of CTCA, MPS and ICA were all derived from an earlier cost-effectiveness analysis of alternative diagnostic evaluation strategies for individuals with stable chest pain syndrome and suspected CAD [15]. The population included in this analysis was comparable to our own population, and the test performance characteristics were all sourced from published multicentre trials, with a subsequent bivariate random effects model utilised to produce unbiased estimates of test sensitivity and specificity whilst preserving the joint distribution or correlation between sensitivity and specificity. The mortality risks associated with CTCA, MPS and ICA were all derived from a previous study that involved a clinical- and cost-effectiveness analysis of CTCA for use amongst patients with chest pain [16].
In the Markov model, data to inform the transition probabilities of patients were all sourced from a previous clinical study exploring cardiovascular outcomes amongst patients undergoing alternative anticoagulation strategies (COMPASS trial) [17] and from NICE technology appraisal guidance on the use of rivaroxaban for preventing atherothrombotic events in people with CAD or PAD [18]. The COMPASS trial was a double-blind superiority trial comparing alternative treatment strategies amongst patients with stable CAD or PAD. In total, 27,395 patients were recruited from 602 centres in 33 countries, and the study provided an excellent source of long-term data for this patient population [17]. Similarly, the NICE technology appraisal guidance provided useful long-term data for population of the model [18]. Death rates associated with the modelled health states were derived from the same studies [17, 18]. The relative risks of patients experiencing a range of clinical adverse events when not undergoing anticoagulation treatment were sourced from an earlier study of CTCA for use amongst patients with chest pain [16] and from the NICE technology appraisal guidance [18]. All clinical data are presented in Table 1.
2.2.2 Utilities
All health states and adverse events included in the model were assigned appropriate utility values, or decrements, respectively. All values were derived from the same clinical study exploring cardiovascular outcomes, referenced previously [17]. In this multi-national study involving 33 countries, the quality of life of patients receiving treatment for stable CAD or PAD with either rivaroxaban or aspirin was assessed using the EQ-5D [17]. Utility values are presented in Table 1.
2.2.3 Costs
The cost of testing with the CADScor System was derived from a Medtech innovation briefing focussing on the use of the test for ruling out CAD and on inputs from the manufacturer (Acarix AB) [8]. The estimated per-test cost of £27.25 was based on a system cost of £2100 per unit (excluding value-added tax) and an assumption that the technology would have a lifespan of 2 years and be used to test three patients per day, for 4 days a week for 41 weeks of the year. Information on lifespan, frequency of testing and number of patients receiving testing was provided by the company. However, it should be noted that patient throughput is likely to vary depending on the setting in which the test is used and the size of the trust delivering testing. Therefore, the cost per test will also vary according to these factors, and the unit cost applied in the model was an estimate based on an assumed number of tests delivered. It should also be noted that the existing cost of the test does not include the cost of training healthcare professionals to use the device, because the company will be providing the training free of charge. Additionally, although healthcare staff would need to spend a short amount of time receiving training on use of the device, the number of patients that would ultimately receive testing from individual staff with the device would render the cost of training on an individual patient level negligible. Therefore, these costs were omitted from the analysis.
Costs for the other diagnostic tests included in the model and the cost of the consultant-led outpatient appointment in the cardiology ward required for delivery of the test were sourced from UK NHS reference costs, 2019 [19]. Medication costs considered in the long-term Markov model were sourced from the British National Formulary, 2019 [20]. Costs related to the health states included in the Markov model were sourced from a population-based cohort study examining long-term healthcare use and costs in patients with stable CAD [21]. Costs of complications included in this model were derived from UK NHS reference costs, 2019 [19], and a previous economic evaluation [13]. All costs included in the model are presented in Table 1. Costs are in sterling (£) for the year 2019. Where costs were derived from a source prior to 2019, costs were inflated accordingly using the Campbell Collaboration Evidence for Policy and Practice Information and Co-ordinating Centre cost converter [22].
2.3 Analysis
Expected costs and health outcomes associated with the two strategies being compared were estimated. Results of the analysis are presented in the form of cost per quality-adjusted life-year (QALY) gained. In total, 10,000 iterations of the model were run in a Monte Carlo simulation to obtain probabilistic results, and results of these analyses are presented in the form of cost-effectiveness planes and cost-effectiveness acceptability curves (CEACs). The impact of individual and multiple parameter variations on the model results were also explored in deterministic sensitivity analyses. Results of these analyses are presented using tornado diagrams. We performed several scenario analyses to address the structural and parameter-related uncertainties. We investigated the impact of different prevalence rates of CAD (i.e., 3, 5 and 10%) amongst patients referred for non-invasive cardiac imaging tests to rule out CAD. A recent study [23] comparing different non-invasive cardiac imaging tests as a gatekeeper versus upfront ICA found that ICA was still performed in about 80% of patients in the non-invasive groups. In our scenario analysis, we assumed ICA would be offered to 80% of patients post-CTCA/MPS because of inconclusive results or for definitive diagnosis of CAD. We also assessed the combined impact of these scenarios. In addition, we assessed the impact of using different methods to estimate the CADScor test cost per use. Instead of estimating the equipment cost per use using annualization of equipment cost, we assumed it could be used for 1000 tests.
3 Results
Results of the base-case analysis, and all sensitivity analyses, are presented in the following subsections, with those from the decision tree component of the model presented first, followed by results from the Markov model.
3.1 Decision Tree
Diagnostic results from the decision tree analysis, presented in Table 2, indicated that implementing an additional line of testing before the use of CTCA resulted in over £12.3 million of savings per each 100,000 population presenting with symptoms of CAD (i.e., 16% reduction in the overall costs of diagnostic testing). This equates to an average diagnostic cost saving of £123 per patient. Our results indicated that per each 100,000 people for whom CAD cannot be ruled out based on clinical assessment or/and ECG, using the CADScor System could lead to five fewer diagnostic intervention-related deaths (22 vs. 27).
3.2 Markov Model
3.2.1 Base-Case Analysis
Table 3 shows the results from the base-case analysis. Findings indicated that the introduction of the CADScor System resulted in per-patient overall cost savings of £131 over a 1-year time horizon. This equates to over £92.6 million cost savings per each annual cohort of patients who have CAD symptoms and need further assessment in England. There is a marginal improvement in QALYs lived per patient over the same time horizon (0.00001). Probabilistic results indicated that the intervention had a > 99% probability of being cost effective at a £20,000 willingness-to-pay (WTP) threshold and a 100% probability of being cost saving. In the scatterplot and CEAC shown in Fig. 3, results from the simulation analysis are clustered within the south-east quadrant of the cost-effectiveness plane, indicating that the intervention is always cost saving and marginally more effective than the comparator.
3.2.2 Sensitivity Analysis
Deterministic sensitivity analyses were also carried out to explore the impact of key parameter variations on the model results. A number of model parameters were varied by 25% (increased and decreased) to examine the effect that each variation would have on the incremental cost of the intervention and on the net monetary benefit (NMB) of the CADScor System. NMB is an alternative way of considering the economic efficiency of a healthcare intervention and is calculated as follows: (incremental benefit × threshold) – incremental cost. A positive incremental NMB indicates that an intervention is cost effective at a given WTP threshold. Results of these sensitivity analyses are presented in the form of tornado diagrams in Fig. 4 (incremental cost) and Fig. 5 (incremental NMB).
Tornado diagram showing the impact of changing the input parameters by ± 25% on the estimated incremental cost of the intervention. CAD coronary artery disease, CC comorbidity and complications, CTCA computed tomography coronary angiography, ICA invasive coronary angiography, ICH intracranial haemorrhage, IS ischaemic stroke, MI myocardial infarction, MPS myocardial perfusion scan
Tornado diagram showing the impact of changing the input parameters by ± 25% on the estimated net monetary benefit of the intervention. CAD coronary artery disease, CC comorbidity and complications, CTCA computed tomography coronary angiography, ICA invasive coronary angiography, ICH intracranial haemorrhage, IS ischaemic stroke, MI myocardial infarction, MPS myocardial perfusion scan
Results from Figs. 4 and 5 indicated that the parameters with the greatest impact on the incremental cost of the intervention included the accuracy of the CADScor, ICA and CTCA tests; the costs of the CADScor, ICA and CTCA tests; and the prevalence of CAD amongst patients presenting with symptoms. Results suggested that increasing the accuracy of the CADScor System and reducing the costs of the test led to further cost savings for the intervention. Results also suggested that the cost savings of the CADScor System improved when the prevalence of CAD increased. The estimated cost savings for different time horizons are reported in Table S1 in the electronic supplementary material (ESM). Results from the scenario analyses are also reported in Table S2 in the ESM. Change in prevalence of CAD and the method used to estimate the cost per use of the CADScor System did not have a significant impact on the estimated outcome; however, changing the percentage of patients who will need ICA post-CTCA/MPS because of inconclusive results or for definitive diagnosis of CAD had a significant impact on the estimated cost savings.
4 Discussion
We conducted a novel analysis to explore the economic and health-related impact of introducing the CADScor System in England to rule out CAD at an early stage in the diagnostic pathway. Results of the analysis showed that the probability of the intervention being cost effective across all WTP thresholds between £0 and 20,000 was > 99% over a 1-year time horizon. Additionally, probabilistic results indicated that the intervention had a 100% probability of being cost saving compared with current diagnostic practice. These results are highlighted in the presented CEAC and cost-effectiveness plane, the latter of which shows that the CADScor System was less costly than current practice in all simulations of the model analysis.
Results also indicated that introduction of the CADScor System resulted in a marginal improvement in QALYs gained over 1 year. This result is driven by the lower number of deaths associated with the introduction of an additional line of testing into the existing testing sequence, i.e., more patients are ruled out initially than with the current testing strategy, some of whom may die from complications related to more invasive tests (CTCA, MPS, ICA). However, as the death rate associated with more invasive tests is very low, the overall QALY difference between the strategies being compared was minimal, whereas the cost savings were substantial. This cost saving is partially because fewer patients would be unnecessarily tested for CAD using a range of different diagnostic tests than with the existing testing strategy. Therefore, the test would appropriately act as a cost-saving rule-out test at an early stage in the diagnostic testing pathway. For this reason, the intervention is highly likely to be cost saving.
Comparable evidence on the cost effectiveness of such a testing strategy is limited. Schmidt et al. [9] conducted a retrospective analysis of pooled data from existing cohorts to explore the potential for the CADScor System to be used as an effective rule-out device to avoid the need to send patients for subsequent testing. The authors found that the device enabled the safe and effective re-classification of patients, and they highlighted the potential for this to result in substantial cost savings. However, these potential economic advantages were not formally explored in the analysis. Similarly, whilst the NICE Medtech innovation briefing (MIB174) of the device presented a detailed assessment of the intervention [8] and highlighted separate studies by Winther et al. [7, 24] exploring the diagnostic accuracy of the test, the NICE assessment was also notable for the absence of any formal economic evaluations. Therefore, the work presented here adds substantially to the evidence base by presenting a robust cost-utility analysis of the introduction of the CADScor System in England.
There are limitations to this analysis. First, the quality-of-life impact of undergoing minimally invasive, and invasive, diagnostic testing was not explored in the decision tree component of the analysis because of a lack of robust data. Instead, we focussed on the main complication (i.e., death), diagnostic outcomes and costs associated with the two strategies being compared. Therefore, the benefits of the CADScor System are likely to be underestimated in this analysis. If other complications and utility decrements associated with undergoing CTCA, MPS and ICA were included in the model, the relative impact of the CADScor System on QALYs gained would likely be improved. A second limitation is that the decision tree analysis only considered the costs associated with testing without considering further costs that may need to be considered in such a diagnostic model, such as additional health service utilization and patient treatment. Thus, we performed several scenario analyses to address the structural and parameter-related uncertainties. Results indicated that changing the percentage of patients who would need ICA post-CTCA/MPS had a significant impact on the estimated cost savings. We used a conservative estimate of performing ICA post-CTCA/MPS in the base-case analysis compared with that reported in a previous study [23]. Therefore, the estimated cost saving is likely a conservative estimate of potential cost savings that could be achieved through the introduction of the CADScor System into the England NHS.
An additional limitation of this analysis is that the patient population is assumed to be homogenous, in that all are assumed to follow the same diagnostic pathway and receive a limited range of treatment options following diagnosis. This does not account for the varying levels of disease and symptom severity that are likely to be present in such a population or the impact of this on diagnostic tests received or on treatment effectiveness. A microsimulation model, which may be designed to consider individual patient characteristics and complex patient pathways, would be best suited to address this issue. Finally, only a small amount of data on the diagnostic accuracy of the CADScor System were available, and the available data were based on a Danish cohort [7, 9] rather than an English population. Ideally, given the focus of the analysis, this evidence would be confirmed and validated amongst a England population to include in the model analysis presented here. However, the clinical guidelines regarding testing for CAD in England are clear [6], and a robust decision tree model was developed to reflect these pathways, utilising published evidence on diagnostic accuracy, and costs, from peer-reviewed literature [8, 9, 14, 15] and routine UK sources [19]. Similarly, economic modelling of the longer-term costs and outcomes associated with CAD is a well-researched area [13, 16,17,18], and these studies were used to inform the analysis presented here. Therefore, the long-term model that was developed can be considered a robust analysis that considers the diagnostic outcomes produced by the decision tree model and extrapolates costs and outcomes over a 1-year time horizon, based on initial diagnosis. These models can be utilised further as additional evidence associated with the CADScor System and related technologies become available.
5 Conclusion
The CADScor System has the potential to be a cost-saving early-stage rule-out test for CAD and is highly likely to result in cost savings for the healthcare system in England.
References
British Heart Foundation in collaboration with the Institute of Applied Health Research, University of Birmingham. Heart and Circulatory Disease Statistics 2019. https://www.bhf.org.uk/what-we-do/our-research/heart-statistics/heart-statisticspublications/cardiovascular-disease-statistics-2019. Accessed 15 Apr 2020.
Cleveland Clinic. Coronary Artery Disease. 2019. https://my.clevelandclinic.org/health/diseases/16898-coronary-arterydisease. Accessed 15 Apr 2020.
Healthline. What is Coronary Artery Disease? 2020. https://www.healthline.com/health/coronary-artery-disease. Accessed 21 Sep 2020.
British Heart Foundation Health Promotion Research Group, Department of Public Health, University of Oxford. Coronary heart disease statistics—a compendium of health statistics. 2012. https://www.bhf.org.uk/informationsupport/publications/statistics/coronary-heart-disease-statistics-2012. Accessed 15 Apr 2020.
Mayo Clinic. Coronary Artery Disease. 2020. https://www.mayoclinic.org/diseases-conditions/coronary-artery-disease/diagnosis-treatment/drc-20350619#:~:text=Nitroglycerin.,II%20receptor%20blockers%20(ARBs). Accessed 21 Sep 2020.
NICE Clinical Guidelines CG95. Recent-onset chest pain of suspected cardiac origin: assessment and diagnosis. (Updated 2016). https://www.nice.org.uk/Guidance/CG95. Accessed 15 Apr 2020.
Winther S, Nissen L, Emil Schmidt S, et al. Diagnostic performance of an acoustic-based system for coronary artery disease risk stratification. Heart. 2018;104(11):928–35.
NICE Medtech Innovation Briefing MIB174. CADScor system for ruling out coronary artery disease in people with symptoms of stable coronary artery disease. 2019. https://www.nice.org.uk/advice/mib174. Accessed 15 Apr 2020.
Schmidt SE, Winther S, Larsen BS, et al. Coronary artery disease risk reclassification by a new acoustic-based score. Int J Cardiovasc Imaging. 2019;35(11):2019–28.
NHS Digital 2019-20 Hospital Episode Statistics, 2019-20. https://digital.nhs.uk/data-and-information/data-tools-andservices/data-services/hospital-episode-statistics. Accessed 21 Sep 2020.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. 2013. https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. Accessed 21 Sep 2020.
Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–95.
Cowie MR, Lamy A, Levy P, et al. Health economic evaluation of rivaroxaban in the treatment of patients with chronic coronary artery disease or peripheral arterial disease. Cardiovasc Res. 2019;116:1918–24.
Lorenzoni L, Bellelli S, Caselli C, et al. Cost-effectiveness analysis of stand-alone or combined non-invasive imaging tests for the diagnosis of stable coronary artery disease: results from the EVINCI study. Eur J Health Econ. 2019;20:1437–49.
Min JK, Gilmore A, Jones EC, et al. Cost-effectiveness of diagnostic evaluation strategies for individuals with stable chest pain syndrome and suspected coronary artery disease. Clin Imaging. 2017;43:97–105.
Ladapo JA, Jaffer FA, Hoffmann U, Thomson CC, Bamberg F, Dec W, Cutler DM, Weinstein MC, Gazelle GS. Clinical outcomes and cost-effectiveness of coronary computed tomography angiography in the evaluation of patients with chest pain. J Am Coll Cardiol. 2009;54(25):2409–22. https://doi.org/10.1016/j.jacc.2009.10.012.
Bosch J, Eikelboom JW, Connolly SJ, et al. Rationale, design and baseline characteristics of participants in the cardiovascular outcomes for people using anticoagulation strategies (COMPASS) trial. Can J Cardiol. 2017;33:1027–35.
NICE Technology Appraisal Guidance TA607. (2019). Rivaroxaban for preventing atherothrombotic events in people with coronary or peripheral artery disease. https://www.nice.org.uk/guidance/ta607. Accessed 15 Apr 2020.
Department of health and social care. 2019. NHS Reference costs [Online]. https://www.england.nhs.uk/national-costcollection/#ncc1819. Accessed 15 Apr 2020.
British National Formulary. 2019. https://www.medicinescomplete.com/mc/?utm_source=bnforg&utm_medium=homepage&utm_campaign=medicinescomplete. Accessed 15 Apr 2020.
Walker S, Asaria M, Manca A, Palmer S, Gale CP, Shah AD, Abrams KR, Crowther M, Timmis A, Hemingway H, Sculpher M. Long-term healthcare use and costs in patients with stable coronary artery disease: a population-based cohort using linked health records (CALIBER). Eur Heart J Qual Care Clin Outcomes. 2016;2(2):125–40. https://doi.org/10.1093/ehjqcco/qcw003.PMID:27042338;PMCID:PMC4816202.
Campbell Collaboration. CCEMG - EPPI-Centre Cost Converter. https://eppi.ioe.ac.uk/costconversion/. Accessed 15 Apr 2020.
Thom H, West NEJ, Hughes V, et al. Cost-effectiveness of initial stress cardiovascular MR, stress SPECT or stress echocardiography as a gate-keeper test, compared with upfront invasive coronary angiography in the investigation and management of patients with stable chest pain: mid-term outcomes from the CECaT randomised controlled trial. BMJ Open. 2014;4:e003419. https://doi.org/10.1136/bmjopen-2013-003419.
Winther S, Schmidt SE, Holm NR, et al. Diagnosing coronary artery disease by sound analysis from coronary stenosis induced turbulent blood flow: diagnostic performance in patients with stable angina pectoris. Int J Cardiovasc Imaging. 2016;32(2):235–45. https://doi.org/10.1007/s10554-015-0753-4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This report is independent research funded by Acarix AB company. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the Department of Health or the company.
Conflicts of interest
MJ, AM, MRH and EM are employees of Optimax Access Ltd and subcontractors to Device Access Ltd. MBH is an employee of Device Access Ltd. Device Access Ltd received funding from Acarix AB to conduct this study.
Availability of data and material
The economic model is the intellectual property of the company but may be available upon request. Please contact the study authors.
Ethics approval
Not applicable.
Consent
Not applicable.
Code availability
The economic model is the intellectual property of the company but may be available upon request. Please contact the study authors.
Author contributions
MJ was responsible for developing and populating the economic model and drafting the final version of the paper. All authors provided inputs for the model and read and approved the final draft of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Javanbakht, M., Mashayekhi, A., Hemami, M.R. et al. Economic Analysis of the CADScor System for Ruling Out Coronary Artery Disease in England. PharmacoEconomics Open 6, 123–135 (2022). https://doi.org/10.1007/s41669-021-00297-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-021-00297-0